Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms
Abstract
1. Introduction
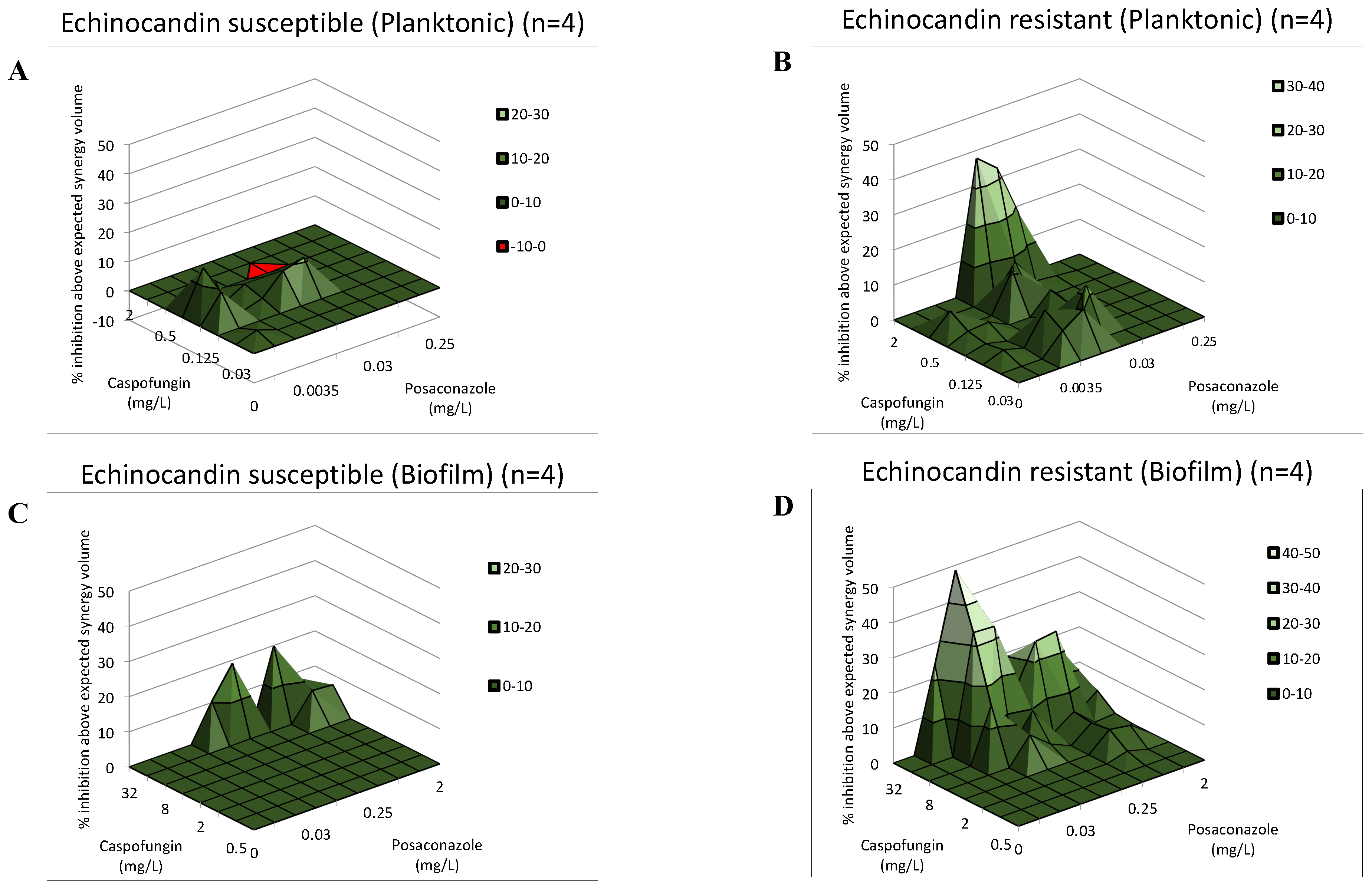
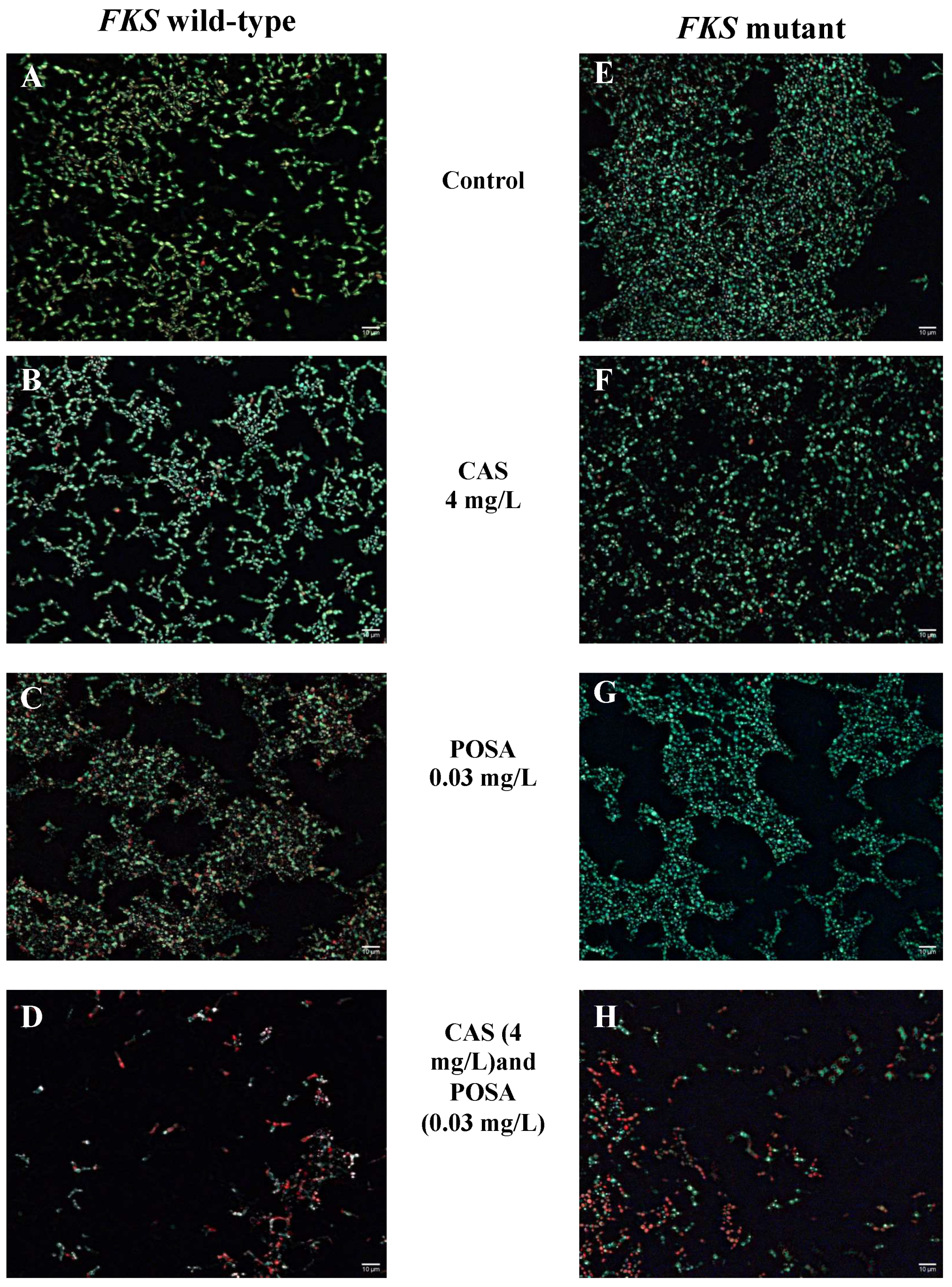
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolates
4.2. Whole Genome Sequencing of Isolates
4.3. Antifungal Susceptibility Testing for Planktonic Cells
4.4. Biofilm Development
4.5. Assessment of Antifungal Susceptibility for Biofilms
4.6. Assessment of Synergy between Caspofungin and Posaconazole
4.7. Biofilm Viability Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. Environmental Candida auris and the Global Warming Emergence Hypothesis. mBio 2021, 12, e00360-21. [Google Scholar] [CrossRef] [PubMed]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimic. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2020, 58, e01503-19. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, S.; Innes, G.; Quinn, M.; Southwick, K.; Ostrowsky, B.; Greenko, J.A.; Lutterloh, E.; Greeley, R.; Magleby, R.; Chaturvedi, V.; et al. Antifungal Resistance Trends of Candida auris Clinical Isolates in New York and New Jersey from 2016 to 2020. Antimic. Agents Chemother. 2022, 66, e0224221. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar]
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and Profiling of Candida auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef]
- Horton, M.V.; Nett, J.E. Candida auris infection and biofilm formation: Going beyond the surface. Curr. Clin. Microbiol. Rep. 2020, 7, 51–56. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Mahmood, S.F. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med. Mycol. 2020, 58, 721–729. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. 1), S1–S25. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Aldejohann, A.M.; Wiese-Posselt, M.; Gastmeier, P.; Kurzai, O. Expert recommendations for prevention and management of Candida auris transmission. Mycoses 2022, 65, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Paul, R.A.; Rudramurthy, S.M.; Kashyap, N.; Bhattacharya, S.; Soman, R.; Shankarnarayan, S.A.; Chavan, D.; Singh, S.; Das, P.; et al. Impact of FKS1 Genotype on Echinocandin In Vitro Susceptibility in Candida auris and In Vivo Response in a Murine Model of Infection. Antimic. Agents Chemother. 2022, 66, e0165221. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimic. Agents 2020, 55, 105901. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Friar, L.; Ditelberg, S.; Kirby, J.E. Evaluation of the synergistic activity of antibacterial and antifungal drugs against Candida auris using an inkjet printer-assisted method. Antimic. Agents Chemother. 2021, 65, e0026821. [Google Scholar] [CrossRef]
- Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics 2021, 10, 355. [Google Scholar] [CrossRef]
- Nagy, F.; Tóth, Z.; Nyikos, F.; Forgács, L.; Jakab, Á.; Borman, A.M.; Majoros, L.; Kovács, R. In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med. Mycol. 2021, 59, 1015–1023. [Google Scholar] [CrossRef]
- Vitale, R.G. Role of Antifungal Combinations in Difficult to Treat Candida Infections. J. Fungi 2021, 7, 731. [Google Scholar] [CrossRef]
- Jahn, L.J.; Simon, D.; Jensen, M.; Bradshaw, C.; Ellabaan, M.M.H.; Sommer, M.O.A. Compatibility of Evolutionary Responses to Constituent Antibiotics Drive Resistance Evolution to Drug Pairs. Mol. Biol. Evol. 2021, 38, 2057–2069. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lehman, V.N.; Averette, A.F.; Perfect, J.R.; Heitman, J. Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PLoS ONE 2013, 8, e57672. [Google Scholar] [CrossRef] [PubMed]
- Denardi, L.B.; Keller, J.T.; Oliveira, V.; Mario, D.; Santurio, J.M.; Alves, S.H. Activity of Combined Antifungal Agents Against Multidrug-Resistant Candida glabrata Strains. Mycopathologia 2017, 182, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Majima, H.; Watanabe, A.; Kamei, K. In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata. J. Fungi 2021, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In Vitro Evaluation of Antifungal Drug Combinations against Multidrug-Resistant Candida auris Isolates from New York Outbreak. Antimic. Agents Chemother. 2020, 64, e02195-19. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimic. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- van Schalkwyk, E.; Mpembe, R.S.; Thomas, J.; Shuping, L.; Ismail, H.; Lowman, W.; Karstaedt, A.S.; Chibabhai, V.; Wadula, J.; Avenant, T.; et al. Epidemiologic Shift in Candidemia Driven by Candida auris, South Africa, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1698–1707. [Google Scholar] [CrossRef]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef]
