Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Effect of BGL on Growth Performance and Nutrients Digestibility in Weaned Pigs upon ETEC Challenge
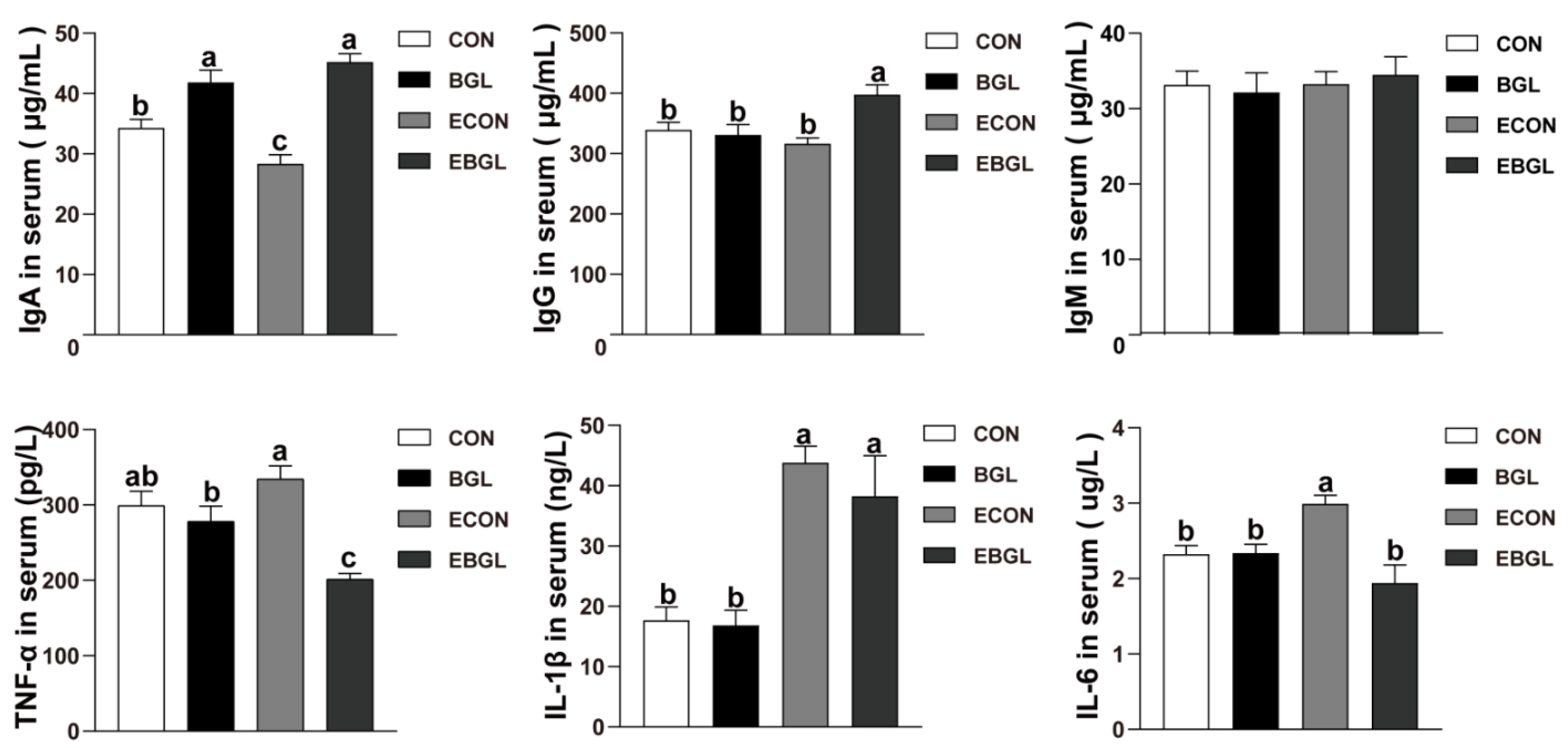
2.2. Effect of BGL on Serum Immunoglobulins and Inflammatory Cytokines in Weaned Pigs upon ETEC Challenge
2.3. Effect of BGL Supplementation on Intestinal Morphology and Mucosal Enzyme Activity in Weaned Pigs upon ETEC Challenge
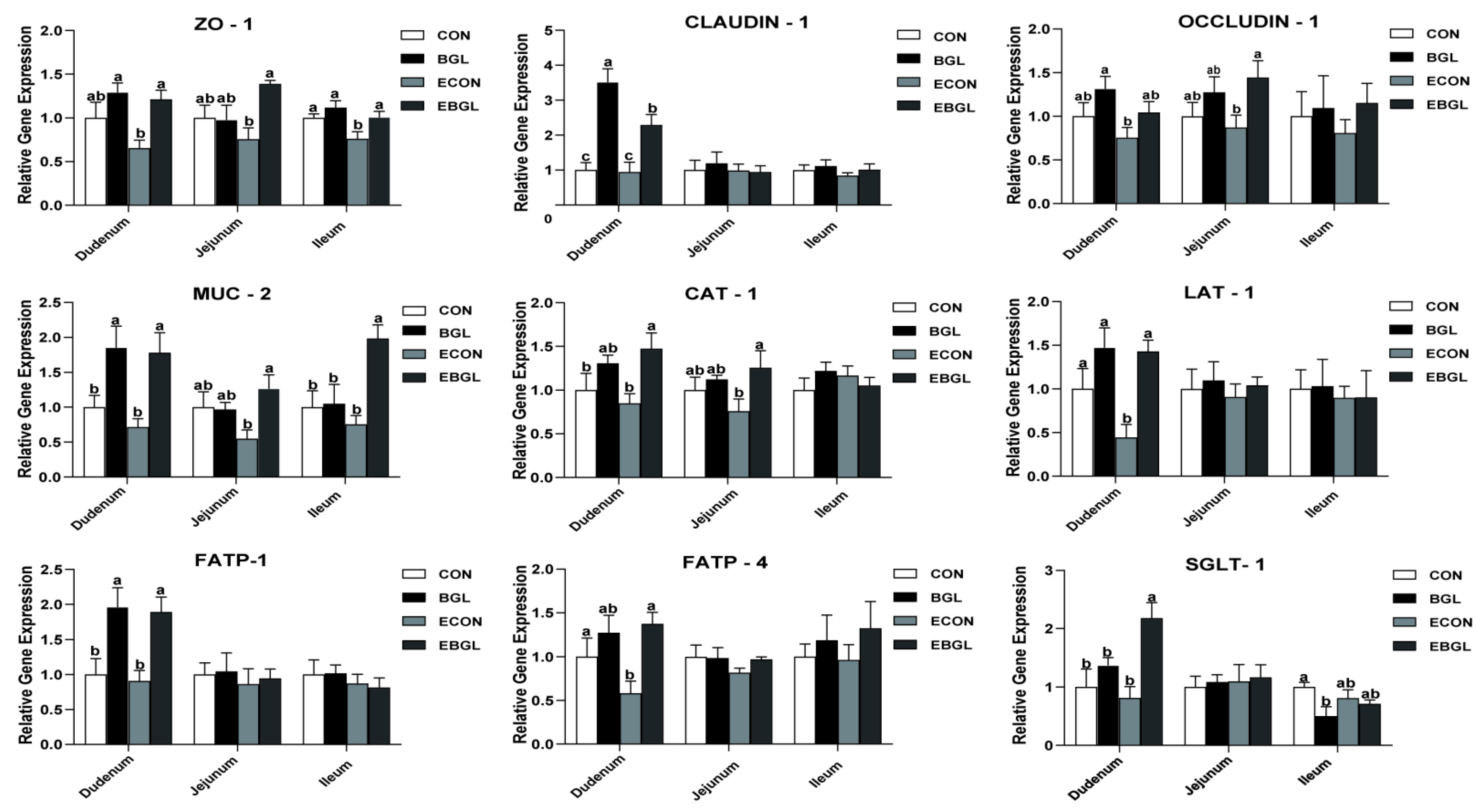
2.4. Effect of BGL Supplementation on Expressions of Critical Genes Involved in Intestinal Epithelium Functions
2.5. Effect of BGL Supplementation on Intestinal Microbial Populations in Weaned Pigs upon ETEC Challenge
3. Discussion
4. Materials and Methods
4.1. Animal Diets and Experimental Design
4.2. Growth Performance Evaluation
4.3. Sample Collection
4.4. Apparent Total Tract Nutrient Digestibility Analysis
4.5. Serum Proinflammatory Cytokines and Immunoglobulin Detection
4.6. Histomorphology Analysis of Each Intestinal Segment
4.7. Enzyme Activity
4.8. Caecal Microbiological Analysis
4.9. Metabolite Concentrations in Cecal Contents
4.10. Isolation and Reverse Transcription of RNA from Intestinal Mucosa and q-PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, E.Ø.; Kudirkiene, E.; Christensen, A.E.; Agerlin, M.V.; Weber, N.R.; Nødtvedt, A.; Nielsen, J.P.; Hartmann, K.T.; Skade, L.; Larsen, L.E.; et al. Post-weaning diarrhea in pigs weaned without medicinal zinc: Risk factors, pathogen dynamics, and association to growth rate. Porc. Health Manag. 2021, 7, 54. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, T.P.V.; Sakellaris, H. Colonization factors of enterotoxigenic Escherichia coli. Adv. Appl. Microbiol. 2015, 90, 155–197. [Google Scholar] [CrossRef]
- Jensen, M.L.; Thymann, T.; Cilieborg, M.S.; Lykke, M.; Mølbak, L.; Jensen, B.B.; Schmidt, M.; Kelly, D.; Mulder, I.; Burrin, D.G.; et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am. J. Physiol. -Gastrointest. Liver Physiol. 2014, 306, G59–G71. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Zhang, L.S.; Martinez, K.; Chang, E.B.; Yang, Q.; Wang, F.; Howles, P.N.; Hokari, R.; Miura, S.; Tso, P. Antibiotics Suppress Activation of Intestinal Mucosal Mast Cells and Reduce Dietary Lipid Absorption in Sprague-Dawley Rats. Gastroenterology 2016, 151, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef]
