Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health of the Population of the Republic of Kazakhstan and the Activities of Healthcare Organizations in 2018: Statistical Collection. Nur-Sultan, 2019–2324 p. Available online: http://www.rcrz.kz/index.php/ru/statistika-zdravookhraneniya-2 (accessed on 11 January 2020).
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Research Institute for Antimicrobial Chemotherapy. Scientific Report on the Results of the Study of Antibiotic Resistance of Bacterial Causative Agents of Nosocomial Infections in Departments with Intensive Use of Antibiotics in Hospitals in Russia (ReVANSH); Research Institute for Antimicrobial Chemotherapy: Smolensk, Russia, 2009. [Google Scholar]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.L.L.; Chua, A.Q.; Teo, J.Q.-M.; Cai, Y.; Lee, W.; Kwa, A.L.-H. Importance of control groups when delineating antibiotic use as a risk factor for carbapenem resistance, extreme-drug resistance, and pan-drug resistance in Acinetobacter baumannii and Pseudomonas aeruginosa: A systematic review and meta-analysis. Int. J. Infect. Dis. 2018, 76, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabi, R.; Momtaz, H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop. Med. Health 2019, 47, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laura, D.M.; Scott, N.L.; Vanner, E.A.; Miller, D.; Flynn, H.W. Genotypic and Phenotypic Antibiotic Resistance in Staphylococcus epidermidis Endophthalmitis. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.J.; Ogyu, A.; Cowling, B.J.; Fukuda, K.; Pang, H.H. Available evidence of antibiotic resistance from extended-spectrum β-lactamase-producing Enterobacteriaceae in paediatric patients in 20 countries: A systematic review and meta-analysis. Bull. World Health Organ. 2019, 97, 486–501B. [Google Scholar] [CrossRef]
