Synergistic Antibacterial and Anti-inflammatory Activities of Ocimum tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens
Abstract
:1. Introduction
2. Results
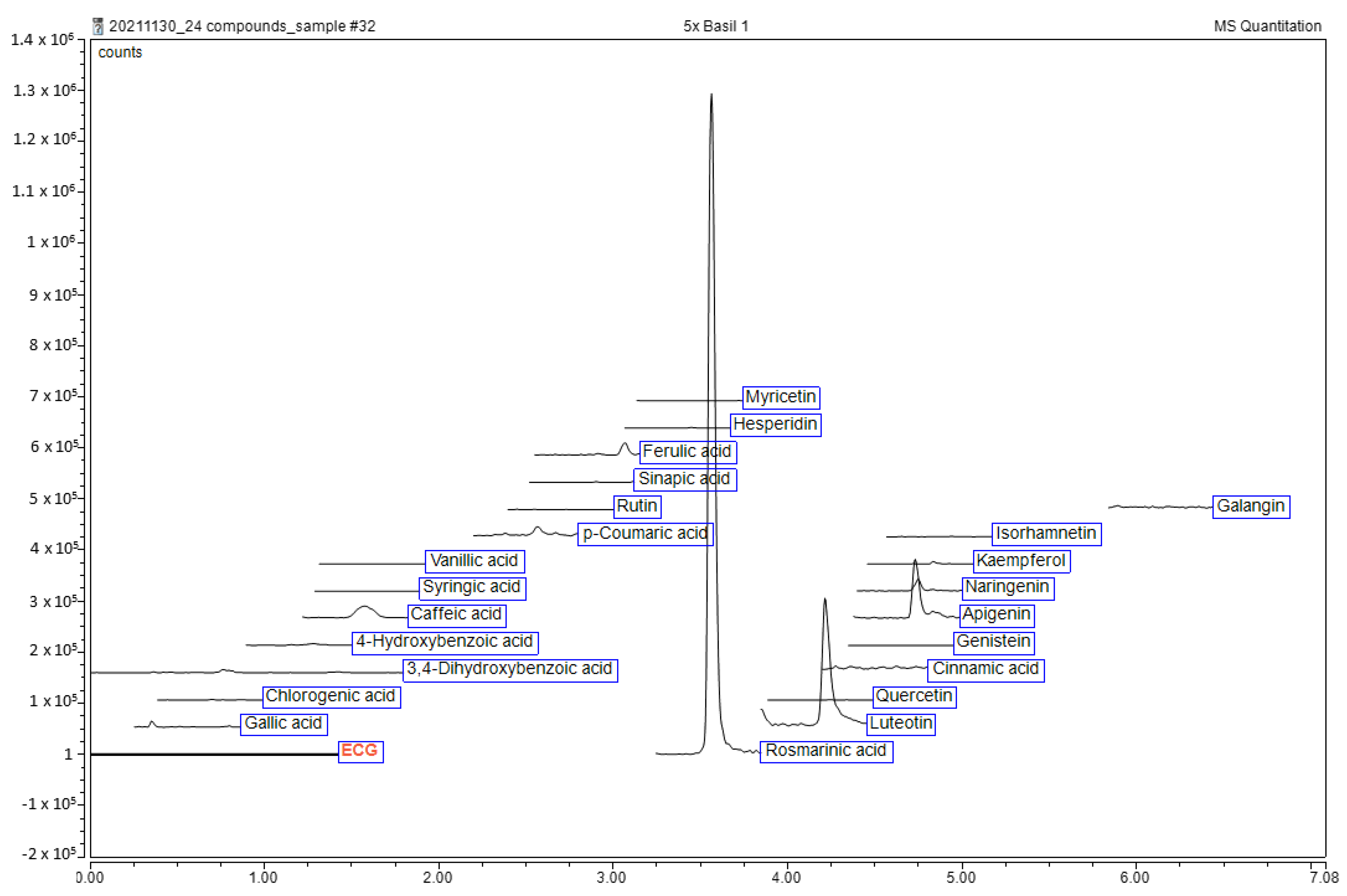
2.1. Qualitative Phytochemical Analysis of Ethanolic Extract of O. tenuiflorum
2.2. MIC and MBC of the Antibacterial Drugs and O. tenuiflorum Extract
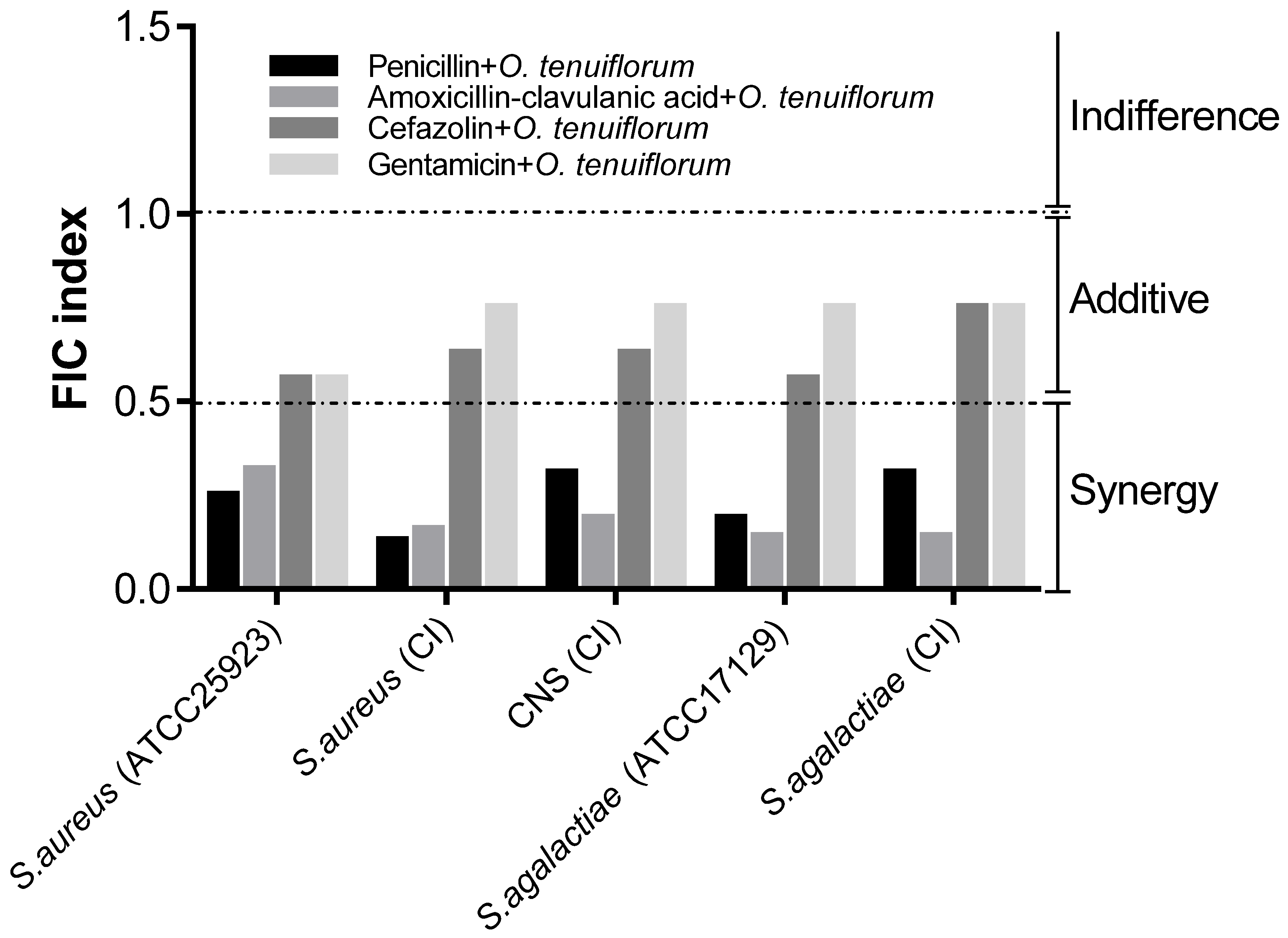
2.3. Evaluation of the Synergistic Effect of Antibacterial Drugs and O. tenuiflorum Extract
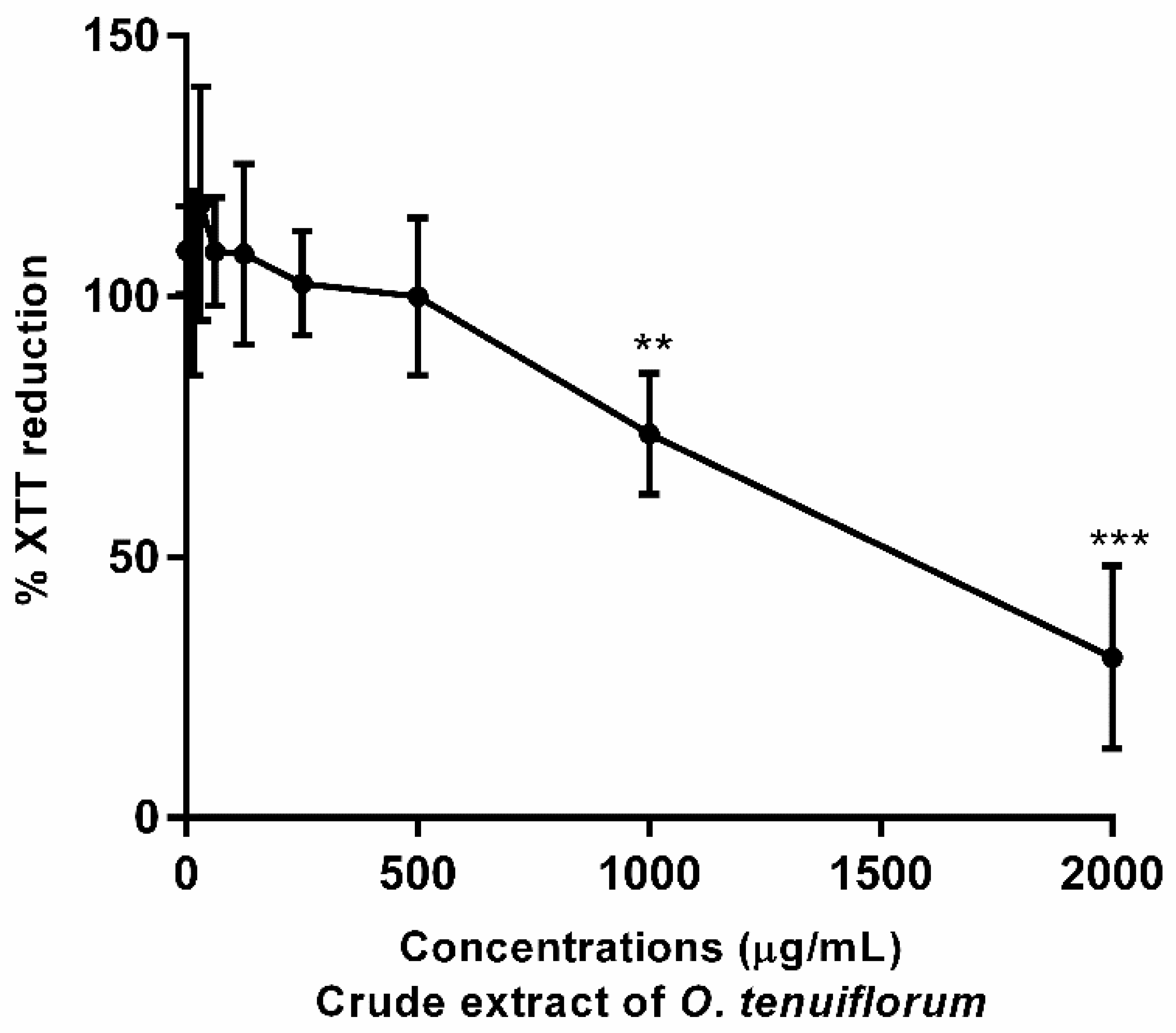
2.4. Cytotoxicity Assay with RAW 264.7 Murine Macrophage Cells
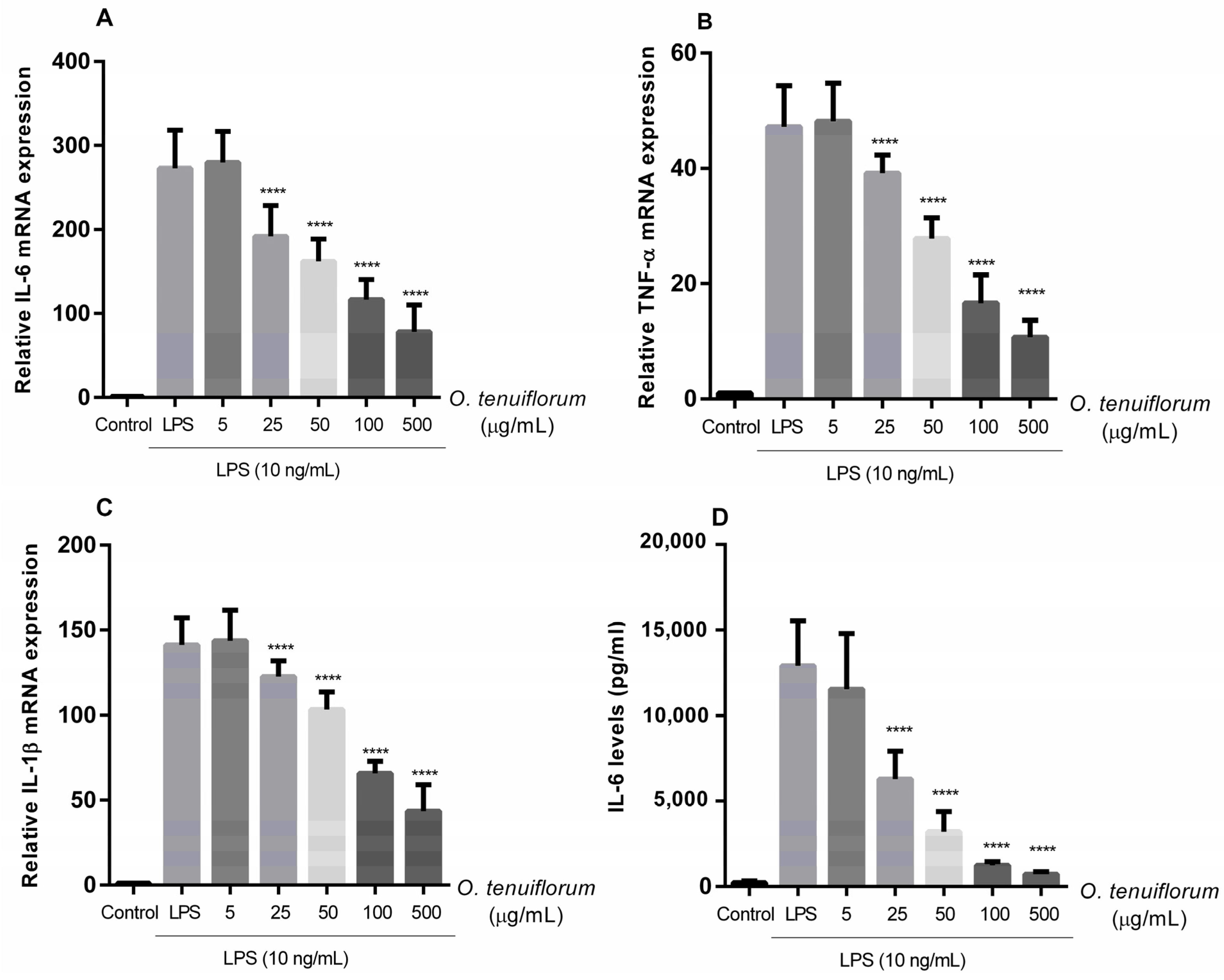
2.5. O. tenuiflorum Extract Suppressed LPS-Induced Expression of Pro-Inflammatory Cytokines in RAW 264.7
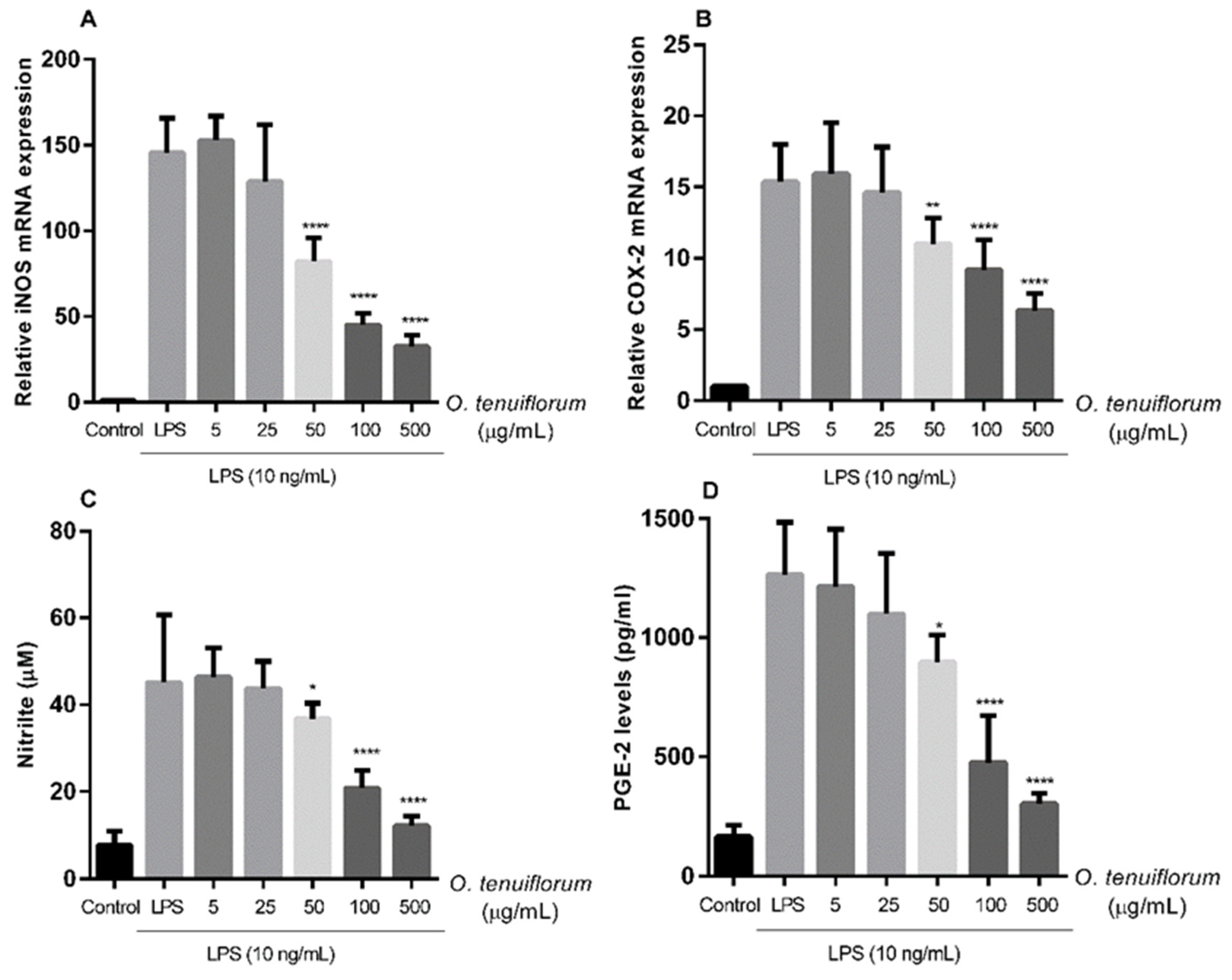
2.6. O. tenuiflorum Extract Suppressed LPS-Induced iNOS and COX-2 Expression and NO and PGE2 Production in RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Cell Cultures, Chemicals, and Reagents
4.2. Plant Materials and Preparation of the Extracts
4.3. Phytochemical Analyses
4.4. Bacterial Culture and Identification
4.5. Antibacterial Drugs and Extract of O. tenuiflorum
4.6. In Vitro Determination of Antibacterial Susceptibility
4.7. Evaluation of Synergistic Effect
4.8. Cell Culture
4.9. Cytotoxicity Assay with RAW 264.7 Cells
4.10. Anti-Inflammatory Activity Determination
4.11. Measurement of Nitric Oxide (NO) Concentration
4.12. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis for Proinflammatory Gene Expression
4.13. Determination of IL-6 and PGE2 Productions by Enzyme-Linked Immunosorbent Assay