- Oberc, A.M.; Fiebig-Comyn, A.A.; Tsai, C.N.; Elhenawy, W.; Coombes, B.K. Antibiotics Potentiate Adherent-Invasive E. coli Infection and Expansion. Inflamm. Bowel Dis. 2019, 25, 711–721. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Tian, G.; Chen, D.; Huang, L.; Zhang, D.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; et al. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet1. J. Anim. Sci. 2019, 97, 4548–4556. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Yu, B.; Yin, H.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Fructooligosaccharides improve growth performance and intestinal epithelium function in weaned pigs exposed to enterotoxigenic Escherichia coli. Food Funct. 2020, 11, 9599–9612. [Google Scholar] [CrossRef]
- Froebel, L.K.; Froebel, L.E.; Duong, T. Refined functional carbohydrates reduce adhesion of Salmonella and Campylobacter to poultry epithelial cells in vitro. Poult. Sci. 2020, 99, 7027–7034. [Google Scholar] [CrossRef]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Franca, M.S.; Williams, S.M.; Gogal, R.M.; Ritz, C.W.; Kim, W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 2018, 97, 2525–2533. [Google Scholar] [CrossRef]
- Yu, E.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yin, H.; Yu, J.; Luo, J.; et al. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge. Br. J. Nutr. 2021, 126, 993–1002. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Baruah, R.; Goyal, A. A food additive with prebiotic properties of an α-d-glucan from lactobacillus plantarum DM5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Li, X.; Zheng, F.; Zeng, X. Yeast β-glucan, a potential prebiotic, showed a similar probiotic activity to inulin. Food Funct. 2020, 11, 10386–10396. [Google Scholar] [CrossRef]
- Chae, J.S.; Shin, H.; Song, Y.; Kang, H.; Yeom, C.-H.; Lee, S.; Choi, Y.S. Yeast (1 → 3)-(1 → 6)-β-d-glucan alleviates immunosuppression in gemcitabine-treated mice. Int. J. Biol. Macromol. 2019, 136, 1169–1175. [Google Scholar] [CrossRef]
- Alexander, M.P.; Fiering, S.N.; Ostroff, G.R.; Cramer, R.A.; Mullins, D.W. Beta-glucan-induced inflammatory monocytes mediate antitumor efficacy in the murine lung. Cancer Immunol. Immunother. 2018, 67, 1731–1742. [Google Scholar] [CrossRef]
