Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae
Abstract
1. Introduction
2. Results
2.1. EGCG Inhibits Drug Resistant V. cholerae Strains
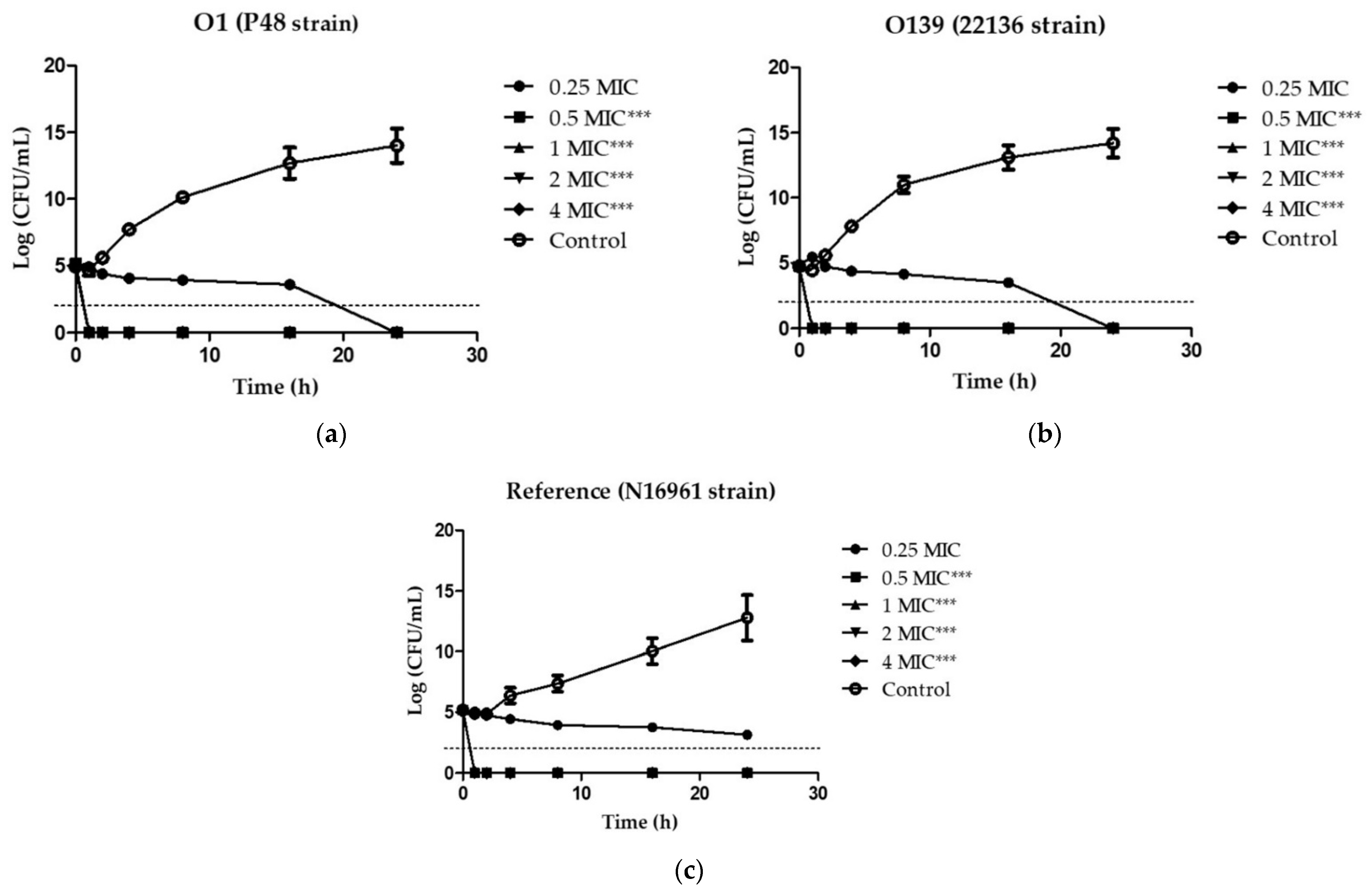
2.2. Analysis of Bacterial Killing Kinetics
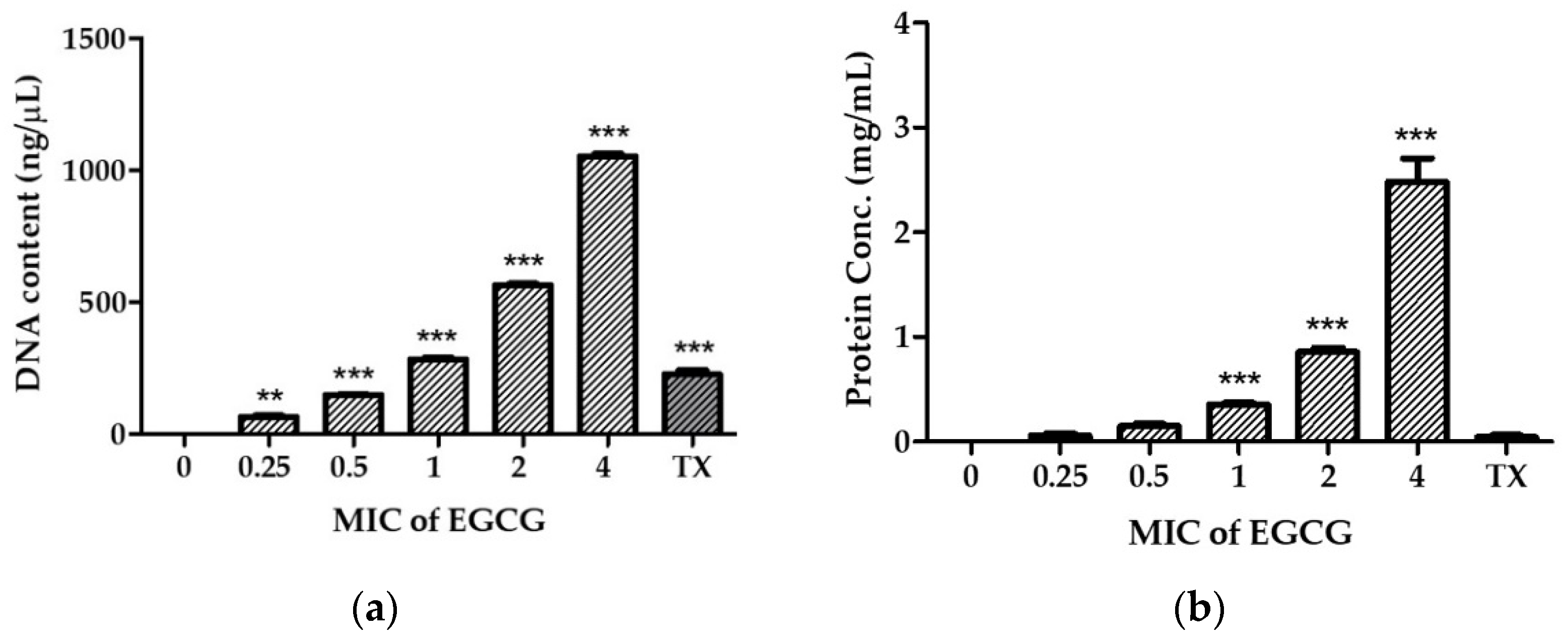
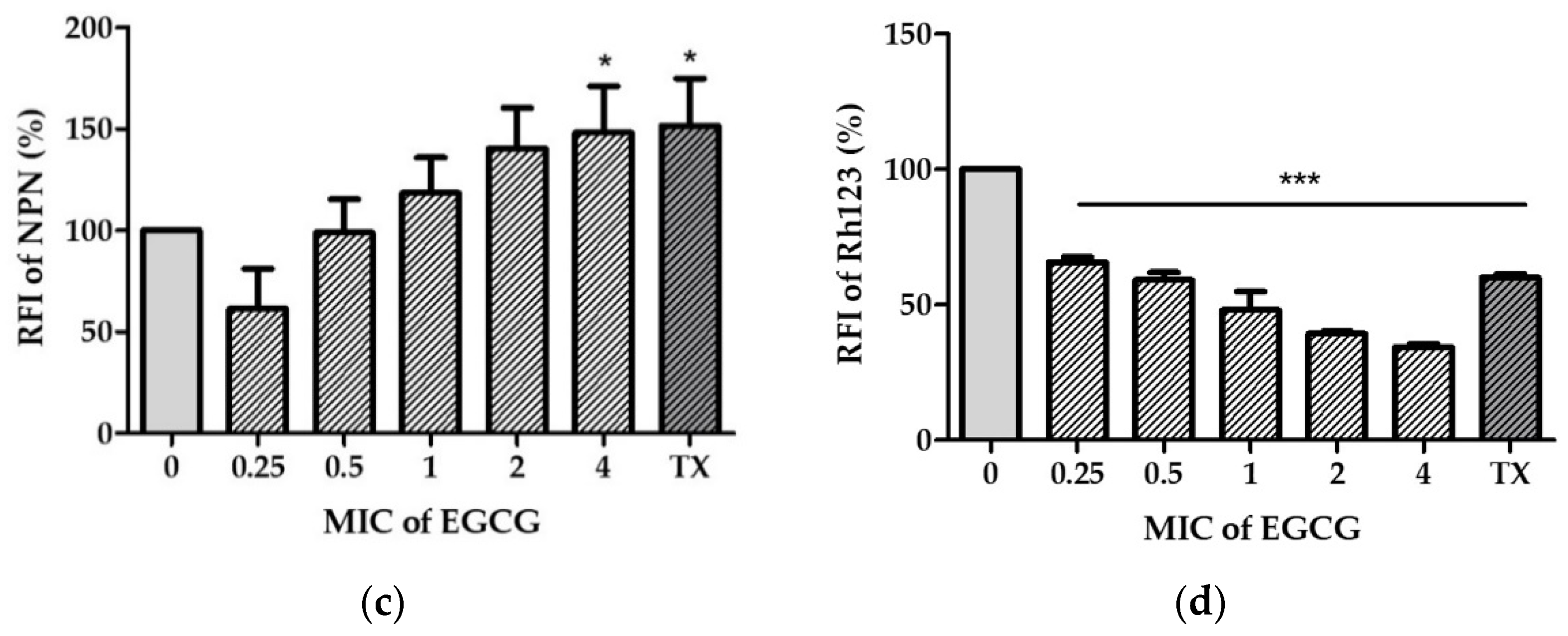
2.3. EGCG Disrupts V. cholerae Membrane Permeability