- Umamaheshwari, S.; Neelambike, S.M.; Shankarnarayan, S.A.; Kumarswamy, K.S.; Gopal, S.; Prakash, H.; Rudramurthy, S.M. Clinical profile, antifungal susceptibility, and molecular characterization of Candida auris isolated from patients in a South Indian surgical ICU. J. Mycol. Med. 2021, 31, 101176. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Naicker, S.D.; Kwenda, S.; Muñoz, J.F.; van Schalkwyk, E.; Wadula, J.; Nana, T.; Ismail, A.; Coetzee, J.; Govind, C.; et al. In Vitro Antifungal Resistance of Candida auris Isolates from Bloodstream Infections, South Africa. Antimic. Agents Chemother. 2021, 65, e0051721. [Google Scholar] [CrossRef]
- Bandara, N.; Samaranayake, L. Emerging and future strategies in the management of recalcitrant Candida auris. Med. Mycol. 2022, 60, myac008. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimic. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimic. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Echinocandin Resistance in Candida Species: A Review of Recent Developments. Curr. Infect. Dis. Rep. 2016, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaid, I.; Asadzadeh, M.; Ahmad, S.; Alobaid, K.; Alfouzan, W.; Bafna, R.; Emara, M.; Joseph, L. Fatal Breakthrough Candidemia in an Immunocompromised Patient in Kuwait Due to Candida auris Exhibiting Reduced Susceptibility to Echinocandins and Carrying a Novel Mutation in Hotspot-1 of FKS1. J. Fungi 2022, 8, 267. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Mokaddas, E.; Ahmad, S.; Abdullah, A.A.; de Groot, T.; Meis, J.F.; Shetty, S.A. Molecular characterisation of Candida auris isolates from immunocompromised patients in a tertiary-care hospital in Kuwait reveals a novel mutation in FKS1 conferring reduced susceptibility to echinocandins. Mycoses 2022, 65, 331–343. [Google Scholar] [CrossRef]
- Tóth, Z.; Forgács, L.; Kardos, T.; Kovács, R.; Locke, J.B.; Kardos, G.; Nagy, F.; Borman, A.M.; Adnan, A.; Majoros, L. Relative Frequency of Paradoxical Growth and Trailing Effect with Caspofungin, Micafungin, Anidulafungin, and the Novel Echinocandin Rezafungin against Candida Species. J. Fungi 2020, 6, 136. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Hussain, K.; Moradi, P.W.; Strauss, G.E.; Myint, K.L.; Zaw, M.H.; Kovanda, L.L.; Petraitiene, R.; et al. In vitro combination therapy with isavuconazole against Candida spp. Med. Mycol. 