Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii
Abstract
:1. Introduction
2. Results
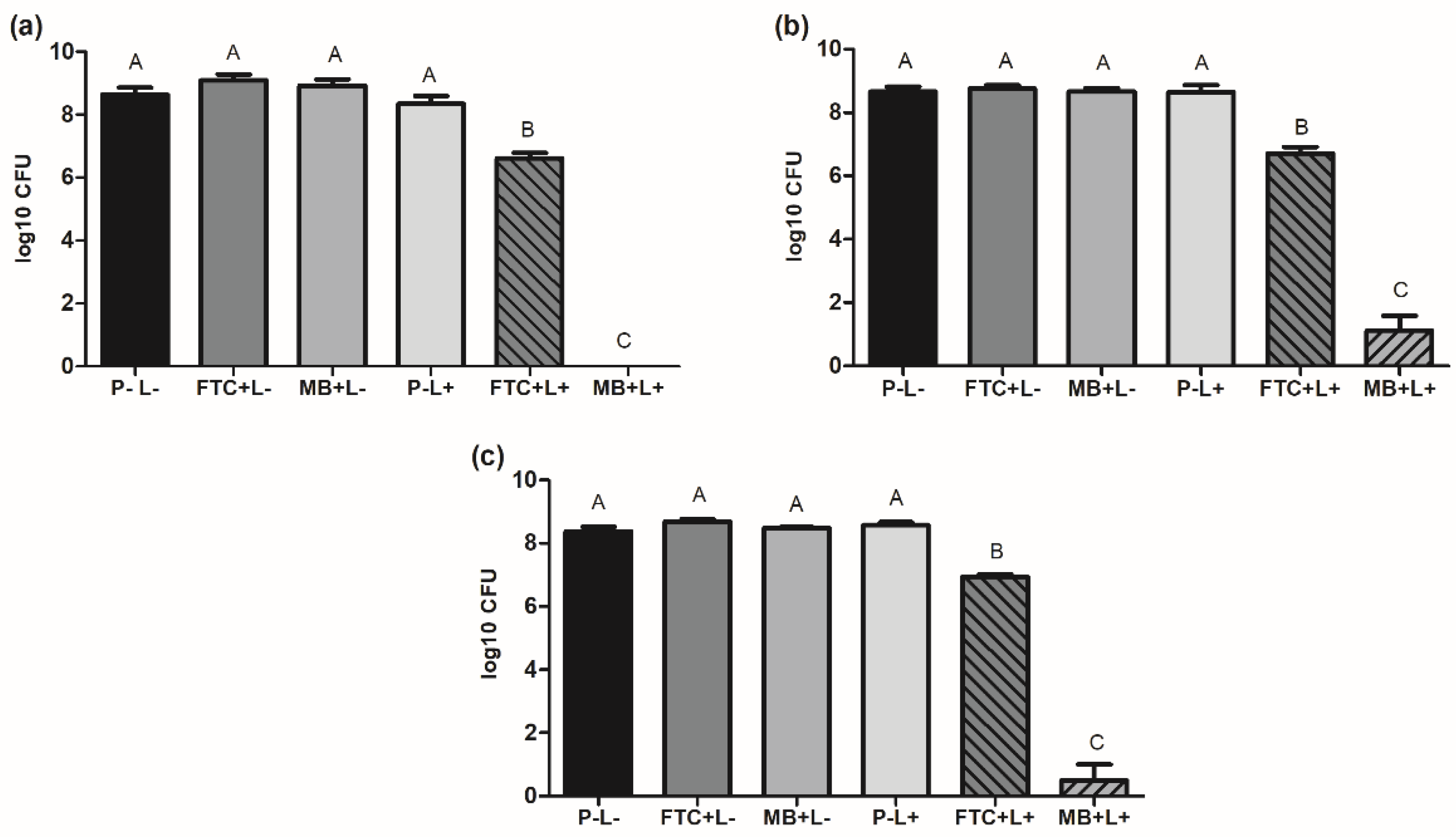
2.1. Effects of aPDT on Planktonic Cultures of A. baumannii Strains
2.2. Effects of aPDT on A. baumannii Biofilms
2.3. Effects of aPDT on Burn Infections in Galleria Mellonella Model
2.3.1. Survival Curve
2.3.2. Health Index
2.4. Absorption of Photosensitizers by the A. baumannii Cells
2.4.1. Analysis of Confocal Microscopy
2.4.2. Test of Absorbance by Spectrophotometry
3. Discussion
4. Materials and Methods
4.1. Strains of A. baumannii
4.2. Preparation of Standardized A. baumannii Suspensions
4.3. Photosensitizers and Light Source
4.4. aPDT Application on Planktonic Cultures of A. baumannii
4.5. aPDT Application on A. baumannii Biofilms
4.6. Analysis of the In Vivo Effects of aPDT in a Burn Model of G. mellonella Infected with A. baumannii
4.6.1. G. mellonella Larvae
4.6.2. Induction of Burn Lesion and Infection of A. baumannii in G. mellonella
4.6.3. aPDT in G. mellonella
4.6.4. Survival Curve of G. mellonella Larvae
4.6.5. G. mellonella Larvae Health Index
4.7. Analysis of Photosensitizer Absorption by A. baumannii Cells
4.8. Analysis of the Internalization of Photosensitizers by A. baumannii Using Confocal Microscopy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter Baumannii Biofilms: Effects of Physicochemical Factors, Virulence, Antibiotic Resistance Determinants, Gene Regulation, and Future Antimicrobial Treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubareck, C.A.; Halat, D.H. Insights into Acinetobacter Baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasr, P. Genetics, Epidemiology, and Clinical Manifestations of Multidrug-Resistant Acinetobacter Baumannii. J. Hosp. Infect. 2020, 104, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter Baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-C.; Wu, R.-X.; Chiu, C.-C.; Yang, Y.-S.; Lee, Y.; Lin, J.-C.; Chang, F.-Y. The Clinical and Microbiological Characteristics of Infections in Burn Patients from the Formosa Fun Coast Dust Explosion. J. Microbiol. Immunol. Infect. 2017. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Apisarnthanarak, A.; Khan, E.; Suwantarat, N.; Ghafur, A.; Tambyahb, P.A. Carbapenem-Resistant Acinetobacter Baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2017, 30, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [Green Version]
- Rani, F.M.; Rahman, N.; Ismail, S.; Alattraqchi, A.; Cleary, D.W.; Clarke, S.C.; Yeo, C.C. Acinetobacter Spp. Infections in Malaysia: A Review of Antimicrobial Resistance Trends, Mechanisms and Epidemiology. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer Membrane Protein A (OmpA) as a Potential Therapeutic Target for Acinetobacter Baumannii Infection. J. Biomed. Sci. 2020, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wareth, G.; Neubauer, H.; Sprague, L.D. Acinetobacter Baumannii—a Neglected Pathogen in Veterinary and Environmental Health in Germany. Vet. Res. Commun. 