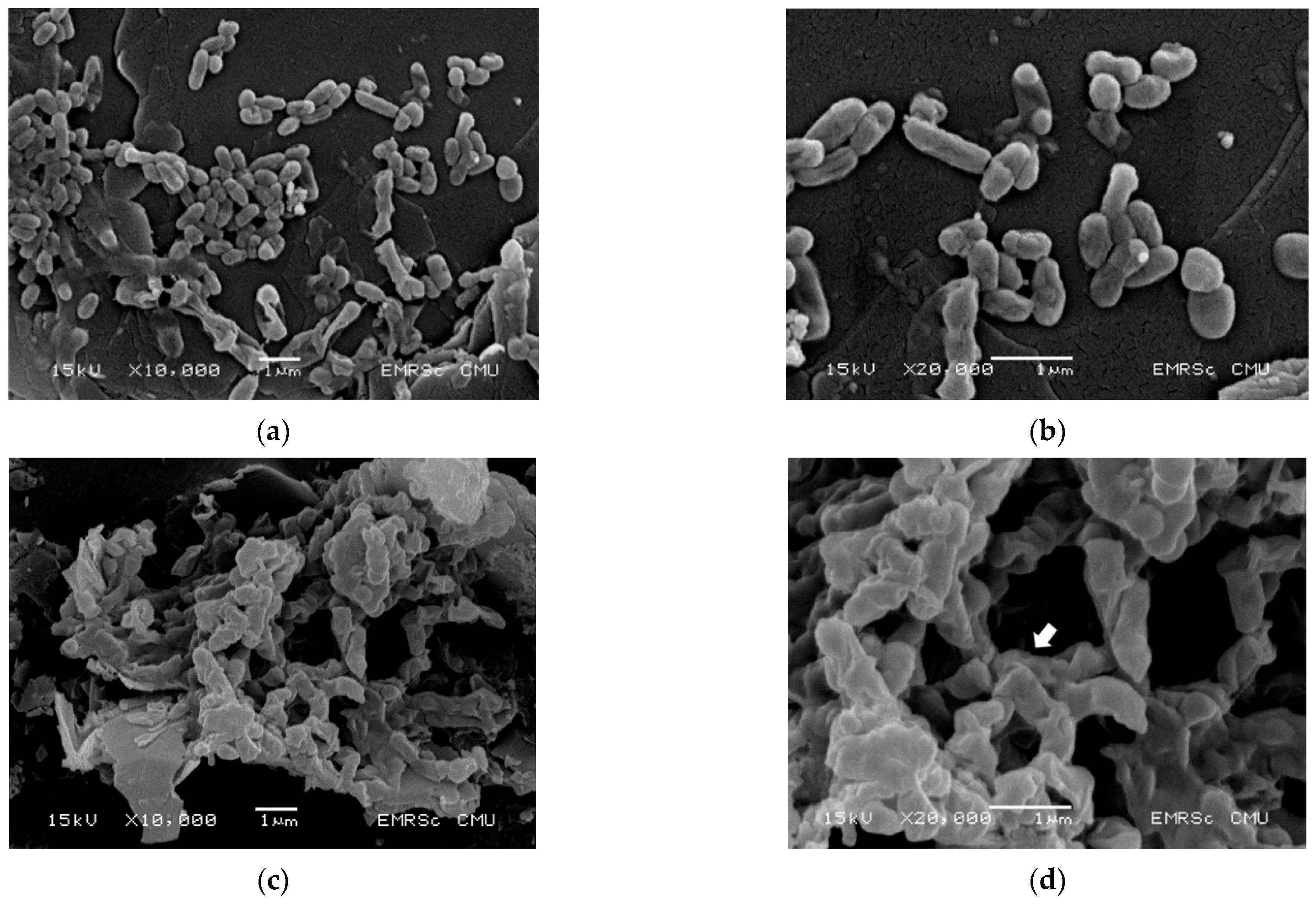
2.4. EGCG Altered the Morphological Characterization of V. cholerae
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains
4.3. Determination of the MIC and the MBC
4.4. Antimicrobial Synergy Testing
4.5. Time-Kill Kinetics Assay
4.6. Outer Membrane Permeabilization Analysis
4.6.1. Determination of Nucleotide and Protein Leakage
4.6.2. Determination of Outer Membrane Disruption
4.6.3. Determination of Cell Membrane Potential
4.7. Scanning Electron Microscopy (SEM) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, D.; Liu, B.; Feng, L.; Ding, P.; Guo, X.; Wang, M.; Cao, B.; Reeves, P.R.; Wang, L. Origins of the current seventh cholera pandemic. Proc. Natl. Acad. Sci. USA 2016, 113, E7730. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cholera Annual Report 2019 Weekly Epidemiological Record; WHO: Genewa, Switzerland, 2020; pp. 441–448. [Google Scholar]
- WHO. Cholera Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cholera (accessed on 4 January 2022).