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Suresh, K.P.; Jayamma, K.S.; Shome, B.R.; Patil, S.S.; Amachawadi, R.G. An Understanding of the Global Status of Major Bacterial Pathogens of Milk Concerning Bovine Mastitis: A Systematic Review and Meta-Analysis (Scientometrics). Pathogens 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Horpiencharoen, W.; Thongratsakul, S.; Poolkhet, C. Risk factors of clinical mastitis and antimicrobial susceptibility test results of mastitis milk from dairy cattle in western Thailand: Bayesian network analysis. Prev. Vet. Med. 2019, 164, 49–55. [Google Scholar] [CrossRef]
- Hinthong, W.; Pumipuntu, N.; Santajit, S.; Kulpeanprasit, S.; Buranasinsup, S.; Sookrung, N.; Chaicumpa, W.; Aiumurai, P.; Indrawattana, N. Detection and drug resistance profile of Escherichia coli from subclinical mastitis cows and water supply in dairy farms in Saraburi Province, Thailand. PeerJ 2017, 5, e3431. [Google Scholar] [CrossRef] [Green Version]
- Suriyasathaporn, W. Epidemiology of subclinical mastitis and their antibacterial susceptibility in smallholder dairy farms, Chiang Mai province, Thailand. J. Anim. Vet. Adv. 2011, 10, 316–321. [Google Scholar]
- Pumipuntu, N.; Tunyong, W.; Chantratita, N.; Diraphat, P.; Pumirat, P.; Sookrung, N.; Chaicumpa, W.; Indrawattana, N. Staphylococcus spp. associated with subclinical bovine mastitis in central and northeast provinces of Thailand. PeerJ 2019, 7, e6587. [Google Scholar] [CrossRef] [Green Version]
