Comparative Analysis of Human and Animal E. coli: Serotyping, Antimicrobial Resistance, and Virulence Gene Profiling
Abstract
1. Introduction
2. Results
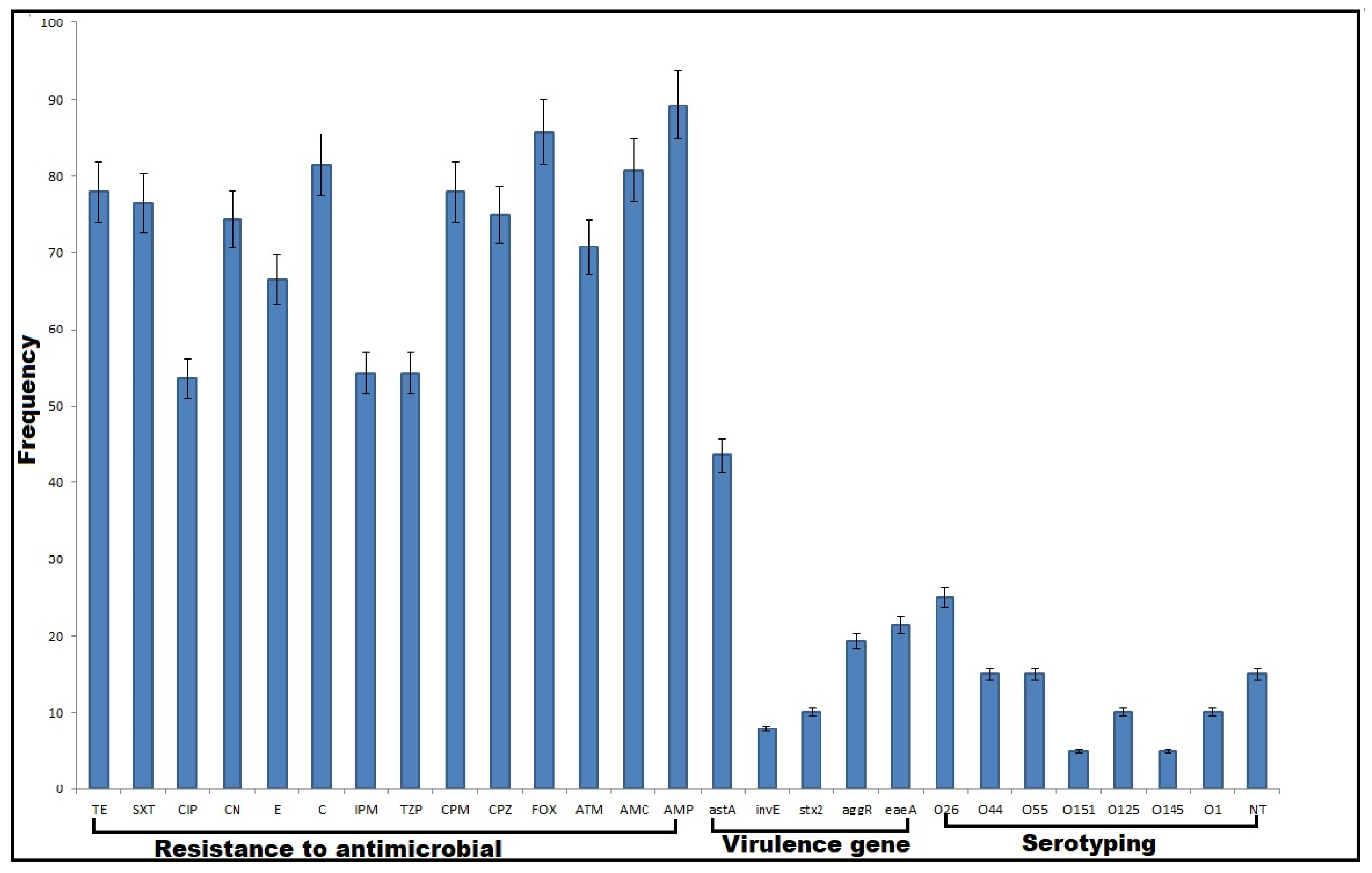
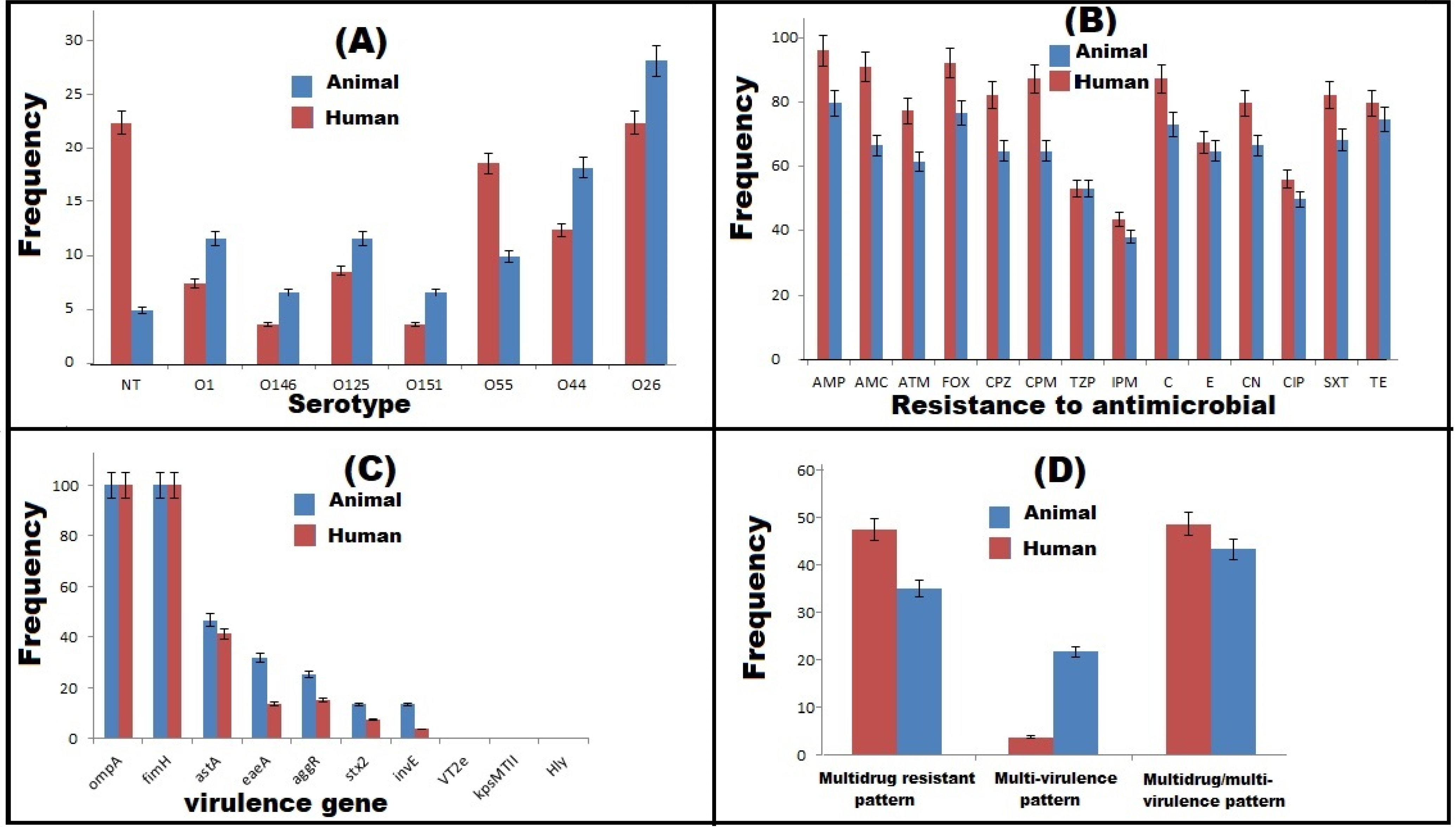
2.1. Phenotypic Characterization of DEC
2.2. Antimicrobial Susceptibility Patterns
2.3. Virulence Gene Profiles
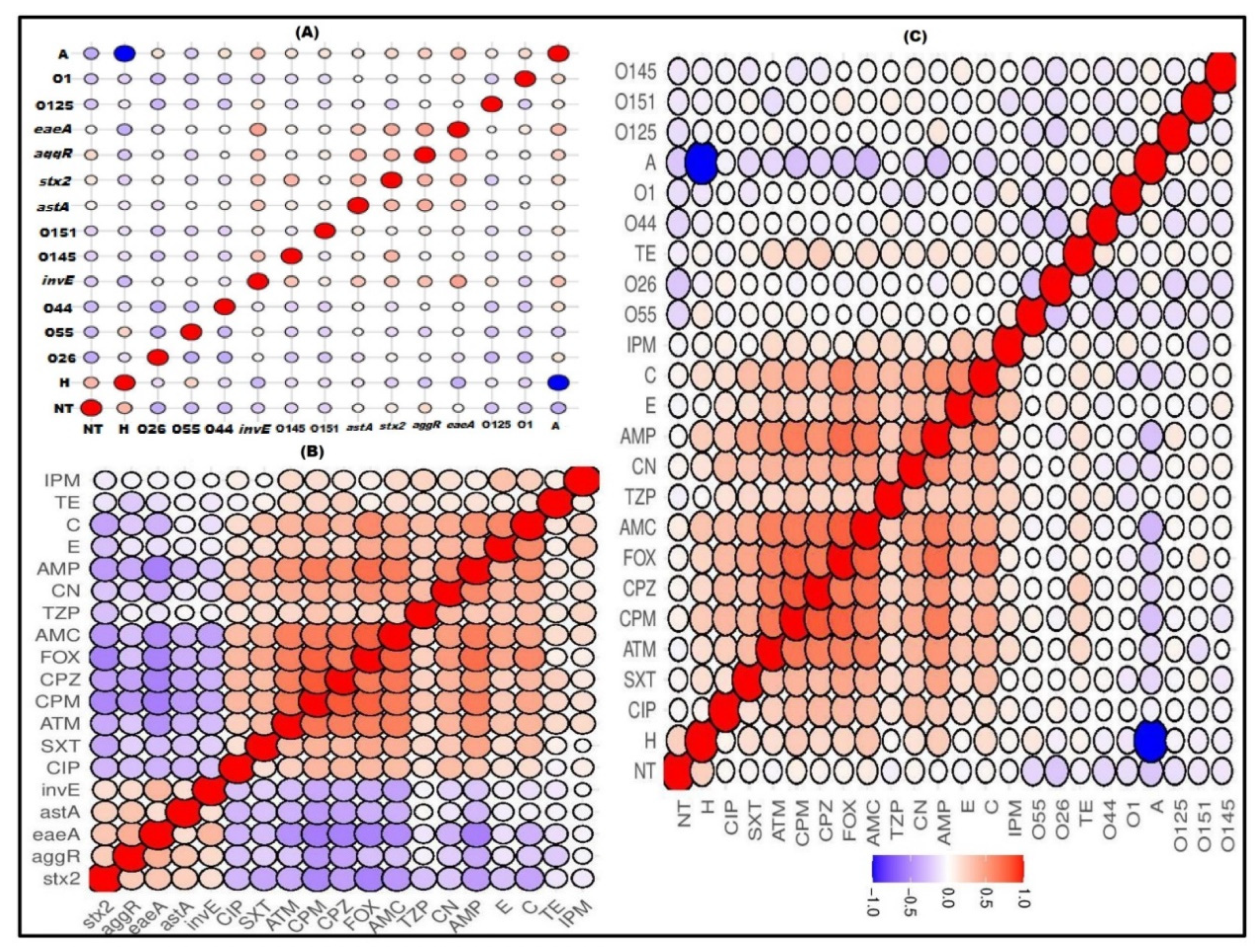
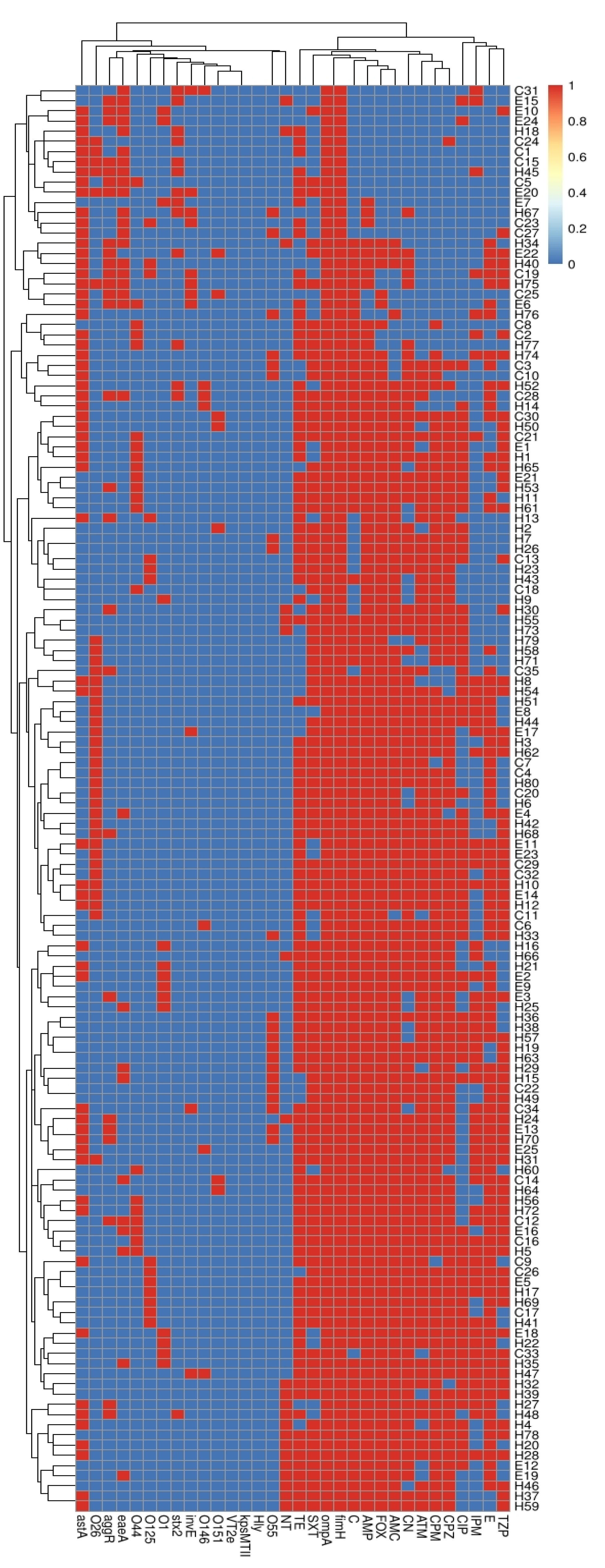
2.4. Correlation between Serotypes, Existence of Virulence Genes and Antimicrobial Resistance
3. Discussion
4. Materials and Methods
4.1. Study Design and DEC Isolation and Identification
4.2. Serotyping
4.3. Antimicrobial Susceptibility Testing
4.4. Determination of Multiple Antibiotic Resistance (MAR) Indices
4.5. Virulence Genotyping of E. coli
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, R.; Brown, P.D. Mltiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 2016, 65, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.; Finlay, B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.S.; Abd El-Hamid, M.I.; Hashem, Y.M.; Erfan, A.M.; Abdelrahman, B.A.; Mahmoud, H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: An in vivo perspective. J. Appl. Microbiol. 2019, 127, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Koczura, R.; Mokracka, J.; Jablonska, L.; Gozdecka, E.; Kubek, M.; Kaznowski, A. Antimicrobial resistance of integron-harboring Escherichia coli isolates from clinical samples, wastewater treatment plant and river water. Sci. Total Environ. 2012, 414, 680–685. [Google Scholar] [CrossRef]
- Hamelin, K.; Bruant, G.; El-Shaarawi, A.; Hill, S.; Edge, T.A.; Fairbrother, J.; Harel, J.; Maynard, C.; Masson, L.; Brousseau, R. Occurrence of virulence and antimicrobial resistance genes in Escherichia coli isolates from different aquatic ecosystems within the St. Clair River and Detroit River areas. Appl. Environ. Microbiol. 2007, 73, 477–484. [Google Scholar] [CrossRef]