- Saha, D.; Karim, M.M.; Khan, W.A.; Ahmed, S.; Salam, M.A.; Bennish, M.L. Single-dose azithromycin for the treatment of cholera in adults. N. Engl. J. Med. 2006, 354, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Bag, S.; Saha, B.; Kumar, P.; Ghosh, T.S.; Dayal, M.; Senapati, T.; Mehra, S.; Dey, P.; Desigamani, A.; et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2019, 116, 6226–6231. [Google Scholar] [CrossRef]
- Rivard, N.; Colwell, R.R.; Burrus, V.; Castanheira, M. Antibiotic Resistance in Vibrio cholerae: Mechanistic Insights from IncC Plasmid-Mediated Dissemination of a Novel Family of Genomic Islands Inserted at trmE. mSphere 2020, 5, e00748-20. [Google Scholar] [CrossRef]
- Shankar, U.; Jain, N.; Majee, P.; Kodgire, P.; Sharma, T.K.; Kumar, A. Exploring Computational and Biophysical Tools to Study the Presence of G-Quadruplex Structures: A Promising Therapeutic Solution for Drug-Resistant Vibrio cholerae. Front. Genet. 2020, 11, 935. [Google Scholar] [CrossRef]
- De, R. Mobile Genetic Elements of Vibrio cholerae and the Evolution of Its Antimicrobial Resistance. Front. Trop. Dis. 2021, 2, 691604. [Google Scholar] [CrossRef]
- Sjölund-Karlsson, M.; Reimer, A.; Folster, J.P.; Walker, M.; Dahourou, G.A.; Batra, D.G.; Martin, I.; Joyce, K.; Parsons, M.B.; Boncy, J.; et al. Drug-resistance mechanisms in Vibrio cholerae O1 outbreak strain, Haiti, 2010. Emerg. Infect. Dis. 2011, 17, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Siriphap, A.; Leekitcharoenphon, P.; Kaas, R.S.; Theethakaew, C.; Aarestrup, F.M.; Sutheinkul, O.; Hendriksen, R.S. Characterization and Genetic Variation of Vibrio cholerae Isolated from Clinical and Environmental Sources in Thailand. PLoS ONE 2017, 12, e0169324. [Google Scholar] [CrossRef]
- Taylor, P.W.; Hamilton-Miller, J.M.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Imai, K.; Nakachi, K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002, 188, 9–13. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef]
- Rawangkan, A.; Kengkla, K.; Kanchanasurakit, S.; Duangjai, A.; Saokaew, S. Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 4014. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure-activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Okubo, S.; Ikigai, H.; Shimamura, T. Antibacterial and anti-hemolysin activities of tea catechins and their structural relatives. Nihon Saikingaku Zasshi 1990, 45, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Aldhaher, Z. Evaluation of Antibacterial Activity of Aqueous Extracts of Pomegranate Peels, Green Tea Leaves and Bay Leaves against Vibrio cholera. Iraqi J. Vet. Med. 2018, 37, 90–95. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ikigai, H.; Suzuki, T.; Suzuki, Y.; Hara, Y.; Shimamura, T. The protective activity of tea catechins against experimental infection by Vibrio cholerae O1. Microbiol. Immunol. 1992, 36, 999–1001. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Dutta, P.; Dastidar, S.; Chatterjee, T. In vitro and in vivo antidiarrhoeal activity of epigallocatechin 3-gallate: A major catechin isolated from indian green tea. Orient. Pharm. Exp. Med. 2008, 8, 171–177. [Google Scholar] [CrossRef]
- Sarwar, S.; Chakraborti, S.; Bera, S.; Sheikh, I.A.; Hoque, K.M.; Chakrabarti, P. The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: Variation in response depends on biotype. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1499–1509. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 January 2022).