- Zhen, W.; Shao, Y.; Wu, Y.; Li, L.; van Pham, H.; Abbas, W.; Wan, Z.; Guo, Y.; Wang, Z. Dietary yeast β-glucan supplementation improves eggshell color and fertile eggs hatchability as well as enhances immune functions in breeder laying hens. Int. J. Biol. Macromol. 2020, 159, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-L.; Chi-Keung Cheung, P. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [Green Version]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Zebeli, Q. Cereal β-glucan alters nutrient digestibility and microbial activity in the intestinal tract of pigs, and lower manure ammonia emission: A meta-analysis. J. Anim. Sci. 2013, 91, 3188–3199. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [Green Version]
- Abo Ghanima, M.M.; Abd El-Aziz, A.H.; Noreldin, A.E.; Atta, M.S.; Mousa, S.A.; El-Far, A.H. β-glucan administration improves growth performance and gut health in New Zealand White and APRI rabbits with different breed responses. PLoS ONE 2020, 15, e0234076. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, S. The growth performance and nonspecific immunity of loach Paramisgurnus dabryanus as affected by dietary β-1,3-glucan. Fish Shellfish Immunol. 2018, 83, 368–372. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Adhikari, R.; Llamas-Moya, S.; Kim, W.K. Effects of combination of mannan-oligosaccharides and β-glucan on growth performance, intestinal morphology, and immune gene expression in broiler chickens. Poult. Sci. 2021, 100, 101483. [Google Scholar] [CrossRef]
- Markina, Y.V.; Gerasimova, E.V.; Markin, A.M.; Glanz, V.Y.; Wu, W.-K.; Sobenin, I.A.; Orekhov, A.N. Sialylated Immunoglobulins for the Treatment of Immuno-Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 5472. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Ravetch, J.V. Diversification of IgG effector functions. Int. Immunol. 2017, 29, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljuca, F.; Gegic, A.; Salkic, N.N.; Pavlovic-Calic, N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn’s disease. Dig. Dis. Sci. 2010, 55, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.J.; Wang, L.X.; Yang, H.S.; Hu, A.; Yin, Y.L. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 2019, 13, 2727–2735. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Yang, Y.; Wang, J.; Jing, Y.; Yang, Q. Impact of TGEV infection on the pig small intestine. Virol. J. 2018, 15, 102. [Google Scholar] [CrossRef]
