Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review
Abstract
1. Introduction
2. Use of Antibiotics in Poultry Production
2.1. Therapeutic Antibiotics
2.2. Metaphylaxis Antibiotics
2.3. Prophylaxis Antibiotics
2.4. Antibiotic Growth Promoters
3. Green Tea Products
3.1. Green Tea Nutrients
3.2. Green Tea Bioactive Compounds

4. The Effect of Green Tea Products Inclusion in Poultry Diets
4.1. Effects on Nutrient Utilisation and Growth Performance
4.2. Effects on Visceral Organs, Carcass, and Meat Quality Traits
4.3. Effects on Health Status of Poultry
4.4. The Effects on Gut Microbes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thema, K.; Mlambo, V.; Snyman, N.; Mnisi, C.M. Evaluating alternatives to zinc-bacitracin antibiotic growth promoter in broilers: Physiological and meat quality responses. Animals 2019, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Mahlake, S.K.; Mnisi, C.M.; Lebopa, C.K.; Kumanda, C. The effect of green tea (Camellia sinensis) leaf powder on growth performance, selected haematological indices, carcass characteristics and meat quality parameters of jumbo quail. Sustainability 2021, 13, 7080. [Google Scholar] [CrossRef]
- Mnisi, C.M.; Marareni, M.; Madibana, M.J. A way forward for the South African quail sector as a potential contributor to food and nutrition security following the aftermath of COVID-19: A review. Agric. Food Secur. 2021, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi-Saraee, M.H.; Seidavi, A.R.; Dadashbeiki, M.; Laudadio, V.; Tufarelli, V. Effect of dietary supplementation with different levels of green tea powder and fish oil or their combination on carcass characteristics in broil chickens. Pak. J. Zool. 2014, 46, 1767–1773. [Google Scholar]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Park, K.D.; Lee, K.H.; Byun, Y.H.; Park, J.H.; Kim, S.H.; Kim, J.H.; Seong, B.L. Biological evaluation of anti-influenza viral activity of semi-synthetic catechin derivatives. Antivir. Res. 2007, 76, 178–185. [Google Scholar] [CrossRef]
- Shomal, T.; Najmeh, M.; Saeed, N. Two weeks of dietary supplementation with green tea powder does not affect performance, D-xylose absorption, and selected serum parameters in broiler chickens. Comp. Clinic. Pathol. 2012, 21, 1023–1027. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef]
- Cully, M. Public health: The politics of antibiotics. Nature 2014, 509, 16–17. [Google Scholar] [CrossRef]
- Durso, L.M.; Cook, K.L. Impacts of antibiotic use in agriculture: What are the benefits and risks? Curr. Opin. Microbiol. 2014, 19, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of analytical methods. Food Cont. 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Bester, L.A.; Essack, S.Y. Prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry suppliers in KwaZulu-Natal, South Africa. J. Antimicrob. Chemother. 2008, 62, 1298–1300. [Google Scholar] [CrossRef]
- Smith, S.D.; Colgan, P.; Yang, F.; Rieke, E.L.; Soupir, M.L.; Moorman, T.B.; Allen, H.K.; Howe, A. Investigating the dispersal of antibiotic resistance associated genes from manure application to soil and drainage waters in stimulated agricultural farmland systems. PLoS ONE 2019, 14, e0222470. [Google Scholar] [CrossRef]