- Barh, D.; Barve, N.; Gupta, K.; Chandra, S.; Jain, N.; Tiwari, S.; Leon-Sicairos, N.; Canizalez-Roman, A.; dos Santos, A.R.; Hassan, S.S.; et al. Exoproteome and secretome derived broad spectrum novel drug and vaccine candidates in Vibrio cholerae targeted by Piper betel derived compounds. PLoS ONE 2013, 8, e52773. [Google Scholar] [CrossRef]
- Tiwari, S.; Barh, D.; Imchen, M.; Rao, E.; Kumavath, R.K.; Seenivasan, S.P.; Jaiswal, A.K.; Jamal, S.B.; Kumar, V.; Ghosh, P.; et al. Acetate Kinase (AcK) is Essential for Microbial Growth and Betel-derived Compounds Potentially Target AcK, PhoP and MDR Proteins in M. tuberculosis, V. cholerae and Pathogenic, E. coli: An in silico and in vitro Study. Curr. Top. Med. Chem. 2018, 18, 2731–2740. [Google Scholar] [CrossRef]
- Acosta-Smith, E.; Leon-Sicairos, N.; Tiwari, S.; Flores-Villaseñor, H.; Canizalez-Roman, A.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Piper betel Compounds Piperidine, Eugenyl Acetate, and Chlorogenic Acid Are Broad-Spectrum Anti-Vibrio Compounds that Are Also Effective on MDR Strains of the Pathogen. Pathogens 2019, 8, 64. [Google Scholar] [CrossRef]
- Hör, M.; Rimpler, H.; Heinrich, M. Inhibition of intestinal chloride secretion by proanthocyanidins from Guazuma ulmifolia. Planta Med. 1995, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Oi, H.; Matsuura, D.; Miyake, M.; Ueno, M.; Takai, I.; Yamamoto, T.; Kubo, M.; Moss, J.; Noda, M. Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 3042–3046. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Miyake, M.; Toba, M.; Okamatsu, H.; Shimizu, S.; Noda, M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol. Immunol. 2002, 46, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial activity of elephant garlic oil against Vibrio cholerae in vitro and in a food model. Biosci. Biotechnol. Biochem. 2009, 73, 1623–1627. [Google Scholar] [CrossRef]
- Yamasaki, S.; Asakura, M.; Neogi, S.B.; Hinenoya, A.; Iwaoka, E.; Aoki, S. Inhibition of virulence potential of Vibrio cholerae by natural compounds. Indian J. Med. Res. 2011, 133, 232–239. [Google Scholar]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef]
- Das, S.; Chourashi, R.; Mukherjee, P.; Kundu, S.; Koley, H.; Dutta, M.; Mukhopadhyay, A.K.; Okamoto, K.; Chatterjee, N.S. Inhibition of growth and virulence of Vibrio cholerae by carvacrol, an essential oil component of Origanum spp. J. Appl. Microbiol. 2021, 131, 1147–1161. [Google Scholar] [CrossRef]
- Pederson, D.B.; Dong, Y.; Blue, L.B.; Smith, S.V.; Cao, M. Water-soluble cranberry extract inhibits Vibrio cholerae biofilm formation possibly through modulating the second messenger 3’,5’—Cyclic diguanylate level. PLoS ONE 2018, 13, e0207056. [Google Scholar] [CrossRef]
- Sanchez, E.; García, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef]
- Yussof, A.; Habiba, U.; Liaw, D.; Chu, T.; Lee, L. Epigallocatechin Gallate-Stearate Enhances the Efficacy of Antibiotics. Open J. Med. Microbiol. 2019, 9, 77–94. [Google Scholar] [CrossRef][Green Version]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Aboulmagd, E.; Al-Mohammed, H.; Al-Badry, S. Synergism and postantibiotic effect of green tea extract and imipenem against methicillin-resistant Staphylococcus aureus. Microbiol. J. 2011, 1, 89–96. [Google Scholar] [CrossRef]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Razqan, G.S.; Kwon, D.H. Antibacterial activity of epigallocatechin-3-gallate (EGCG) and its synergism with β-lactam antibiotics sensitizing carbapenem-associated multidrug resistant clinical isolates of Acinetobacter baumannii. Phytomedicine 2017, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.I.N.; Zhang, J.; Xu, D.U.O.; Li, Y.; Liu, Y.; Zhang, X.I.N.; Zhang, R.; Wu, Z.; Weng, P. Antimicrobial Effect of Tea Polyphenols against Foodborne Pathogens: A Review. J. Food Prot. 2021, 84, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.S.; Meena, K.S.; Ramesh, C. 13 Inhibition of virulence potential of Vibrio cholerae by herbal compounds. In Post-Genomic Approaches in Drug and Vaccine Development; River Publishers: Gistrup, Denmark, 2015; pp. 347–374. [Google Scholar]