- Wu, T.; Shi, Y.; Zhang, Y.; Zhang, M.; Zhang, L.; Ma, Z.; Zhao, D.; Wang, L.; Yu, H.; Hou, Y.; et al. Lactobacillus rhamnosus LB1 Alleviates Enterotoxigenic Escherichia coli-Induced Adverse Effects in Piglets by Improving Host Immune Response and Anti-Oxidation Stress and Restoring Intestinal Integrity. Front. Cell. Infect. Microbiol. 2021, 11, 724401. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwon, C.H.; Park, B.C.; Lee, C.Y.; Han, J.H. Effects of a lipid-encapsulated zinc oxide dietary supplement, on growth parameters and intestinal morphology in weanling pigs artificially infected with enterotoxigenic Escherichia coli. J. Anim. Sci. Technol. 2015, 57, 4. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.L.; Keita, A.V.; Parsons, B.N.; Prorok-Hamon, M.; Knight, P.; Winstanley, C.; O’ Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Soluble plantain fibre blocks adhesion and M-cell translocation of intestinal pathogens. J. Nutr. Biochem. 2013, 24, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef] [PubMed]
- Stüber, E.; Büschenfeld, A.; von Freier, A.; Arendt, T.; Fölsch, U.R. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL-Fas interaction: Effect of pentoxifylline on the development of mucosal atrophy. Gut 1999, 45, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Saito, A.C.; Higashi, T.; Fukazawa, Y.; Otani, T.; Tauchi, M.; Higashi, A.Y.; Furuse, M.; Chiba, H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 2021, 32, 722–738. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Nanayakkara, S.; Kaye, D.M. Pathogenesis and treatment of the cardiorenal syndrome: Implications of L-arginine-nitric oxide pathway impairment. Pharmacol. Ther. 2015, 154, 1–12. [Google Scholar] [CrossRef]
- Persaud, A.; Cormerais, Y.; Pouyssegur, J.; Rotin, D. Dynamin inhibitors block activation of mTORC1 by amino acids independently of dynamin. J. Cell Sci. 2018, 131, jcs211755. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Poppelreuther, M.; Ehehalt, R.; Füllekrug, J. Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS ONE 2012, 7, e45087. [Google Scholar] [CrossRef]