- Littmann, J.; Buyx, A.; Cars, O. Antibiotic resistance: An ethical challenge. Int. J. Antimicrob. Agents 2015, 46, 359–361. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Sanchez, A.Z.; Ortega, O.A.C.; Avalos, J.O.; Flores, J.G.E.; Gonzalez-Ronquillo, M.; Vargas-Bello-Perez, E. Effect of different growth stages of rapeseed (Brassica, L.) on nutrient intake and digestibility, nitrogen balance, and rumen fermentation kinetics in sheep diets. Ital. J. Anim. Sci. 2021, 20, 698–706. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, 93–106. [Google Scholar] [CrossRef]
- Boamah, V.E.; Agyare, C.; Odoi, H.; Dalsgaard, A. Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J. Antimicrob. 2016, 2, 120. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.S.; Eldaly, E.A.; El-Abbasy, M.T.; Ikenaka, Y.; Nakayama, S.; Ishizuka, M. Antibiotic residues in food: The African scenario. Jpn. J. Vet. Res. 2013, 61, S13–S22. [Google Scholar] [PubMed]
- Adesokan, H.K.; Akinseye, V.; Adesokan, G.A. Food safety training is associated with improved knowledge and behaviours among foodservice establishments’ workers. Int. J. Food Sci. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Newman, M.J.; Yeboah-Manu, D. Epidemiological aspects of nonhuman antibiotic usage and resistance: Implications for the control of antibiotic resistance in Ghana. Trop. Med. Int. Health 2012, 17, 462–468. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef]
- Jang, S.I.; Jun, M.H.; Lillehoj, H.S.; Dalloul, R.A.; Kong, I.K.; Kim, S.; Min, W. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 2007, 114, 172–175. [Google Scholar] [CrossRef]
- Bousquet-Melou, A.; Ferran, A.; Toutan, P.L. Prophylaxis and metaphylaxis in veterinary antimicrobial therapy. In Proceedings of the 5th International Conference on Antimicrobial Agents Veterinary Medical, Tel Aviv, Israel, 11–15 May 2010. [Google Scholar]
- Elliot, J.; Glotter, M.; Ruane, A.C.; Boote, K.J.; Hatfield, J.L.; Jones, J.W.; Rosenzweig, C.; Smith, L.A.; Foster, I. Characterizing agricultural impacts of recent large-scale US droughts and changing technology and management. Agric. Syst. 2018, 159, 275–281. [Google Scholar] [CrossRef]
- Lugsomya, K.; Chatsuwan, T.; Niyomtham, W.; Tummaruk, P.; Hampson, D.J.; Prapasarakul, N. Routine prophylactic antimicrobial use is associated with increased phenotypic and genotypic resistance in commensal Escherichia coli isolates recovered from healthy fattening pigs on Farms in Thailand. Microb. Drug. Resist. 2018, 24, 213–223. [Google Scholar] [CrossRef]
- Simon, K.; Verwoolde, M.B.; Zhang, J.; Smidt, H.; de Vries Reilingh, G.; Kemp, B.; Lammers, A. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult. Sci. 2016, 95, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.V.; Kiet, B.T.; Phu, D.H.; Van, N.T.B.; Hien, V.B.; Thwaites, G.; Carrique-Mas, J.; Choisy, M. Effects of prophylactic and therapeutic antimicrobial uses in small-scale chicken flocks. Zoonoses Public Health 2021, 68, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C.M. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Heritage, J. Antibiotic Growth-Promoters in Food Animals. In Assessing Quality and Safety of Animal Feeds; FAO: Rome, Italy, 2004; pp. 129–152. [Google Scholar]