- Rasko, D.A.; Rosovitz, M.J.; Myers, G.S.; Mongodin, E.F.; Fricke, W.F.; Gajer, P.; Crabtree, J.; Sebaihia, M.; Thomson, N.R.; Chaudhuri, R.; et al. The pangenome structure of Escherichia coli: Comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 2008, 190, 6881–6893. [Google Scholar]
- Broach, W.H.; Egan, N.; Wing, H.J.; Payne, S.M.; Murphy, E.R. VirF-independent regulation of Shigella virB transcription is mediated by the small RNA RyhB. PLoS ONE 2012, 7, e33529. [Google Scholar] [CrossRef]
- Wang, J.; Stanford, K.; McAllister, T.A.; Johnson, R.P.; Chen, J.; Hou, H.; Zhang, G.; Niu, Y.D. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 Shiga toxin–producing Escherichia coli. Foodborne Pathog Dis. 2016, 13, 316–324. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Johnson, J.R.; O’Bryan, T.T.; Kuskowski, M.; Maslow, J.N. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect Immun. 2001, 69, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Oundo, J.O.; Hibino, T.; Saidi, S.M.; Hinenoya, A.; Osawa, K.; Shirakawa, T.; Osawa, R.; Yamasaki, S. High Prevalence of Diarrheagenic Escherichia coli among Children with Diarrhea in Kenya. Jpn. J. Infect. Dis. 2017, 24, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Acosta, G.J.; Vigo, N.I.; Durand, D.; Riveros, M.; Arango, S.; Zambruni, M.; Ochoa, T.J. Diarrheagenic Escherichia coli: Prevalence and Pathotype Distribution in Children from Peruvian Rural Communities. Am. J. Trop. Med. Hyg. 2016, 7, 574–579. [Google Scholar] [CrossRef]
- Qato, M.; Como, N.; Meta, E.; Pipero, P.; Ostreni, V.; Kraja, D. Prevalence of diarrheagenic Escherichia coli in young children from rural South Africa: The Mal-ED cohort. Int. J. Infect. Dis. 2014, 21, 149. [Google Scholar] [CrossRef][Green Version]