- Namiki, K.; Wongsirisin, P.; Yokoyama, S.; Sato, M.; Rawangkan, A.; Sakai, R.; Iida, K.; Suganuma, M. (−)-Epigallocatechin gallate inhibits stemness and tumourigenicity stimulated by AXL receptor tyrosine kinase in human lung cancer cells. Sci. Rep. 2020, 10, 2444. [Google Scholar] [CrossRef]
- M100-S25; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2015.
- Qaiyumi, S. Macro-and microdilution methods of antimicrobial susceptibility testing. In Antimicrobial Susceptibility Testing Protocols; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 75–79. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Ahmadi, M.H. Global status of tetracycline resistance among clinical isolates of Vibrio cholerae: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2021, 10, 115. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically Approved Drugs Inhibit the Staphylococcus aureus Multidrug NorA Efflux Pump and Reduce Biofilm Formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Foerster, S.; Unemo, M.; Hathaway, L.J.; Low, N.; Althaus, C.L. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef]
- Soudeiha, M.A.H.; Dahdouh, E.A.; Azar, E.; Sarkis, D.K.; Daoud, Z. In vitro Evaluation of the Colistin-Carbapenem Combination in Clinical Isolates of A. baumannii Using the Checkerboard, Etest, and Time-Kill Curve Techniques. Front. Cell. Infect. Microbiol. 2017, 7, 209. [Google Scholar] [CrossRef]
- Sateriale, D.; Facchiano, S.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Paolucci, M.; Volpe, M.G.; Salvatore, P.; Pagliarulo, C. In vitro Synergy of Polyphenolic Extracts from Honey, Myrtle and Pomegranate against Oral Pathogens, S. mutans and R. dentocariosa. Front. Microbiol. 2020, 11, 1465. [Google Scholar] [CrossRef]
- Petersen, P.J.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2007, 59, 347–349. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, J.; Gao, H.; Wang, Z.; Dong, N.; Ma, Q.; Shan, A. Antimicrobial Properties and Membrane-Active Mechanism of a Potential α-Helical Antimicrobial Derived from Cathelicidin PMAP-36. PLoS ONE 2014, 9, e86364. [Google Scholar] [CrossRef]
- Wang, J.; Chou, S.; Xu, L.; Zhu, X.; Dong, N.; Shan, A.; Chen, Z. High specific selectivity and Membrane-Active Mechanism of the synthetic centrosymmetric α-helical peptides with Gly-Gly pairs. Sci. Rep. 2015, 5, 15963. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Staiano-Coico, L.; Melamed, M.R. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc. Natl. Acad. Sci. USA 1981, 78, 2383. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T. Staining of living bacteria with rhodamine 123. FEMS Microbiol. Lett. 1984, 21, 153–157. [Google Scholar] [CrossRef][Green Version]
- Leal, A.M.; de Queiroz, J.D.; de Medeiros, S.R.; Lima, T.K.; Agnez-Lima, L.F. Violacein induces cell death by triggering mitochondrial membrane hyperpolarization in vitro. BMC Microbiol. 2015, 15, 115. [Google Scholar] [CrossRef] [PubMed]
| No. | Strain | Serogroup/Serotype/Serovar | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|
| 1 | N16961 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 2 | P33 | V. cholerae O1 El Tor Ogawa | 62.5 | 125 |
| 3 | P34 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 4 | P35 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 5 | P36 | V. cholerae O1 El Tor Ogawa | 62.5 | 125 |
| 6 | P38 | V. cholerae O1 El Tor Inaba | 250 | 500 |
| 7 | P39 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 8 | P41 | V. cholerae O1 El Tor Ogawa | 62.5 | 125 |