- Nasir, Z.; Grashorn, M.A. Alternatives to antibiotics: Do we really have some alternatives. In Proceeding XXIII World’s Poultry Congress 2008. World. Poult. Sci. J. 2008, 64, 165. [Google Scholar]
- Attia, Y.A.; Zeweil, H.S.; Alsaffar, A.A.; El-Shafy, A.S. Effect of non-antibiotic feed additives as an alternative to flavomycin on productive, meat quality and blood plasma traits of broiler chicks. Eur. Poult. Sci. 2011, 75, 40–48. [Google Scholar]
- Patterson, J.A.; Burkholder, K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef]
- Crisol-Martínez, E.; Stanley, D.; Geier, M.S.; Hughes, R.J.; Moore, R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 4547–4559. [Google Scholar] [CrossRef]
- Yang, C.J.; Yang, I.Y.; Oh, D.H.; Bae, I.H.; Cho, S.G.; Kong, I.G.; Uuganbayar, D.; Nou, I.S.; Choi, K.S. Effect of green tea by-product on performance and body composition in broiler chicks. Asian-Australas. J. Anim Sci. 2003, 16, 867–872. [Google Scholar] [CrossRef]
- Khan, S.H. The use of green tea (Camellia sinensis) as a phytogenic substance in poultry diets. Onderstepoort. J. Vet. Res. 2014, 81, 1–8. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Abdel-Azeem, S.M.; Al-Mohesen, I.A.; Ibrahim, A.M.H. Analysis of total phenolic compounds in tea and fruits using diazotized aminobenzenes colorimetric spots. Food Chem. 2020, 332, 127392. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, F.; Yang, Y.; Tu, Z.; Lin, J.; Ye, Y.; Xu, P. Oxygen-enriched fermentation improves the taste of black tea by reducing the bitter and astringent metabolites. Food Res. Int. 2021, 148, 110613. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Jolvis Pou, K.R. A review of withering in the processing of black tea. J. Biosyst. Eng. 2016, 41, 365–372. [Google Scholar] [CrossRef]
- Abdo, Z.M.A.; Hassan, R.A.; El-Salam, A.A.; Helmy, S.A. Effect of adding green tea and its aqueous extract as natural antioxidants to laying hen diet on productive, reproductive performance and egg quality during storage and its content of cholesterol. Egypt. Poult. Sci. 2010, 30, 1121–1149. [Google Scholar]
- Thinh, N.H.; Vinh, N.T.; Linh, N.V.; Phuong Giang, N.T.; Doan, B.H.; Dang, P.K. Effect of dietary supplementation with green tea powder on performance characteristic, meat organoleptic quality and cholesterol content of broilers. Livest. Res. Rural Develop. 2018, 30, 160. [Google Scholar]
- NRC. Nutrient Requirements of Poultry. In National Research Council, 9th ed.; National Academy Press: Washington, DC, USA, 1994; p. 234. [Google Scholar]
- Saeed, M.; Khan, M.S.; Kamboh, A.A.; Alagawany, M.; Khafaga, A.F.; Noreldin, A.E.; Qumar, M. Metabolism and nutrition. L-theanine: An astounding sui generis amino acid in poultry nutrition. Poult. Sci. 2020, 99, 5625–5636. [Google Scholar] [CrossRef]