- Canizalez-Roman, A.; Flores-Villaseñor, H.M.; Gonzalez-Nuñez, E.; Velazquez-Roman, J.; Vidal, J.E.; Muro-Amador, S.; Alapizco-Castro, G.; Díaz-Quiñonez, J.A.; León-Sicairos, N. Surveillance of Diarrheagenic Escherichia coli Strains Isolated from Diarrhea Cases from Children, Adults and Elderly at Northwest of Mexico. Front. Microbiol. 2016, 7, 1924. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Le-Van, P.; Le-Huy, C.; Gia, K.N.; Weintraub, A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 2005, 43, 755–760. [Google Scholar] [CrossRef]
- Gascón, J.; Vargas, M.; Schellenberg, D.; Urassa, H.; Casals, C.; Kahigwa, E.; Aponte, J.J.; Mshinda, H.; Vila, J. Diarrhea in children under 5 years of age from Ifakara, Tanzania: A case-control study. J. Clin. Microbiol. 2000, 38, 4459–4462. [Google Scholar] [CrossRef]
- Rebecca, P.; Sharia, M.; Berry, J.; Brosi, K.L. Climatic Drivers of Diarrheagenic Escherichia coli Incidence: A Systematic Review and Meta-analysis. J. Infect. Dis. 2016, 214, 6–15. [Google Scholar] [CrossRef]
- Zaidi, M.B.; Zamora, E.; Diaz, P.; Tollefson, L.; Fedorka-Cray, P.J.; Headrick, M.L. Risk factors for fecal quinolone-resistant Escherichia coli in Mexican children. Antimicrob. Agents Chemother. 2003, 47, 1999–2001. [Google Scholar] [CrossRef][Green Version]
- Baquero, F.; Martinez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Lupo, A.; Coyne, S.; Berendonk, T.U. Origin and evolution of antibiotic resistance: The common mechanisms of emergence and spread in water bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zheng, W.; Yu, Y.; Huang, W.; Zheng, S.; Zhang, Y.; Guan, X.; Zhuang, Y.; Chen, N.; Topp, E. Class 1 integrons, selected virulence genes, and antibiotic resistance in Escherichia coli isolates from the Minjiang River, Fujian Province, China. Appl. Environ. Microbiol. 2011, 77, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.H.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms 2020, 22, 1285. [Google Scholar] [CrossRef] [PubMed]