- Bakhit, M.; Del Mar, C.; Scott, A.M.; Hoffmann, T. An analysis of reporting quality of prospective studies examining community antibiotic use and resistance. Trials 2018, 19, 656. [Google Scholar] [CrossRef]
- Fresnadillo-Martínez, M.J.; García-Sánchez, E.; García-Merino, E.; Martín-Del-Rey, A.; Rodríguez-Encinas, A.; Rodríguez-Sánchez, G.; García-Sánchez, J.E. Matematical modeling of antibiotic resistance: Perspectives from a meta-analysys. Rev. Esp. Quimioter. 2012, 25, 172–179. (In Spanish) [Google Scholar]
- Yousfi, K.; Bekal, S.; Usongo, V.; Touati, A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1353–1362. [Google Scholar] [CrossRef]
- Noguchi, N. Antimicrobial Resistance and Infection Control for Gram-positive Bacteria. YAKUGAKU ZASSHI 2021, 141, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Yang, F.; Li, D.M.; Sun, T.T.; Shang, P.P.; Zheng, J.J.; Peng, Y.H. A study of bacterial distribution and drug resistance in skin and soft tissue infection. Zhonghua Yi Xue Za Zhi 2019, 99, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Galli, L.; Venturini, E.; Bassi, A.; Gattinara, G.C.; Chiappini, E.; Defilippi, C.; Diociaiuti, A.; Esposito, S.; Garazzino, S.; Giannattasio, A.; et al. Common Community-acquired Bacterial Skin and Soft-tissue Infections in Children: An Intersociety Consensus on Impetigo, Abscess, and Cellulitis Treatment. Clin. Ther. 2019, 41, 532–551.e17. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.J.; Wylam, M.E. Methicillin-Resistant Staphylococcus aureus Infection and Treatment Options. Methods Mol. Biol. 2020, 2069, 229–251. [Google Scholar] [CrossRef]