2017, 55, 859–868. [Google Scholar]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimic. Agents Chemother. 2017, 61, e01056-17. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Deshpande, L.M.; Rhomberg, P.R.; Utt, E.A.; Castanheira, M. Evaluation of Synergistic Activity of Isavuconazole or Voriconazole plus Anidulafungin and the Occurrence and Genetic Characterization of Candida auris Detected in a Surveillance Program. Antimic. Agents Chemother. 2021, 65, e02031-20. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jiang, S.; Tan, L.; Shi, H.; Yang, L.; Sun, Y.; Wang, X. Antifungal Activity of Minocycline and Azoles Against Fluconazole-Resistant Candida Species. Front. Microbiol. 2021, 12, 649026. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Garcia-Effron, G.; Lewis, R.E.; Gamarra, S.; Leventakos, K.; Perlin, D.S.; Kontoyiannis, D.P. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J. Infect. Dis. 2011, 204, 626–635. [Google Scholar] [CrossRef]
- Heath, C.H.; Dyer, J.R.; Pang, S.; Coombs, G.W.; Gardam, D.J. Candida auris Sternal Osteomyelitis in a Man from Kenya Visiting Australia, 2015. Emerg. Infect. Dis. 2019, 25, 192–194. [Google Scholar] [CrossRef]
- Stathi, A.; Loukou, I.; Kirikou, H.; Petrocheilou, A.; Moustaki, M.; Velegraki, A.; Zachariadou, L. Isolation of Candida auris from cystic fibrosis patient, Greece, April 2019. Eurosurveillance 2019, 24, 1900400. [Google Scholar] [CrossRef]
- Shaukat, A.; Al Ansari, N.; Al Wali, W.; Karic, E.; El Madhoun, I.; Mitwally, H.; Hamed, M.; Alutra-Visan, F. Experience of treating Candida auris cases at a general hospital in the state of Qatar. IDCases 2020, 23, e01007. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Li, X.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimic. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Isolates of the Emerging Pathogen Candida auris Present in the UK Have Several Geographic Origins. Med. Mycol. 2017, 55, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Bozó, A.; Gesztelyi, R.; Domán, M.; Kardos, G.; Nagy, F.; Tóth, Z.; Majoros, L. Effect of caspofungin and micafungin in combination with farnesol against Candida parapsilosis biofilms. Int. J. Antimic. Agents 2016, 47, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Approved Standard M27-A3 3rd ed.; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- Nagy, F.; Tóth, Z.; Daróczi, L.; Székely, A.; Borman, A.M.; Majoros, L.; Kovács, R. Farnesol increases the activity of echinocandins against Candida auris biofilms. Med. Mycol. 