- Alessi, A.M.; Gray, V.; Farquharson, F.M.; Flores-López, A.; Shaw, S.; Stead, D.; Wegmann, U.; Shearman, C.; Gasson, M.; Collie-Duguid, E.S.R.; et al. β-Glucan is a major growth substrate for human gut bacteria related to Coprococcus eutactus. Environ. Microbiol. 2020, 22, 2150–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ya, T.; Zhang, Q.; Chu, F.; Merritt, J.; Bilige, M.; Sun, T.; Du, R.; Zhang, H. Immunological evaluation of Lactobacillus casei Zhang: A newly isolated strain from koumiss in Inner Mongolia, China. BMC Immunol. 2008, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Ramírez, L.M.; Hernández-Ochoa, B.; Gómez-Manzo, S.; Marcial-Quino, J.; Cárdenas-Rodríguez, N.; Centeno-Leija, S.; García-Garibay, M. Evaluation of Immunomodulatory Activities of the Heat-Killed Probiotic Strain Lactobacillus casei IMAU60214 on Macrophages In Vitro. Microorganisms 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Lackraj, T.; Kim, J.I.; Tran, S.-L.; Barnett Foster, D.E. Differential modulation of flagella expression in enterohaemorrhagic Escherichia coli O157: H7 by intestinal short-chain fatty acid mixes. Microbiology 2016, 162, 1761–1772. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Kim, S.H.; Choi, Y.J.; Soriano, A.P.; Cho, K.K.; Lee, K.; Bae, G.S.; Lee, S.S. Increased propionate concentration in Lactobacillus mucosae-fermented wet brewers grains and during in vitro rumen fermentation. J. Appl. Microbiol. 2017, 123, 29–40. [Google Scholar] [CrossRef]
- Xia, Z.; Han, Y.; Wang, K.; Guo, S.; Wu, D.; Huang, X.; Li, Z.; Zhu, L. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017, 16, 62. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (U.S.). Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 9780309224239. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Franklin, M.A.; Mathew, A.G.; Vickers, J.R.; Clift, R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs. 24 days of age. J. Anim. Sci. 2002, 80, 2904–2910. [Google Scholar] [CrossRef]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef]

| ITEM | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | BGL | ECON | EBGL | BGL | ETEC | Interaction | ||
| 1–25 d | ||||||||
| ADFI (g/d) | 427.11 | 429.16 | 459.58 | 459.77 | 22.47 | |||
| ADG (g/d) | 280.47 | 267.2 | 286.48 | 302.13 | 15.61 | |||
| F: G | 1.58 | 1.62 | 1.56 | 1.55 | 0.04 | |||
| 25–28 d | ||||||||
| ADFI (g) | 631.68 | 602.26 | 537.85 | 597.22 | 24.96 | 0.78 | 0.35 | 0.4 |
| ADG (g) | 519.9 a | 476.67 ab | 361.33 b | 511.11 ab | 27.9 | 0.31 | 0.24 | 0.08 |
| F: G | 1.25 | 1.3 | 1.53 | 1.24 | 0.07 | 0.39 | 0.41 | 0.22 |
| ITEM | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | BGL | ECON | EBGL | |||
| DM (%) | 88.43 | 88.69 | 88.98 | 89.17 | 0.40 | 0.93 |