- Elfaky, M.A.; Abdel-Hamid, M.I.; Khalifa, E.; Alshareef, W.A.; Mosbah, R.A.; Elazab, S.T.; Ghoneim, M.M.; Al-Sanea, M.M.; Bendary, M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: Insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022, 106, 1691–1703. [Google Scholar] [CrossRef]
- Ghaly, M.F.; Nasr, Z.M.; Abousaty, A.I.; Seadawy, H.G.; Shaheen, M.A.A.; Albogami, S.; Al-Sanea, M.M.; Bendary, M.M. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics 2021, 10, 1453. [Google Scholar] [CrossRef]
- Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics 2021, 10, 1342. [Google Scholar] [CrossRef]
- Ammar, A.M.; Attia, A.M.; Abd El-Hamid, M.I.; El-Shorbagy, I.M.; Abd El-Kader, S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016, 62, 7–15. [Google Scholar]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2020, 10, 25. [Google Scholar] [CrossRef]
- Ghaly, M.; Shaheen, A.; Bouhy, A.; Bendary, M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020, 202, 1231–1240. [Google Scholar] [CrossRef]
- Ghaith, D.M.; Zafer, M.M.; Said, H.M.; Elanwary, S.; Elsaban, S.; Al-Agamy, M.H.; Bohol, M.F.F.; Bendary, M.M.; Al-Qahtani, A.; Al-Ahdal, M.N. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.; Elsohaby, I.; Ghaith, D.M.; Alshareef, W.A. What is behind phylogenetic analysis of hospital, community and livestock associated methicillin-resistant Staphylococcus aureus? Transbound Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [PubMed]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aurues recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic Diversity of Campylobacter jejuni Isolated from Avian and Human Sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef]
- Rahman, M.A.; Samad, M.A.; Kabir, M.L. Bacterio-pathological studies on salmonellosis, colibacillosis and pasteurellosis in natural and experimental infections in chickens. Bangladesh J. Vet. Med. 2004, 2, 1–8. [Google Scholar] [CrossRef]