2019, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Casas, A.; Di Venosa, G.; Hasan, T. Al Batlle Mechanisms of Resistance to Photodynamic Therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic Therapy for Localized Infections—State of the Art. NIH Public Access 2010, 6, 170–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-Mediated Photodynamic Inactivation of Candida Albicans in a Murine Model of Oral Candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, A.; Salvadori, A.; Bressenot, A.; Bezdetnaya, L.; Guillemin, F.; D’Hallewin, M.A. How to Avoid Local Side Effects of Bladder Photodynamic Therapy: Impact of the Fluence Rate. J. Urol. 2013, 190, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, F.P.; Maisch, T. Photodynamic Inactivation for Controlling Candida Albicans Infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, J.C.; Fuchs, B.B.; Muhammed, M.; Coleman, J.J.; Suleiman, J.M.; Vilela, S.F.; Costa, A.C.; Rasteiro, V.M.; Jorge, A.O.; Mylonakis, E. Oral Candida Albicans Isolates from HIV-Positive Individuals Have Similar in Vitro Biofilm-Forming Ability and Pathogenicity as Invasive Candida Isolates. BMC Microbiol. 2011, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- Junqueira, J.C.; Ribeiro, M.A.; Rossoni, R.D.; Barbosa, J.O.; Querido, S.M.R.; Jorge, A.O.C. Antimicrobial Photodynamic Therapy: Photodynamic Antimicrobial Effects of Malachite Green on Staphylococcus, Enterobacteriaceae, and Candida. Photomed. Laser Surg. 2010, 28, S-67–S-72. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene Oxide Mediated Delivery of Methylene Blue for Combined Photodynamic and Photothermal Therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy–What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Bayona, A.M.D.P.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design Features for Optimization of Tetrapyrrole Macrocycles as Antimicrobial and Anticancer Photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terra-Garcia, M.; de Souza, C.M.; Ferreira Gonçalves, N.M.; Pereira, A.H.C.; de Barros, P.P.; Borges, A.B.; Miyakawa, W.; Strixino, J.F.; Junqueira, J.C. Antimicrobial Effects of Photodynamic Therapy with Fotoenticine on Streptococcus Mutans Isolated from Dental Caries. Photodiagnosis Photodyn. Ther. 2021, 34, 102303. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.D. Avaliação Da Eficiência Fotodinâmica De Fotossensibilizadores Com Aplicação Em Terapia Fotodinâmica. Doctoral Dissertation, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Figueiredo-Godoi, L.M.A.; Menezes, R.T.; Carvalho, J.S.; Garcia, M.T.; Segundo, A.G.; Jorge, A.O.C.; Junqueira, J.C. Exploring the Galleria Mellonella Model to Study Antifungal Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2019, 27, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, J.C. Galleria Mellonella as a Model Host for Human Pathogens: Recent Studies and New Perspectives. Virulence 2012, 3, 474–476. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria Mellonella as an Infection Model: An in-Depth Look at Why It Works and Practical Considerations for Successful Application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The Greater Wax Moth Galleria Mellonella: Biology and Use in Immune Studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Mylonakis, E.; Borghi, E. Galleria Mellonella Experimental Model: Advances and Future Directions. Pathog. Dis. 2021, 79, ftab021. [Google Scholar] [CrossRef]
- Chibebe Jr, J.; Sabino, C.P.; Tan, X.; Junqueira, J.C.; Wang, Y.; Fuchs, B.B.; Jorge, A.O.C.; Tegos, G.P.; Hamblin, M.R.; Mylonakis, E. Selective Photoinactivation of Candida Albicans in the Non-Vertebrate Host Infection Model Galleria Mellonella. BMC Microbiol. 2013, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- Chibebe Junior, J.; Fuchs, B.B.; Sabino, C.P.; Junqueira, J.C.; Jorge, A.O.C.; Ribeiro, M.S.; Gilmore, M.S.; Rice, L.B.; Tegos, G.P.; Hamblin, M.R.; et al. Photodynamic and Antibiotic Therapy Impair the Pathogenesis of Enterococcus Faecium in a Whole Animal Insect Model. PLoS ONE 2013, 8, e55926. [Google Scholar] [CrossRef] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.; Sellera, F.P.; dos Anjos, C.; Baptista, M.D.S.; Lincopan, N. Global Priority Multidrug-Resistant Pathogens Do Not Resist Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.M.; de Barros, P.P.; de Bernardes, R.C.; Alves, S.R.; Ramanzini, N.P.; Figueiredo-Godoi, L.M.A.; Prado, A.C.C.; Jorge, A.O.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy against Clinical Isolates of Carbapenem-Susceptible and Carbapenem-Resistant Acinetobacter Baumannii. Lasers Med. Sci. 2019, 34, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.H.; Walsh, L.J.; Figueredo, C.M.; George, R. Evaluation of Effectiveness of Photosensitizers Used in Laser Endodontics Disinfection: A Systematic Review. Transl. Biophotonics 2021, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Guo, X.; Wang, L.; Zeng, J.; Qiu, H.; Tan, Y.; Chen, D.; Zhao, H.; Gu, Y. Enhanced Antimicrobial Activity through the Combination of Antimicrobial Photodynamic Therapy and Low-Frequency Ultrasonic Irradiation. Adv. Drug Deliv. Rev. 2022, 183, 114168. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Pereira, A.H.C.; Figueiredo-Godoi, L.M.A.; Jorge, A.O.C.; Strixino, J.F.; Junqueira, J.C. Photodynamic Therapy Mediated by Chlorin-Type Photosensitizers against Streptococcus Mutans Biofilms. Photodiagnosis Photodyn. Ther. 2018, 24, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Krajczewski, J.; Rucińska, K.; Townley, H.E.; Kudelski, A. Role of Various Nanoparticles in Photodynamic Therapy and Detection Methods of Singlet Oxygen. Photodiagnosis Photodyn. Ther. 2019, 26, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Hosseini, N.; Boluki, E.; Chiniforush, N.; Bahador, A. Photoelimination Potential of Chitosan NanoparticlesIndocyanine Green Complex Against the Biological Activities of Acinetobacter Baumannii Strains: A