- Nunes, A.R.; Alves, M.G.; Moreira, P.I.; Oliveira, P.F.; Silva, B.M. Impact of Green Tea (Camellia sinensis L.) Consumption in Diabetes Mellitus-Induced Neurodegeneration. In Green Tea and Health: Antioxidant Properties, Consumption and Role in Disease Prevention; Powell, N., Ed.; Nova Science Publishers: Covilha, Portugal, 2015; pp. 1–33. [Google Scholar] [CrossRef]
- Shim, K.F.; Vohra, P. A review of the nutrition of Japanese quail. World. Poult. Sci. J. 1984, 40, 261–274. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Polyphenolic chemistry of tea and coffee: A century of progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef]
- Rahman, N.H.A.; Chieng, B.W.; Ibrahim, N.A.; Rahman, N.A. Extraction and characterization of cellulose nanocrystals from tea leaf waste fibers. Polymers 2017, 9, 588. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, B.A.; Nyachoti, C.M.; Campbell, L.D.; Guenter, W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult. Sci. 2005, 84, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, D.; Chaudhry, A.S.; Seal, C.J. Chemical composition, plant secondary metabolites, and minerals of green and black teas and the effect of different tea-to-water ratios during their extraction on the composition of their spent leaves as potential additives for ruminants. J. Agric. Food Chem. 2013, 61, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Saptadip, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2002, 41, 65–93. [Google Scholar] [CrossRef]
- Serafini, M.; Del-Rio, D.; Yao, D.N.; Bettuzzi, S.; Peluso, I. Health Benefits of Tea. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ahmad, R.S.; Butt, M.S.; Huma, N.; Sultan, M.T.; Arshad, M.U.; Mushtaq, Z.; Saeed, F. Quantitative and qualitative portrait of green tea catechins (Gtc) through Hplc. Int. J. Food Prop. 2014, 17, 1626–1636. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, Z.; Zhao, J. Identification of green tea′s (Camelia sinensis L.) quality level according to measurement of main catechins and caffeine contents by HPLC and support vector classification pattern recognition. J. Pharmaceut. Biomed. Anal. 2008, 48, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Abdallah, F.; Abdel-hamid, T.; Hernandez-Santana, A. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br. Poult. Sci. 2016, 57, 714–722. [Google Scholar] [CrossRef]
- Kara, K.; Guclu, B.K.; Baytok, E.; Senturk, M. Effects of grape pomace supplementation to laying hen diet on performance, egg quality, egg lipid peroxidation and some biochemical parameters. J. Appl. Anim. Res. 2016, 44, 303–310. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ali, R.; Sultan, J.I.; Ali, A.; Kamran, Z.; Khan, S.A.; Ahsan, U. Impact of replacing grape polyphenol with vitamin E on growth performance, relative organs weight and antioxidant status of broilers. J. Anim. Plant. Sci. 2014, 24, 1579–1583. [Google Scholar]
- Rice-Evans, C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc. Soc. Exp. Biol. Med. 2010, 6, 220–262. [Google Scholar] [CrossRef]
- Yamamoto, Y. Inhibitory effect of tea polyphenols on cancer metastasis. J. Jan. Soc. Food Sci. Technol. 2000, 47, 567–572. [Google Scholar] [CrossRef]