2020, 58, 404–407. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the assessment of in vitro and in vivo Antifungal Combinations. J. Fungi (Switzerland) 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Verweij, P.E.; TeDorsthorst, D.T.; Meis, J.F.; Mouton, J.W. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: Comparison of different drug interaction models. Med. Mycol. 2005, 43, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Shipman, C., Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990, 14, 181–205. [Google Scholar] [CrossRef]
- Rhoden, E.; Ng, T.F.F.; Campagnoli, R.; Nix, W.A.; Konopka-Anstadt, J.; Selvarangan, R.; Briesach, L.; Oberste, M.S.; Weldon, W.C. Antifungal Triazole Posaconazole Targets an Early Stage of the Parechovirus A3 Life Cycle. Antimic. Agents Chemother. 2020, 64, e02372-19. [Google Scholar] [CrossRef]
- Kovács, R.; Nagy, F.; Tóth, Z.; Forgács, L.; Tóth, L.; Váradi, G.; Tóth, G.K.; Vadászi, K.; Borman, A.M.; Majoros, L.; et al. The Neosartorya fischeri Antifungal Protein 2 (NFAP2): A New Potential Weapon against Multidrug-Resistant Candida auris Biofilms. Int. J. Mol. Sci. 2021, 22, 771. [Google Scholar] [CrossRef]
| Isolates | Clade | Isolation Source | FKS Mutations |
|---|---|---|---|
| Ca_1 | South Asian | wound swab | HS1 WT HS2 R1354H |
| Ca_2 | South Asian | perianal swab | HS1 WT HS2 R1354H |
| Ca_3 | South Asian | Central line | HS1 S639Y HS2 WT |
| Ca_4 | South Asian | wound swab | HS1 S639P HS2 WT |
| Ca_5 | South Asian | Unknown | HS1 WT HS2 WT |
| Ca_6 | East Asian | Unknown | HS1 WT HS2 WT |
| Ca_7 | South African | Tracheostomy | HS1 WT HS2 WT |
| Ca_8 | South American | Blood | HS1 WT HS2 WT |
| Isolates | Planktonic Cells Median MIC (Range) of Drug Used (50% OD492nm Reduction in Turbidity) | Biofilms Median MIC (Range) of Drug Used (50% OD492nm Reduction in Metabolic Activity) | ||||||
|---|---|---|---|---|---|---|---|---|
| Alone | In Combination | Alone | In Combination | |||||
| Caspofungin (mg/L) | Posaconazole (mg/L) | Caspofungin (mg/L) | Posaconazole (mg/L) | Caspofungin (mg/L) | Posaconazole (mg/L) | Caspofungin (mg/L) | Posaconazole (mg/L) | |
| Ca_1 | >2 a | >0.25 b | 1 (0.03–1) | 0.002 | >32 c | >2 a (1–>2) | 0.5 | 0.015 |