Preliminary In Vitro Study in Burn Wound Infections. J. Lasers Med. Sci. 2020, 11, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter Baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Maslova, E.; Shi, Y.; Sjöberg, F.; Azevedo, H.S.; Wareham, D.W.; McCarthy, R.R. An Invertebrate Burn Wound Model That Recapitulates the Hallmarks of Burn Trauma and Infection Seen in Mammalian Models. Front. Microbiol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Lu, Z.; Huang, L.; Zhiyentayev, T.; Franklin, M.J.; Baer, D.G.; Hamblin, M.R. Photodynamic Therapy for Acinetobacter Baumannii Burn Infections in Mice. Antimicrob. Agents Chemother. 2009, 53, 3929–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera, D.; Aguilera-Correa, J.-J.; García-Coca, M.; Mahillo-Fernández, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. The Galleria Mellonella Infection Model as a System to Investigate the Virulence of Candida Auris Strains. Pathog. Dis. 2020, 78, 51–87. [Google Scholar] [CrossRef] [PubMed]
- Wand, M.E.; Bock, L.J.; Turton, J.F.; Nugent, P.G.; Sutton, J.M. Acinetobacter Baumannii Virulence Is Enhanced in Galleria Mellonella Following Biofilm Adaptation. J. Med. Microbiol. 2012, 61, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From Moths to Caterpillars: Ideal Conditions for Galleria Mellonella Rearing for in Vivo Microbiological Studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.C.B.P.; Chibebe Junior, J.; Pereira, C.A.; Machado, A.K.D.S.; Beltrame Junior, M.; Junqueira, J.C.; Jorge, A.O.C. Susceptibility of Planktonic Cultures of Streptococcus Mutans to Photodynamic Therapy with a Light-Emitting Diode. Braz. Oral Res. 2010, 24, 413–418. [Google Scholar] [CrossRef]
- Loh, J.M.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria Mellonella Larvae as an Infection Model for Group A Streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Kishen, A. Photophysical, Photochemical, and Photobiological Characterization of Methylene Blue Formulations for Light-Activated Root Canal Disinfection. J. Biomed. Opt. 2007, 12, 034029. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. https://doi.org/10.3390/antibiotics11050619
Figueiredo-Godoi LMA, Garcia MT, Pinto JG, Ferreira-Strixino J, Faustino EG, Pedroso LLC, Junqueira JC. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics. 2022; 11(5):619. https://doi.org/10.3390/antibiotics11050619
Chicago/Turabian StyleFigueiredo-Godoi, Lívia M. A., Maíra T. Garcia, Juliana G. Pinto, Juliana Ferreira-Strixino, Eliseu Gabriel Faustino, Lara Luise Castro Pedroso, and Juliana C. Junqueira. 2022. "Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii" Antibiotics 11, no. 5: 619. https://doi.org/10.3390/antibiotics11050619
APA StyleFigueiredo-Godoi, L. M. A., Garcia, M. T., Pinto, J. G., Ferreira-Strixino, J., Faustino, E. G., Pedroso, L. L. C., & Junqueira, J. C. (2022). Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics, 11(5), 619. https://doi.org/10.3390/antibiotics11050619