- Ammar, A.M.; Abdel-hamid, M.I.; Eid, S.E.; El-Oks, S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Rev. Med. Vet. 2015, 166, 304–314. [Google Scholar]
- Saidi, B.; Mafirakureva, P.; Mbanga, J. Antimicrobial resistance of E. coli isolated from chickens with colibacillosis in and around Harare, Zimbabwe. Avian Dis. 2013, 57, 152–154. [Google Scholar] [CrossRef]
- Eid, S.E.; Erfan, A.M. Characterization of E. coli associated with high mortality of poultry flocks. Assiut Vet. Med. J. 2013, 59, 51–61. [Google Scholar]
- Lima-filho, J.V.; Martins, L.V.; Nascimento, D.C.; Venturea, R.F.; Batista, J.E.; Silva, A.F.; Ralph, M.T.; Vazr, V.; Rabello, C.B.; Silva, M.; et al. Zoonotic potential of multidrug-resistant extraintestinal pathogenic E. coli obtained from healthy poultry carcasses in Salvador, Brazil. Braz. J. Infect. Dis. 2013, 17, 54–61. [Google Scholar] [CrossRef]
- Kim, T.E.; Jeong, Y.W.; Cho, S.H.; Kim, S.J.; Kwon, H.J. Chronological study of antibiotic resistances and their relevant genes in Korean avian pathogenic E. coli isolates. J. Clin. Microbiol. 2007, 45, 3309–3315. [Google Scholar] [CrossRef][Green Version]
- Sharada, R.; Ruban, S.W.; Thiyageeswaran, M. Isolation, characterization and antibiotic resistance pattern of E. coli isolated from poultry. Am.-Eurasian J. Sci. Res. 2010, 5, 18–22. [Google Scholar]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Characterization of Methicillin Resistant Staphylococcus aureus isolated from human and animal samples in Egypt. Cell. Mol. Biol. 2016, 29, 94–100. [Google Scholar]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Vogwill, T.; MacLean, R.C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach. Evol. Appl. 2015, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55. [Google Scholar] [PubMed]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [PubMed]