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-Resistant Staphylococcus aureus in Hospitals: Latest Trends and Treatments Based on Bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Chen, L.; Liu, Y.; Tan, L.; Shen, J.; Zhang, W. Antimicrobial resistance and molecular characterization of methicillin-resistant coagulase-negative staphylococci from public shared bicycles in Tianjin, China. J. Glob. Antimicrob. Resist. 2019, 19, 231–235. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [Green Version]
- Intravia, J.M.; Osterman, M.N.; Tosti, R. Antibiotic Management and Antibiotic Resistance in Hand Infections. Hand Clin. 2020, 36, 301–305. [Google Scholar] [CrossRef]
- Shah, R.A.; Hsu, J.I.; Patel, R.R.; Mui, U.N.; Tyring, S.K. Antibiotic resistance in dermatology: The scope of the problem and strategies to address it. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Xu, Y.; Chen, J.; Zhou, A.; Sun, L.; Gao, Y.; Zhang, Y.; Gu, T.; Ning, X. Bioactive multi-engineered hydrogel offers simultaneous promise against antibiotic resistance and wound damage. Int. J. Biol. Macromol. 2020, 164, 4466–4474. [Google Scholar] [CrossRef]
- Jabbour, J.-F.; Sharara, S.L.; Kanj, S.S. Treatment of multidrug-resistant Gram-negative skin and soft tissue infections. Curr. Opin. Infect. Dis. 2020, 33, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.-F.; Kanj, S.S. Gram-Negative Skin and Soft Tissue Infections. Infect. Dis. Clin. N. Am. 2020, 35, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Moffarah, A.S.; Mohajer, M.A.; Hurwitz, B.L.; Armstrong, D.G. Skin and Soft Tissue Infections. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Kolesnichenko, S.I.; Lavrinenko, A.V.; Akhmaltdinova, L.L. Bloodstream Infection Etiology among Children and Adults. Int. J. Microbiol. 2021, 2021, 6657134. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S. Novel antibiotic treatment for skin and soft tissue infection. Curr. Opin. Infect. Dis. 2014, 27, 116–124. [Google Scholar] [CrossRef]