| 9 | P42 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 10 | P43 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 11 | P44 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 12 | P45 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 13 | P46 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 14 | P47 | V. cholerae O1 El Tor Ogawa | 62.5 | 125 |
| 15 | P48 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 16 | 22115 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 17 | 22116 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 18 | 22118 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 19 | 22125 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 20 | 22126 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 21 | 22127 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 22 | 22135 | V. cholerae O139 | 62.5 | 125 |
| 23 | 22136 | V. cholerae O139 | 125 | 250 |
| 24 | 22137 | V. cholerae O139 | 125 | 250 |
| 25 | 22138 | V. cholerae O139 | 125 | 250 |
| 26 | 22144 | V. cholerae non-O1, non-O139 | 125 | 250 |
| 27 | 4053022001 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 28 | 4053023816 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 29 | 4053023817 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 30 | 4053023818 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 31 | 4053023822 | V. cholerae O1 El Tor Ogawa | 62.5 | 125 |
| 32 | 4053023823 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 33 | 4053023826 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 34 | 4053023828 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 35 | 4053023829 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 36 | 4053023830 | V. cholerae O1 El Tor Ogawa | 125 | 250 |
| 37 | 4053024283 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 38 | 4053024290 | V. cholerae O1 El Tor, Inaba | 125 | 250 |
| 39 | 4053024292 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 40 | 4053024293 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 41 | 4053024294 | V. cholerae O1 El Tor Inaba | 62.5 | 125 |
| 42 | 4053024295 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 43 | 4053024296 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 44 | 4053024297 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| 45 | 4053024299 | V. cholerae O1 El Tor Inaba | 125 | 250 |
| Strain | MIC (µg/mL) of Extracts (a) | FIC (a) | MIC (µg/mL) of Tetracycline (b) | FIC (b) | FICI | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Alone | Combination | Alone | Combination | |||||
| P48 (O1) | 125.0 | 0.97 | 0.008 | 62.5 | 0.061 | 0.001 | 0.009 | Synergistic |
| 22136 (O139) | 125.0 | 0.97 | 0.008 | 0.78 | 0.008 | 0.01 | 0.018 | Synergistic |
| N16961 (Reference) | 125.0 | 0.97 | 0.008 | 3.91 | 0.004 | 0.001 | 0.009 | Synergistic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siriphap, A.; Kiddee, A.; Duangjai, A.; Yosboonruang, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Rawangkan, A. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics 2022, 11, 518. https://doi.org/10.3390/antibiotics11040518
Siriphap A, Kiddee A, Duangjai A, Yosboonruang A, Pook-In G, Saokaew S, Sutheinkul O, Rawangkan A. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics. 2022; 11(4):518. https://doi.org/10.3390/antibiotics11040518
Chicago/Turabian StyleSiriphap, Achiraya, Anong Kiddee, Acharaporn Duangjai, Atchariya Yosboonruang, Grissana Pook-In, Surasak Saokaew, Orasa Sutheinkul, and Anchalee Rawangkan. 2022. "Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae" Antibiotics 11, no. 4: 518. https://doi.org/10.3390/antibiotics11040518
APA StyleSiriphap, A., Kiddee, A., Duangjai, A., Yosboonruang, A., Pook-In, G., Saokaew, S., Sutheinkul, O., & Rawangkan, A. (2022). Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics, 11(4), 518. https://doi.org/10.3390/antibiotics11040518