- Leelahapongsathon, K.; Schukken, Y.H.; Suriyasathaporn, W. Quarter, cow, and farm risk factors for intramammary infections with major pathogens relative to minor pathogens in Thai dairy cows. Trop Anim. Health Prod. 2014, 46, 1067–1078. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Bovine mastitis prevention and control in the post-antibiotic era. Trop Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.A.; Afolayan, A.J.; Okoh, A.I. Synergistic interaction of Helichrysum pedunculatum leaf extracts with antibiotics against wound infection associated bacteria. Biol. Res. 2009, 42, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adwan, G.; Abu-Shanab, B.; Adwan, K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug–resistant Pseudomonas aeruginosa strains. Asian Pac. J. Trop. Med. 2010, 3, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patino, A.; Baizabal-Aguirre, V.M. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef]
- Cai, M.; Shi, Y.; Zheng, T.; Hu, S.; Du, K.; Ren, A.; Jia, X.; Chen, S.; Wang, J.; Lai, S. Mammary epithelial cell derived exosomal MiR-221 mediates M1 macrophage polarization via SOCS1/STATs to promote inflammatory response. Int. Immunopharmacol. 2020, 83, 106493. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Souza, M.T.; Blagitz, M.G.; Souza, F.N.; Batista, C.F.; Alves, A.J.; Fernandes, A.C.C.; Sanchez, E.M.R.; Ordinola-Ramirez, C.M.; da Costa, L.; et al. Milk lymphocyte profile and macrophage functions: New insights into the immunity of the mammary gland in quarters infected with Corynebacterium bovis. BMC Vet. Res. 2021, 17, 282. [Google Scholar] [CrossRef]