- Singh, D.K.; Banerjee, S.; Porter, T.D. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J. Nutr. Biochem. 2009, 20, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, N.S.P.J. Green tea extract: Chemistry, antioxidant properties and food application-A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Song, J.M.; Seong, B.L. Tea catechins as a potential alternative anti-infectious agent. Expert Rev. Anti-Infect. Ther. 2007, 5, 497–506. [Google Scholar] [CrossRef]
- Hara, Y. Effect of tea polyphenols on the intestinal flora. Food Process. 1993, 28, 29. [Google Scholar] [CrossRef]
- Hayajneh, F.M.F. Natural feed additives for broiler chickens. S. Afr. J. Anim. Sci. 2019, 49, 869–870. [Google Scholar] [CrossRef]
- Suganya, T.; Senthilkumar, S.; Deepa, K.; Muralidharan, J.; Gomathi, G.; Gobiraju, S. Herbal feed additives in poultry. Int. J. Sci. Environ. Technol. 2016, 5, 1137–1145. [Google Scholar]
- Madhupriya, V.; Shamsudeen, P.; Manohar, G.R.; Senthilkumar, S.; Soundarapandiyan, V.; Moorthy, M. Phyto feed additives in poultry nutrition–A Review. Int. J. Sci. Environ. Technol. 2018, 7, 815–822. [Google Scholar]
- Shahid, W.; Ahmad, A.; Mangaiyarkarasi, R.; Omer, M.; Shahina, N.; Abdurraheem, U.; Rahmanullah, S.; Zahra, Y. Effect of polyphenolic rich green tea extract as antioxidant on broiler performance during 0–4 weeks. Int. J. Adv. Res. 2013, 9, 177–181. [Google Scholar]
- Yoshino, K.; Tomita, I.; Sano, M.; Oguni, I.; Hara, Y.; Nakano, M. Effects of long term dietary supplement of tea polyphenols on lipid peroxide levels in rats. J. Am. Aging Assoc. 1996, 17, 79–85. [Google Scholar] [CrossRef]
- Tuzcu, M.; Sahin, N.; Karatepe, M.; Cikim, G.; Kilinc, U.; Sahin, K. Epigallocatechin-3-gallate supplementation can improve antioxidant status in stressed quail. Br. Poult. Sci. 2008, 49, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Ariana, M.; Samie, A.; Edriss, M.A.; Jahanian, R. Effects of powder and extract form of green tea and marigold, and α-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plant. Res. 2011, 5, 2710–2716. [Google Scholar]
- Kara, K.; Şentürk, M.; Guclu, B.K.; Sariözkan, S.; Eren, M. Effect of catechins on fattening performance, meat quality, some antioxidant and blood parameters and fattening costs in Japanese quail (Coturnix coturnix japonica). Br. Poult. Sci. 2016, 57, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef]
- Molan, A.L.; Sivakumaran, S.; Spencer, P.A.; Meagher, L.P. Green tea flavan-3-ols and oligomeric proanthocyanidins inhibit the motility of infective larvae of Teladorsagia circumcincta and Trichostrongylus colubriformis in vitro. Res. Vet. Sci. 2004, 77, 239–243. [Google Scholar] [CrossRef]
- Shimizu, M.; Wada, S.; Hayashi, T.; Arisawa, M.; Ikegaya, K.; Ogaku, S.; Yano, S.; Morita, N. Studies on hypoglycemic constituents of Japanese tea. J. Pharm. Soc. Jpn. 2000, 108, 964–970. [Google Scholar] [CrossRef]