- Ukuhor, H.O. The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. J. Infect. Public Health 2020, 14, 53–60. [Google Scholar] [CrossRef]
- Martiny, D.; Busson, L.; Wybo, I.; El Haj, R.A.; Dediste, A.; Vandenberg, O. Comparison of the Microflex LT and Vitek MS Systems for Routine Identification of Bacteria by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2012, 50, 1313–1325. [Google Scholar] [CrossRef] [Green Version]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF Mass Spectrometry in Clinical Diagnostic Microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef]
- CLSI M100 ED32:2022; Performance Standards for Antimicrobial Disk Susceptibility Tests. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED32:2022&scope=user (accessed on 22 April 2022).
| Group of Patients | Age | Mean Age ± SD | Total Examined | Number of Positive Samples |
|---|---|---|---|---|
| Children | 0–12 months | 0.33 m | 1 | 8 (100%) |
| 1–18 years | 12 ± 7.4 | 7 | ||
| Adults | 18–75 years | 43.9 ± 17.9 | 102 | 102 (100%) |
| Infection Localization | Children | Adults | ||
|---|---|---|---|---|
| Abs. | % | Abs. | % | |
| Purulent wound discharge | 2 | 25 | 20 | 19.60 |
| Phlegmon | 2 | 25 | 18 | 17.64 |
| Abscesses | 2 | 25 | 16 | 15.68 |
| Boils | 2 | 25 | 13 | 12.75 |
| Atheroma | 10 | 9.80 | ||
| Postsurgery complications | 8 | 7.84 | ||
| Purulent appendicitis | 4 | 3.92 | ||
| Paraproctitis | 4 | 3.92 | ||
| Purulent complications in hematology | 4 | 3.92 | ||
| Erysepals | 3 | 2.94 | ||
| Purulent complications in cancer | 2 | 1.76 | ||
| Total | 8 | 100 | 102 | 100 |
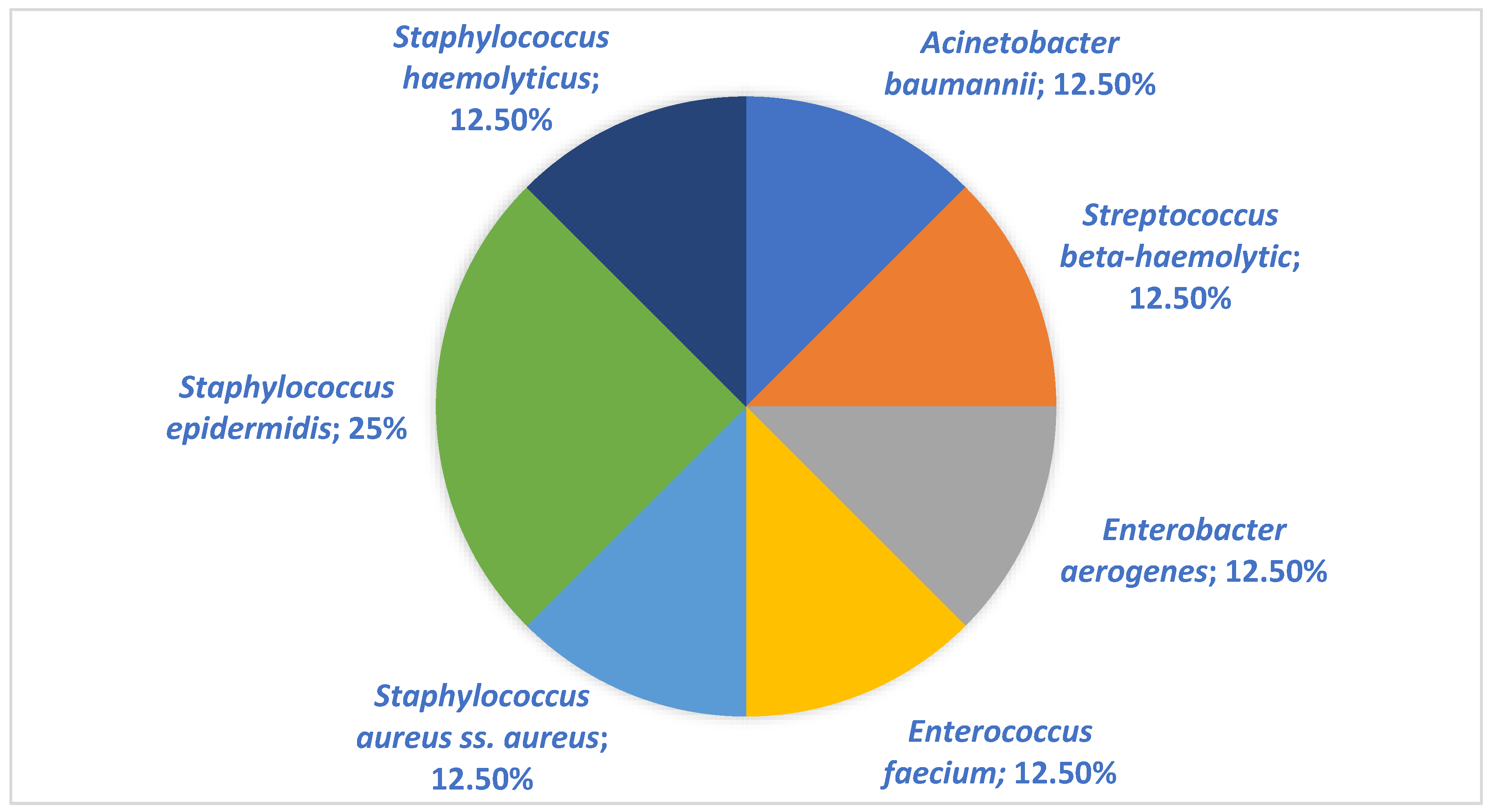
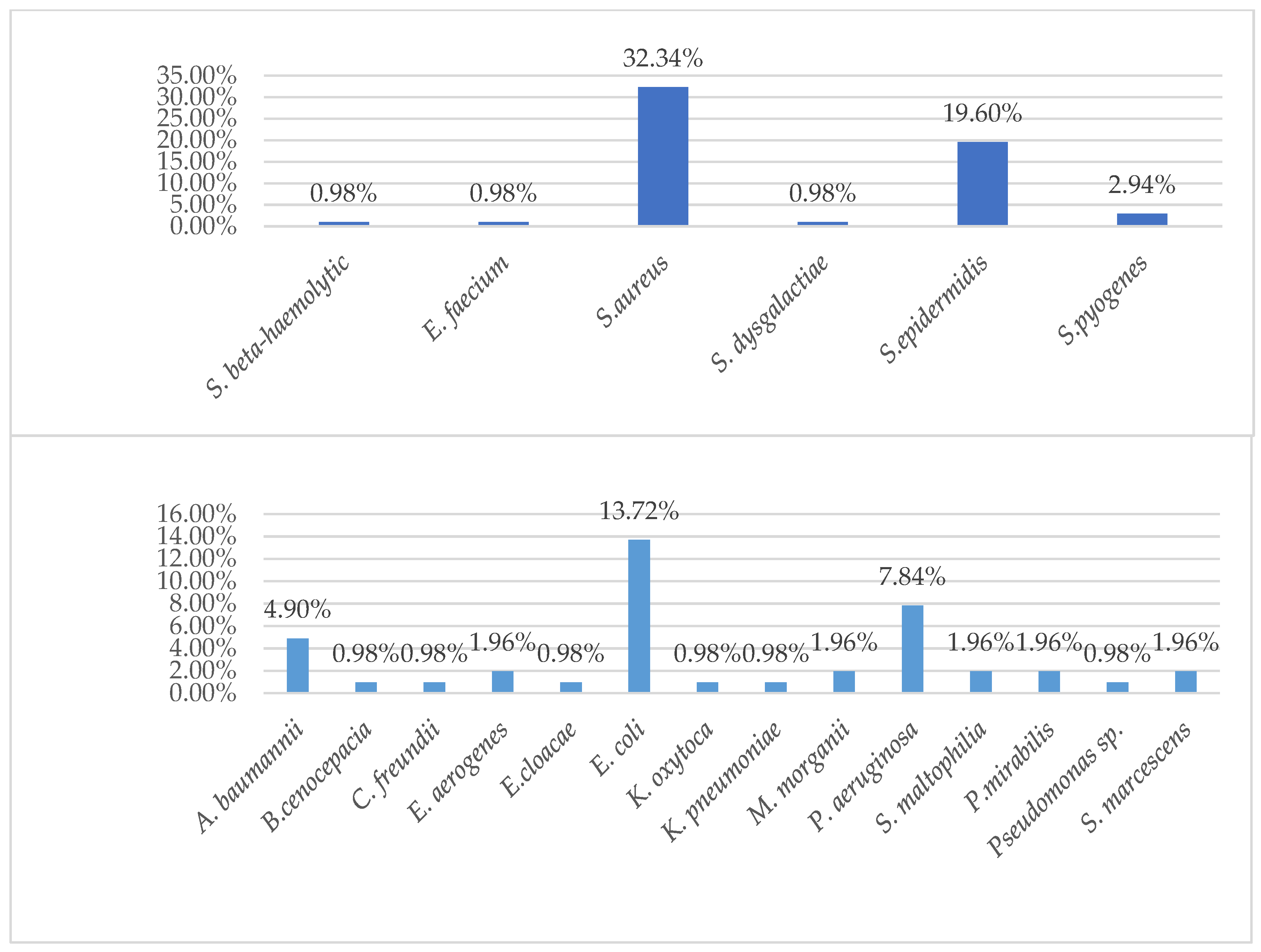
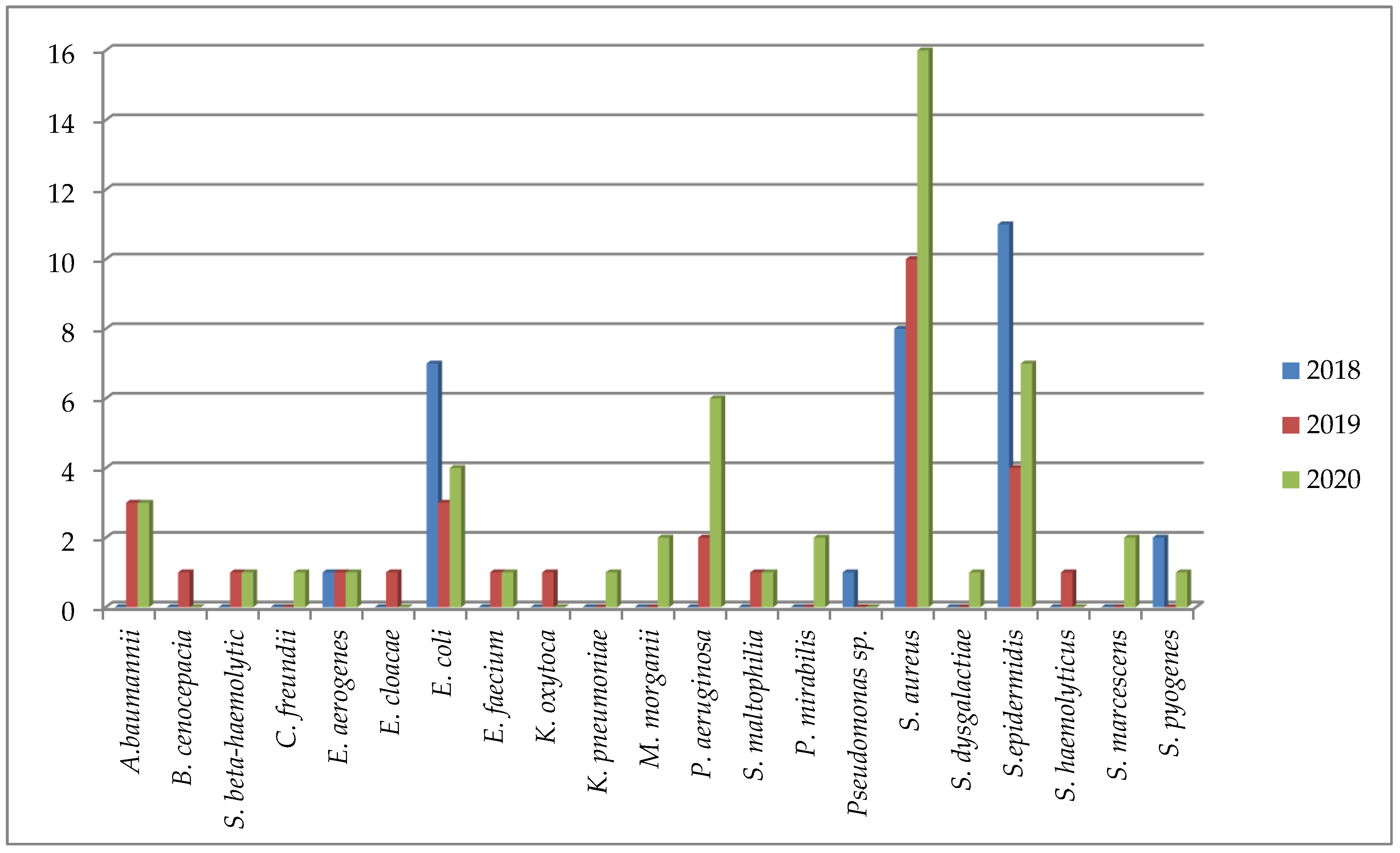