- Cho, S.H.; Oh, K.H.; Kim, S.H.; Oh, H.B.; Park, M.S. Distribution of Virulence Genes and Their Association of Serotypes in Pathogenic Escherichia coli Isolates From Diarrheal Patients in Korea. Osong Public Health Res. Perspect. 2010, 1, 29–35. [Google Scholar] [CrossRef]
- De-Francesco, M.A.; Caracciolo, S.; Gargiulo, F.; Manca, N. Phenotypes, genotypes, serotypes and molecular epidemiology of erythromycin-resistant Streptococcus agalactiae in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1741–1747. [Google Scholar] [CrossRef]
- Schuab, R.B.; Arêas, G.P.; Souza, V.C.; Barros, R.R. Molecular epidemiology of Streptococcus agalactiae recovered from significant bacteriuria. Infect. Dis. 2015, 47, 637–642. [Google Scholar] [CrossRef]
- Yan, Y.; Hu, H.; Lu, T.; Fan, H.; Hu, Y.; Li, G.; Zhang, X.; Shi, Y.; Xia, R. Investigation of serotype distribution and resistance genes profile in group B Streptococcus isolated from pregnant women: A Chinese multicenter cohort study. APMIS 2016, 124, 794–799. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Carter, M.E.; Donnelly, W.J.C.; Lenard, F.C. Veterinary Microbiology and Microbial Diseases, 1st ed.; Blackwell Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Sabat, G.; Rose, P.; Hickey, W.J.; Harkin, J.M. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl. Environ. Microbiol. 2000, 66, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Document M100-S15; 15th International Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Clinical and Laboratory Standards Institute. M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Krumperman, P.H. Multiple Antibiotic Resistance Indexing of Escherichia coli to Identify High-Risk Sources of Fecal Contamination of Foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Tambekar, D.; Dhanorkar, D.; Gulhane, S.; Khandelwal, V.; Dudhane, M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 1562–1565. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kiebling, S.; Alt, K.; Antáo, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Inter. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Piva, I.C.; Pereira, A.L.; Ferraz, L.R.; Silva, R.S.; Vieira, A.C.; Blanco, J.E.; Blanco, M.; Blanco, J.; Giugliano, L.G. Virulence Markers of Enteroaggregative Escherichia coli Isolated from Children and Adults with Diarrhea in Brasília. Brazil. J. Clin. Microbiol. 2003, 41, 1827–1832. [Google Scholar] [CrossRef]
- Dipineto, L.; Santaniello, A.; Fontanella, M.; Lagos, K.; Fioretti, A.; Menna, L.F. Presence of Shiga toxin-producing Escherichia coli O157:H7 in living layer hens. Let. Appl. Microbiol. 2006, 43, 293–295. [Google Scholar] [CrossRef]
- Ghanbarpour, R.; Salehi, M. Determination of Adhesin Encoding Genes in Escherichia coli Isolates from Omphalitis of Chicks. Am. J. Anim. Vet. Sci. 2010, 5, 91–96. [Google Scholar] [CrossRef]
- Johnson, W.M.; Pollard, D.R.; Lior, H.; Tyler, S.D.; Rozee, K.R. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J. Clin. Microbiol. 1990, 28, 2351–2353. [Google Scholar] [CrossRef]
- Ewers, C.; Janßen, T.; Kießling, S.; Philipp, H.C.; Wieler, L.H. Rapid Detection of Virulence-Associated Genes in Avian Pathogenic Escherichia coli by Multiplex Polymerase Chain Reaction. Avian Dis. 2005, 49, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Zeng, L.; Huang, W.; Wu, Q.; Fan, Y.; Zheng, S.; Peng, L.; Han, J.; Huang, Y.; Zhou, H.; et al. Detection and Categorization of Diarrheagenic Escherichia coli with Auto-microfluidic Thin-film Chip Method. Sci. Rep. 2018, 8, 12926. [Google Scholar] [CrossRef] [PubMed]
| Target Virulence | Primers Sequences (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| ompA | F: AGCTATCGCGATTGCAGTG | 919 | 59 |
| R: GGTGTTGCCAGTAACCGG | |||
| kpsMTII | F: CAGGTAGCGTCGAACTGTA | 280 | 59 |
| R: CATCCAGACGATAAGCATGAGCA | |||
| hly | F: AACAAGGATAAGCACTGTTCTGGCT | 1177 | 60 |
| R: ACCATATAAGCGGTCATTCCCGTCA | |||