- Riollet, C.; Rainard, P.; Poutrel, B. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 2000, 7, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Atakisi, O.; Oral, H.; Atakisi, E.; Merhan, O.; Metin Pancarci, S.; Ozcan, A.; Marasli, S.; Polat, B.; Colak, A.; Kaya, S. Subclinical mastitis causes alterations in nitric oxide, total oxidant and antioxidant capacity in cow milk. Res. Vet. Sci. 2010, 89, 10–13. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Yu, D.; Changyong, E.; Yang, J. Morin Protects LPS-Induced Mastitis via Inhibiting NLRP3 Inflammasome and NF-κB Signaling Pathways. Inflammation 2020, 43, 1293–1303. [Google Scholar] [CrossRef]
- Taga, I.; Lan, C.Q.; Altosaar, I. Plant essential oils and mastitis disease: Their potential inhibitory effects on pro-inflammatory cytokine production in response to bacteria related inflammation. Nat. Prod. Commun. 2012, 7, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, U.K.; Mukherjee, R. Activity of cyclooxygenase-2 and nitric oxide in milk leucocytes following intramammary inoculation of a bio-response modifier during bovine Staphylococcus aureus subclinical mastitis. Vet. Res. Commun. 2014, 38, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, E.; Wei, Z.; Liang, D.; Wang, W.; Wang, T.; Guo, M.; Zhang, N.; Yang, Z. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Febs. J. 2014, 281, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Gulbe, G.; Pilmane, M.; Saulīte, V.; Doniņa, S.; Jermolajevs, J.; Peškova, L.; Valdovska, A. Cells and Cytokines in Milk of Subclinically Infected Bovine Mammary Glands after the Use of Immunomodulatory Composition GLP 810. Mediat. Inflamm. 2020, 2020, 8238029. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.D.; Shah, T.M.; Nauriyal, D.S.; Kunjadia, A.P.; Joshi, C.G. Evaluation of a topical herbal drug for its in-vivo immunomodulatory effect on cytokines production and antibacterial activity in bovine subclinical mastitis. Ayu 2014, 35, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, R.; Dash, P.K.; Ram, G.C. Immunotherapeutic potential of Ocimum sanctum (L.) in bovine subclinical mastitis. Res. Vet. Sci. 2005, 79, 37–43. [Google Scholar] [CrossRef]
- Shafi, T.; Bansal, B.; Gupta, D. In vitro antibacterial activity and minimum inhibitory concentration of ocimum sanctum leaves against common bovine mastitis pathogens. J. Dairy Vet. Anim. Res. 2018, 7, 322–324. [Google Scholar]
- Suresh, A.; Rao, T.C.; Solanki, S.; Suresh, M.V.; Menon, B.; Raghavendran, K. The holy basil administration diminishes the NF-kB expression and protects alveolar epithelial cells from pneumonia infection through interferon gamma. Phytother. Res. 2022. [Google Scholar] [CrossRef]
- Chaiyana, W.; Punyoyai, C.; Sriyab, S.; Prommaban, A.; Sirilun, S.; Maitip, J.; Chantawannakul, P.; Neimkhum, W.; Anuchapreeda, S. Anti-Inflammatory and Antimicrobial Activities of Fermented Ocimum sanctum Linn. Extracts against Skin and Scalp Microorganisms. Chem. Biodivers. 2022, 19, e202100799. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. 2004, 18, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Taraktsoglou, M.; Szalabska, U.; Magee, D.A.; Browne, J.A.; Sweeney, T.; Gormley, E.; MacHugh, D.E. Transcriptional profiling of immune genes in bovine monocyte-derived macrophages exposed to bacterial antigens. Vet. Immunol. Immunopathol. 