| Ca_2 | >2 a | 0.25 | 1 | 0.002 | >32 c | 0.06 | 2 | 0.015 |
| Ca_3 | >2 a | 0.125 (0.06–0.125) | 1 (1–2) | 0.008 | >32 c | 0.06 | 8 (8–16) | 0.008 (0.015–0.008) |
| Ca_4 | >2 a | 0.125 | 0.03 | 0.015 (0.008–0.03) | 32 (32–>32 c) | 1 (0.125–1) | 0.5 (0.5–1) | 0.008 |
| Ca_5 | 0.5 | 0.125 | 0.5 (0.25–0.5) | 0.06 | >32 c | >2 a | 8 | 0.5 |
| Ca_6 | 0.5 | >0.25 b | 1 | 0.0009 | 32 (32–>32 c) | >2 a | 0.5 | 0.008 |
| Ca_7 | 0.5 | >0.25 b | 0.5 | 0.0009 | >32 c | 0.25 (0.25–0.5) | 0.5 | 0.008 |
| Ca_8 | 1 | >0.25 b | 1 | 0.0009 | >32 c | 1 | 2 (1–2) | 0.06 |
| Isolate | Planktonic Cells | Biofilms | ||
|---|---|---|---|---|
| FICI | FICI | |||
| Median (Range) of FICI | Interaction | Median (Range) of FICI | Interaction | |
| Ca_1 | 0.31 (0.31–0.37) | Synergy | 0.155 (0.061–0.1876) | Synergy |
| Ca_2 | 0.37 (0.37–0.49) | Synergy | 0.5 (0.375–0.75) | Synergy |
| Ca_3 | 0.49 (0.5–0.56) | Synergy | 0.5 (0.5–0.75) | Synergy |
| Ca_4 | 0.247 | Synergy | 0.091 (0.038–0.315) | Synergy |
| Ca_5 | 1.24 (1–1.24) | Indifferent | 0.375 (0.25–0.5) | Synergy |
| Ca_6 | 2.001 | Indifferent | 0.033 | Synergy |
| Ca_7 | 2.001 | Indifferent | 0.0378 (0.031–0.038) | Synergy |
| Ca_8 | 1.002 | Indifferent | 0.25 (0.185–0.281) | Synergy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, N.; Kovács, F.; Balázs, B.; Borman, A.M.; Bozó, A.; Jakab, Á.; Tóth, Z.; Kobaissi, O.; Majoros, L.; Kovács, R. Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms. Antibiotics 2022, 11, 1601. https://doi.org/10.3390/antibiotics11111601
Balla N, Kovács F, Balázs B, Borman AM, Bozó A, Jakab Á, Tóth Z, Kobaissi O, Majoros L, Kovács R. Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms. Antibiotics. 2022; 11(11):1601. https://doi.org/10.3390/antibiotics11111601
Chicago/Turabian StyleBalla, Noémi, Fruzsina Kovács, Bence Balázs, Andrew M. Borman, Aliz Bozó, Ágnes Jakab, Zoltán Tóth, Ola Kobaissi, László Majoros, and Renátó Kovács. 2022. "Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms" Antibiotics 11, no. 11: 1601. https://doi.org/10.3390/antibiotics11111601
APA StyleBalla, N., Kovács, F., Balázs, B., Borman, A. M., Bozó, A., Jakab, Á., Tóth, Z., Kobaissi, O., Majoros, L., & Kovács, R. (2022). Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms. Antibiotics, 11(11), 1601. https://doi.org/10.3390/antibiotics11111601