- Koo, M.W.L.; Cho, C.H. Pharmacological effects of green tea on the gastrointestinal system. Eur. J. Pharmacol. 2004, 500, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kara, K.; Guçlu, B.K.; Senturk, M.; Konca, Y. Influence of catechin (flavan-3-ol) addition to breeder quail (Coturnix coturnix japonica) diets on productivity, reproductive performance, egg quality and yolk oxidative stability. J. Appl. Anim. Res. 2016, 44, 436–441. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W.; Zhu, S.; Tsai, S.; Li, J.; Tracey, K.J.; Wang, P.; Fan, S.; Sama, A.E.; Wang, H. Green tea catechin quenches the fluorescence of bacterial-conjugated alexa fluor dyes. Inflamm. Allergy. Drug. Targets 2013, 12, 308–314. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, M.; Alkhaleefah, F.; Sheriff, D.M. Antimicrobial effects of epi-gallo-catechin-gallate and epicatechins of green tea on planktonic and biofilm forms of Staphylococcus aureus, including MRSA. Nat. Sci. 2013, 11, 70–79. [Google Scholar]
- Bakkir, L.K.; Yassen, R.T.; Mustaffa, R.M. In vitro and in vivo study of green tea and black tea antimicrobial activity on methicillin resistant Staphylococcus aureus. Bas. J. Vet. Res. 2011, 10, 55014. [Google Scholar] [CrossRef][Green Version]
- Al-Kayali, K.K.; Razooqi, B.M.; Mtaab, A.S. Antibacterial activity of aqueous extract of green tea on bacteria isolated from children with impetigo. Diyala. J. Med. 2011, 1, 37–43. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.M.S.; da Cunha Xavier, J.; Dos Santos, J.F.S.; de Matos, Y.M.L.S.; Tintino, S.R.; de Freitas, T.S.; Coutinho, H.D.M. Evaluation of antibacterial and modifying action of catechin antibiotics in resistant strains. Microb. Pathog. 2018, 115, 175–178. [Google Scholar] [CrossRef]
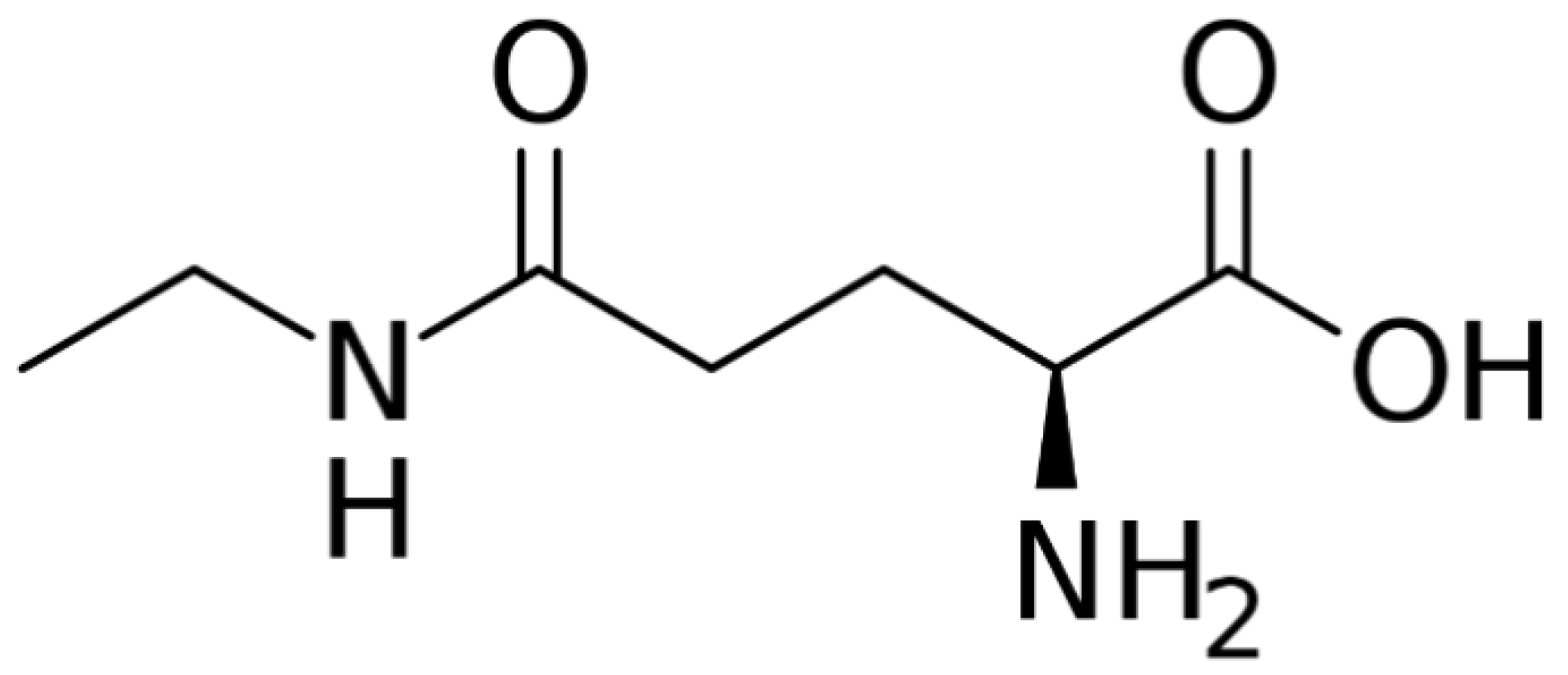
| Chemical Composition | Green Tea Powder | Black Tea Powder | Spent Green Tea Leaves | Spent Black Tea Leaves |
|---|---|---|---|---|
| Dry matter | 90.7 | 94.2 | 13.4 | 12.6 |
| Crude protein | 22.9 | 24.2 | 24.6 | 23.4 |
| Neutral detergent fibre | 32.5 | 32.3 | 40.5 | 47.4 |
| Acid detergent fibre | 21.1 | 30.9 | 29.4 | 41.0 |
| Organic matter | - | 93.9 | 95.7 | 96.1 |
| Ether extract | 2.08 | 1.26 | 2.31 | 1.35 |
| Tannin | 22.3 | - | - | - |
| Metabolizable energy (Kcal/kg) | 2816 | 1529 | 1765 | 1574 |
| Source | [48] | [56] | [56] | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlake, S.K.; Mnisi, C.M.; Kumanda, C.; Mthiyane, D.M.N.; Montso, P.K. Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review. Antibiotics 2022, 11, 565. https://doi.org/10.3390/antibiotics11050565
Mahlake SK, Mnisi CM, Kumanda C, Mthiyane DMN, Montso PK. Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review. Antibiotics. 2022; 11(5):565. https://doi.org/10.3390/antibiotics11050565
Chicago/Turabian StyleMahlake, Steve Kgotlelelo, Caven Mguvane Mnisi, Cebisa Kumanda, Doctor Mziwenkosi Nhlanhla Mthiyane, and Peter Kotsoana Montso. 2022. "Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review" Antibiotics 11, no. 5: 565. https://doi.org/10.3390/antibiotics11050565
APA StyleMahlake, S. K., Mnisi, C. M., Kumanda, C., Mthiyane, D. M. N., & Montso, P. K. (2022). Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review. Antibiotics, 11(5), 565. https://doi.org/10.3390/antibiotics11050565