2011, 140, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Liu, Z.; Jiang, A.; Li, S.; Wu, D.; Zhang, Y.; Zhu, X.; Zhou, E.; Wei, Z.; et al. Sodium Butyrate Alleviates Lipopolysaccharide-Induced Inflammatory Responses by Down-Regulation of NF-κB, NLRP3 Signaling Pathway, and Activating Histone Acetylation in Bovine Macrophages. Front. Vet. Sci. 2020, 7, 579674. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, W.; Liu, B.; Li, T.; Wu, J.; Fu, C.; Shen, Y.; Pei, L.; Cao, J. Live S. aureus and heat-killed S. aureus induce different inflammation-associated factors in bovine endometrial tissue in vitro. Mol. Immunol. 2021, 139, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural Agents against Bovine Mastitis Pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Gomes, F.; Rodrigues, M.E.; Martins, N.; Ferreira, I.; Henriques, M. Phenolic Plant Extracts Versus Penicillin G: In Vitro Susceptibility of Staphylococcus aureus Isolated from Bovine Mastitis. Pharmaceuticals 2019, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Ranjani, S.; Priya, P.S.; Veerasami, M.; Hemalatha, S. Novel Polyherbal Nanocolloids to Control Bovine Mastitis. Appl. Biochem. Biotechnol. 2022, 194, 246–265. [Google Scholar] [CrossRef]
- Dyson, R.; Charman, N.; Hodge, A.; Rowe, S.M.; Taylor, L.F. A survey of mastitis pathogens including antimicrobial susceptibility in southeastern Australian dairy herds. J. Dairy Sci. 2022, 105, 1504–1518. [Google Scholar] [CrossRef]
- Lan, T.; Liu, H.; Meng, L.; Xing, M.; Dong, L.; Gu, M.; Wang, J.; Zheng, N. Antimicrobial susceptibility, phylotypes, and virulence genes of Escherichia coli from clinical bovine mastitis in five provinces of China. Food Agric. Immunol. 2020, 31, 406–423. [Google Scholar] [CrossRef] [Green Version]