| Pathogen | Children (n = 8) (%) | Adults (n = 102) (%) |
|---|---|---|
| Acinetobacter baumannii | 1 (12.5%) | 6 (5.88%) |
| Burkholderia cenocepacia (genomovar III) | 1 (0.98%) | |
| Streptococcus beta-haemolytic | 1 (12.5%) | 1 (0.98%) |
| Enterobacter aerogenes | 2 (1.96%) | |
| Escherichia coli | 14 (13.72%) | |
| Enterococcus faecium | 2 (1.96%) | |
| Pseudomonas aeruginosa | 1 (12.5%) | 8 (7.84%) |
| Stenotrophomonas maltophilia | 2 (1.96%) | |
| Proteus mirabilis | 1 (12.5%) | 2 (1.96%) |
| Staphylococcus aureus ss. aureus | 1 (12.5%) | 33 (32.34%) |
| Staphylococcus epidermidis | 2 (25.0%) | 21 (20.59%) |
| Staphylococcus haemolyticus | 1 (12.5%) | 1 (0.98%) |
| Streptococcus pyogenes | 3 (2.94%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliyeva, S.S.; Lavrinenko, A.V.; Tishkambayev, Y.; Zhussupova, G.; Issabekova, A.; Begesheva, D.; Simokhina, N. Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020. Antibiotics 2022, 11, 659. https://doi.org/10.3390/antibiotics11050659
Kaliyeva SS, Lavrinenko AV, Tishkambayev Y, Zhussupova G, Issabekova A, Begesheva D, Simokhina N. Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020. Antibiotics. 2022; 11(5):659. https://doi.org/10.3390/antibiotics11050659
Chicago/Turabian StyleKaliyeva, Sholpan S., Alyona V. Lavrinenko, Yerbol Tishkambayev, Gulzira Zhussupova, Aissulu Issabekova, Dinara Begesheva, and Natalya Simokhina. 2022. "Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020" Antibiotics 11, no. 5: 659. https://doi.org/10.3390/antibiotics11050659
APA StyleKaliyeva, S. S., Lavrinenko, A. V., Tishkambayev, Y., Zhussupova, G., Issabekova, A., Begesheva, D., & Simokhina, N. (2022). Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020. Antibiotics, 11(5), 659. https://doi.org/10.3390/antibiotics11050659