| CP (%) | 86.53 | 86.49 | 87.18 | 87.62 | 0.68 | 0.93 |
| EE (%) | 84.96 | 84.89 | 86.34 | 86.56 | 0.58 | 0.65 |
| Ash (%) | 67.65 | 70.6 | 69.55 | 70.91 | 0.86 | 0.58 |
| GE (%) | 88.56 | 88.76 | 89.17 | 89.27 | 0.43 | 0.94 |
| ITEM | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | BGL | ECON | EBGL | BGL | ETEC | Interaction | ||
| Duodenum | ||||||||
| Villus height, μm | 402.98 ab | 461.37 a | 378.85 b | 454.45 a | 13.79 | 0.02 | 0.55 | 0.74 |
| Crypt depth, μm | 135.42 | 139.42 | 156.99 | 154.57 | 5.4 | 0.95 | 0.1 | 0.78 |
| V:C | 3.12 ab | 3.52 a | 2.73 b | 3.19 ab | 0.12 | 0.07 | 0.12 | 0.92 |
| Jejunum | ||||||||
| Villus height, μm | 413.89 a | 442.74 a | 299.70 b | 423.62 a | 13.68 | <0.01 | <0.01 | <0.01 |
| Crypt depth, μm | 137.96 | 123.95 | 144.87 | 126.52 | 5.34 | 0.15 | 0.67 | 0.84 |
| V:C | 3.11 ab | 3.50 a | 2.62 b | 3.45 a | 0.12 | 0.01 | 0.22 | 0.33 |
| Ileum | ||||||||
| Villus height, μm | 318.70 ab | 340.07 a | 265.98 b | 344.75 a | 10.87 | 0.01 | 0.21 | 0.14 |
| Crypt depth, μm | 137.96 | 123.95 | 144.87 | 126.52 | 5.34 | 0.15 | 0.67 | 0.84 |
| V:C | 2.42 ab | 3.05 a | 2.23 b | 2.79 ab | 0.14 | 0.04 | 0.42 | 0.9 |
| ITEM | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | BGL | ECON | EBGL | BGL | ETEC | Interaction | ||
| Duodenum | ||||||||
| Lactase (U/L) | 78.59 a | 66.08 c | 72.92 b | 66.03 c | 1.42 | 0.001 | 0.006 | 0.001 |
| Sucrase (U/L) | 255.48 a | 254.47 a | 234.90 b | 264.43 a | 2.76 | 0.001 | 0.16 | <0.01 |
| Maltase (U/L) | 175.56 c | 188.19 b | 166.91 d | 197.63 a | 2.82 | 0.03 | 0.88 | <0.01 |
| Jejunum | ||||||||
| Lactase (U/L) | 107.58 a | 103.48 a | 93.15 b | 102.80 a | 1.77 | 0.001 | 0.13 | 0.14 |
| Sucrase (U/L) | 353.32 a | 352.25 a | 328.36 b | 368.95 a | 4.43 | 0.01 | 0.56 | 0.07 |
| Maltase (U/L) | 286.45 b | 290.57 ab | 274.63 c | 298.55 a | 2.36 | <0.01 | 0.57 | 0.007 |
| Ileum | ||||||||
| Lactase (U/L) | 39.85 a | 40.21 a | 31.15 b | 32.09 b | 1.22 | 0.72 | <0.01 | 0.87 |
| Sucrase (U/L) | 294.08 bc | 315.82 a | 283.07 c | 306.39 ab | 3.27 | <0.01 | 0.03 | 0.86 |
| Maltase (U/L) | 214.18 c | 229.17 b | 202.78 c | 243.26 a | 3.77 | <0.01 | 0.76 | 0.008 |
| ITEM | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | BGL | ECON | EBGL | BGL | ETEC | Interaction | ||
| microbial populations (lg(copies/g)) | ||||||||
| Total bacteria | 11.18 | 11.03 | 11.18 | 11.21 | 0.05 | 0.5 | 0.35 | 0.34 |