- Bolte, J.; Zhang, Y.; Wente, N.; Krömker, V. In Vitro Susceptibility of Mastitis Pathogens Isolated from Clinical Mastitis Cases on Northern German Dairy Farms. Vet. Sci. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 7th ed.; Approve Standard M7-A7, CLSI; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; CLSI Supplement VET01; CLSI: Wayne, PA, USA, 2015.
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 141, 1–4. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. Anti-inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Induced Mastitis in Mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Lu, W.; Li, X.; Wu, Z.; Wang, G.; Dong, W.; Tan, C.; Liu, M. Apigenin and Ampicillin as Combined Strategy to Treat Severe Streptococcus suis Infection. Molecules 2021, 26, 1980. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Xu, N.N.; Sun, W.; Zhao, Y.; Li, C.Y.; Guo, M.Y. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget 2017, 8, 28481–28493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gao, X.; Song, X.; Zhou, W.; Hong, W.; Tian, C.; Liu, Y.; Liu, M. Luteolin Showed a Resistance Elimination Effect on Gentamicin by Decreasing MATE mRNA Expression in Trueperella pyogenes. Microb. Drug. Resist. 2019, 25, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of Drying Processes on Phenolics and In Vitro Health-Related Activities of Indigenous Plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; Hartigan, P.; Fanning, S.; Fitzpatrick, E. Veterinary Microbiology and Microbial Disease; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing; CLSI document M100-S17 2005; CLSI: Wayne, PA, USA, 2007.