| stx2 | F: CCATGACAACGGACAGCAGTT | 779 | 61 |
| R: CCTGTCAACTGAGCAGCACTTTG | |||
| fimH | F: TGCAGAACGGATAAGCCGTGG | 508 | 62 |
| R: GCAGTCACCTGCCCTCCGGTA | |||
| vt2e | F: CCT TAA CTA AAA GGA ATA TA | 230 | 63 |
| R: CTG GTG GTG TAT GAT TAA TA | |||
| astA | F: TGCCATCAACACAGTATATCC | 116 | 64 |
| R: TCAGGTCGCGAGTGACGGC | |||
| invE | F: CGATAGATGGCGAGAAATTATATCCCG | 766 | 65 |
| R:CGATCAAGAATCCCTAACAGAAGAATCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendary, M.M.; Abdel-Hamid, M.I.; Alshareef, W.A.; Alshareef, H.M.; Mosbah, R.A.; Omar, N.N.; Al-Sanea, M.M.; Alhomrani, M.; Alamri, A.S.; Moustafa, W.H. Comparative Analysis of Human and Animal E. coli: Serotyping, Antimicrobial Resistance, and Virulence Gene Profiling. Antibiotics 2022, 11, 552. https://doi.org/10.3390/antibiotics11050552
Bendary MM, Abdel-Hamid MI, Alshareef WA, Alshareef HM, Mosbah RA, Omar NN, Al-Sanea MM, Alhomrani M, Alamri AS, Moustafa WH. Comparative Analysis of Human and Animal E. coli: Serotyping, Antimicrobial Resistance, and Virulence Gene Profiling. Antibiotics. 2022; 11(5):552. https://doi.org/10.3390/antibiotics11050552
Chicago/Turabian StyleBendary, Mahmoud M., Marwa I. Abdel-Hamid, Walaa A. Alshareef, Hanan M. Alshareef, Rasha A. Mosbah, Nasreen N. Omar, Mohammad M. Al-Sanea, Majid Alhomrani, Abdulhakeem S. Alamri, and Walaa H. Moustafa. 2022. "Comparative Analysis of Human and Animal E. coli: Serotyping, Antimicrobial Resistance, and Virulence Gene Profiling" Antibiotics 11, no. 5: 552. https://doi.org/10.3390/antibiotics11050552
APA StyleBendary, M. M., Abdel-Hamid, M. I., Alshareef, W. A., Alshareef, H. M., Mosbah, R. A., Omar, N. N., Al-Sanea, M. M., Alhomrani, M., Alamri, A. S., & Moustafa, W. H. (2022). Comparative Analysis of Human and Animal E. coli: Serotyping, Antimicrobial Resistance, and Virulence Gene Profiling. Antibiotics, 11(5), 552. https://doi.org/10.3390/antibiotics11050552