| Escherichia coli | 8.35 b | 8.14 b | 9.88 a | 9.50 a | 0.22 | 0.42 | 0.001 | 0.81 |
| Lactobacillus | 8.28 ab | 8.75 a | 7.93 b | 8.60 a | 0.1 | 0.004 | 0.17 | 0.58 |
| Bifidobacterium | 6.09 | 6.28 | 6.2 | 6.09 | 0.13 | 0.88 | 0.88 | 0.59 |
| Bacillus | 9.16 | 9.18 | 9.09 | 9.03 | 0.04 | 0.8 | 0.18 | 0.6 |
| VFA (g/g) | ||||||||
| Acetic acid | 3.36 ab | 3.65 a | 2.59 b | 3.14 ab | 0.19 | 0.25 | 0.09 | 0.72 |
| Propanoic acid | 1.73 ab | 1.74 ab | 1.40 b | 2.00 a | 0.09 | 0.07 | 0.83 | 0.08 |
| Butyric acid | 0.78 | 0.95 | 0.82 | 0.78 | 0.05 | 0.59 | 0.6 | 0.41 |
| Ingredients | % | Nutrient Level | Contents |
|---|---|---|---|
| Corn | 28.31 | Digestible energy (calculated, MJ/kg) | 14.78 |
| Extruded corn | 24.87 | Crude Protein (%) | 19.68 |
| Soybean meal | 8.5 | Calcium (%) | 0.81 |
| Extruded full-fat soybean | 10.3 | Available phosphorus (%) | 0.55 |
| Fish meal | 4.2 | Lysine | 1.35 |
| Whey powder | 7 | Methionine | 0.42 |
| Soybean protein concentrate | 8 | Methionine + cysteine | 0.6 |
| Soybean oil | 2 | Threonine | 0.79 |
| Sucrose | 4 | Tryptophan | 0.22 |
| Limestone | 0.9 | ||
| Dicalcium phosphate | 0.5 | ||
| NaCl | 0.3 | ||
| L-Lysine HCl (78%) | 0.47 | ||
| DL-Methionine | 0.15 | ||
| L-Threonine (98.5%) | 0.13 | ||
| Tryptophan (98%) | 0.03 | ||
| Chloride choline | 0.1 | ||
| Vitamin premix 1 | 0.04 | ||
| Mineral premix 2 | 0.2 | ||
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Luo, Y.; Yu, B.; Zheng, P.; Yu, J.; Huang, Z.; Mao, X.; Luo, J.; Yan, H.; He, J. Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. Antibiotics 2022, 11, 519. https://doi.org/10.3390/antibiotics11040519
Zhou Y, Luo Y, Yu B, Zheng P, Yu J, Huang Z, Mao X, Luo J, Yan H, He J. Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. Antibiotics. 2022; 11(4):519. https://doi.org/10.3390/antibiotics11040519
Chicago/Turabian StyleZhou, Yuankang, Yuheng Luo, Bing Yu, Ping Zheng, Jie Yu, Zhiqing Huang, Xiangbing Mao, Junqiu Luo, Hui Yan, and Jun He. 2022. "Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli" Antibiotics 11, no. 4: 519. https://doi.org/10.3390/antibiotics11040519
APA StyleZhou, Y., Luo, Y., Yu, B., Zheng, P., Yu, J., Huang, Z., Mao, X., Luo, J., Yan, H., & He, J. (2022). Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. Antibiotics, 11(4), 519. https://doi.org/10.3390/antibiotics11040519