- Mohammadzadeh, A.; Farnia, P.; Ghazvini, K.; Behdani, M.; Rashed, T.; Ghanaat, J. Rapid and low-cost colorimetric method using 2,3,5-triphenyltetrazolium chloride for detection of multidrug-resistant Mycobacterium tuberculosis. J. Med. Microbiol. 2006, 55, 1657–1659. [Google Scholar] [CrossRef] [Green Version]
- Gülmez, D.; Çakar, A.; Şener, B.; Hasçelik, G.; Karakaya, J.; Gülmez, D. Comparison of different antimicrobial susceptibility testing methods for Stenotrophomonas maltophilia and results of synergy testing. J. Infect. Chemother. 2010, 16, 322–328. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies--methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phochantachinda, S.; Chatchaisak, D.; Temviriyanukul, P.; Chansawang, A.; Pitchakarn, P.; Chantong, B. Ethanolic Fruit Extract of Emblica officinalis Suppresses Neuroinflammation in Microglia and Promotes Neurite Outgrowth in Neuro2a Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 6405987. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Gołąbek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chantong, B.; Kratschmar, D.V.; Nashev, L.G.; Balazs, Z.; Odermatt, A. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J. Neuroinflamm. 2012, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantong, B.; Kratschmar, D.V.; Lister, A.; Odermatt, A. Dibutyltin promotes oxidative stress and increases inflammatory mediators in BV-2 microglia cells. Toxicol. Lett. 2014, 230, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kai, S.; Matsuyama, T.; Adachi, T.; Fukuda, K.; Hirota, K. General anesthetics inhibit LPS-induced IL-1 β expression in glial cells. PLoS ONE 2013, 8, e82930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.-W.; Chou, C.-H.; Wu, X.-M.; Chen, Z.-W.; Chen, Y.-H.; Chang, Y.-Y.; Wu, V.-C.; Rose-John, S.; Hung, C.-S.; Lin, Y.-H. Interleukin-6 plays a critical role in aldosterone-induced macrophage recruitment and infiltration in the myocardium. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165627. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strains * | Penicillin | Amoxicillin-Clavulanic Acid | Cefazolin | Gentamicin | O. tenuiflorum Leaves Extract | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| S. aureus (ATCC25923) | 0.25 | 1 | 0.0625 | 0.125 | 4 | 16 | 4 | 32 | 15.6 | 62.5 |
| S. aureus (CI) | 16 | 128 | 4 | 16 | 64 | 256 | 16 | 128 | 31.2 | 125 |
| CNS (CI) | 0.125 | 1 | 8 | 64 | 1 | 8 | 16 | 128 | 3.9 | 15.6 |
| S. agalactiae (ATCC17129) | 0.5 | 16 | 0.0625 | 8 | 4 | 128 | 32 | 128 | 7.8 | 125 |
| S. agalactiae (CI) | 2 | 32 | 1 | 32 | 32 | 256 | 64 | 256 | 31.2 | 500 |
| E. coli (ATCC25922) | - | - | 16 | 32 | 4 | 128 | 2 | 16 | >1000 | - |
| E. coli (CI) | - | - | 32 | 128 | 16 | 256 | 2 | 16 | >1000 | - |
| Genes | Primers | Sequences | Reference |
|---|---|---|---|
| GAPDH (mouse) | forward | CTCGTGGAGTCTACTGGTGT | [65] |
| reverse | GTCATCATACTTGGCAGGTT | ||
| iNOS (mouse) | forward | ATGAGGTACTCAGCGTGCTCCAC | [66] |
| reverse | CCACAATAGTACAATACTACTTGG | ||
| IL-1β (mouse) | forward | CGACAAAATACCTGTGGCCT | [67] |
| reverse | TTCTTTGGGTATTGCTTGGG | ||
| IL-6 (mouse) | forward | GGAGGCTTAATTACACATGTT | [65] |
| reverse | TGATTTCAAGATGAATTGGAT | ||
| TNF-α (mouse) | forward | TTCTGTCTACTGAACTTCGG | [65] |
| reverse | GTATGAGATAGCAAATCGGC | ||
| COX-2 (mouse) | forward | AAGAGCATCGCAGAGGT | [68] |
| reverse | CCCATTAGCAGCCAGTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichok, J.; Yingbun, N.; Kowawisetsut, T.; Kornmatitsuk, S.; Suttisansanee, U.; Temviriyanukul, P.; Chantong, B. Synergistic Antibacterial and Anti-inflammatory Activities of Ocimum tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens. Antibiotics 2022, 11, 510. https://doi.org/10.3390/antibiotics11040510
Srichok J, Yingbun N, Kowawisetsut T, Kornmatitsuk S, Suttisansanee U, Temviriyanukul P, Chantong B. Synergistic Antibacterial and Anti-inflammatory Activities of Ocimum tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens. Antibiotics. 2022; 11(4):510. https://doi.org/10.3390/antibiotics11040510
Chicago/Turabian StyleSrichok, Janejira, Natthika Yingbun, Teerada Kowawisetsut, Sudsaijai Kornmatitsuk, Uthaiwan Suttisansanee, Piya Temviriyanukul, and Boonrat Chantong. 2022. "Synergistic Antibacterial and Anti-inflammatory Activities of Ocimum tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens" Antibiotics 11, no. 4: 510. https://doi.org/10.3390/antibiotics11040510
APA StyleSrichok, J., Yingbun, N., Kowawisetsut, T., Kornmatitsuk, S., Suttisansanee, U., Temviriyanukul, P., & Chantong, B. (2022). Synergistic Antibacterial and Anti-inflammatory Activities of Ocimum tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens. Antibiotics, 11(4), 510. https://doi.org/10.3390/antibiotics11040510






