Surgical Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery: A RAND/UCLA Appropriateness Method Consensus Study
Abstract
1. Introduction
2. Methods
2.1. RAND/UCLA Appropriateness Method
2.2. Recruitment of Panelists
2.3. Generation of Scenarios
2.4. Two-Round Consensus Process
3. Results
3.1. SCENARIO #1—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Clean Plastic Surgery in Elective Procedures with Exclusive Skin and Subcutis Involvement
3.2. SCENARIO #2—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Clean-Contaminated/Contaminated Plastic Surgery in Elective Procedures with Exclusive Skin and Subcutis Involvement
3.3. SCENARIO #3—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Elective Plastic Surgery with the Use of Local Flaps
3.4. SCENARIO #4—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Elective Plastic Surgery with the Use of Grafts
3.5. SCENARIO #5—Antimicrobial Prophylaxis in Pediatric Patients Undergoing any Type of Prolonged Elective Plastic Surgery
3.6. SCENARIO #6—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery for Acute Burns
3.7. SCENARIO #7—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery following Clean Contused Lacerated Wounds without Bone Exposure
3.8. SCENARIO #8—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery following High-Risk Contused Lacerated Wounds or with Bone Exposure
3.9. SCENARIO #9—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery following Contused Lacerated Wound Involving the Oral Mucosa
3.10. SCENARIO #10—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery following Human Bite
3.11. SCENARIO #11—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery following Animal Bite
3.12. SCENARIO #12—Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery with Tissue Expander Insertion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krizek, T.J.; Koss, N.; Robson, M.C. The current use of prophylactic antibiotics in plastic and reconstructive surgery. Plast. Reconstr. Surg. 1975, 55, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Krizek, T.J.; Gottlieb, L.J.; Koss, N.; Robson, M.C. The use of prophylactic antibacterials in plastic surgery: A 1980s update. Plast. Reconstr. Surg. 1985, 76, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Lyle, W.G.; Outlaw, K.; Krizek, T.J.; Koss, N.; Payne, W.G.; Robson, M.C. Prophylactic antibiotics in plastic surgery: Trends of use over 25 years of an evolving specialty. Aesthet. Surg. J. 2003, 23, 177–183. [Google Scholar] [CrossRef]
- Porco, T.C.; Gao, D.; Scott, J.C.; Shim, E.; Enanoria, W.T.; Galvani, A.P.; Lietman, T.M. When does overuse of antibiotics become a tragedy of the commons? PLoS ONE 2012, 7, e46505. [Google Scholar] [CrossRef]
- Davey, P.; Brown, E.; Charani, E.; Fenelon, L.; Gould, I.M.; Holmes, A.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic proscribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013, 4, CD00354. [Google Scholar]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D. The RAND/UCLA Adeguateness Method User’s Manual; The RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- Hicks, N.R. Some observations on attempts to measure appropriateness of care. BMJ 1994, 309, 730. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Ariyan, S.; Martin, J.; Lal, A.; Cheng, D.; Borah, G.L.; Chung, K.C.; Conly, J.; Havlik, R.; Lee, W.A.; McGrath, M.H.; et al. Antibiotic prophylaxis for preventing surgical-site infection in plastic surgery: An evidence-based consensus conference statement from the American Association of Plastic Surgeons. Plast. Reconstr. Surg. 2015, 135, 1723–1739. [Google Scholar] [CrossRef]
- Wright, T.I.; Baddour, L.M.; Berbari, E.F.; Roenigk, R.K.; Phillips, P.K.; Jacobs, M.A.; Otley, C.C. Antibiotic prophylaxis in dermatologic surgery: Advisory statement 2008. J. Am. Acad. Dermatol. 2008, 59, 464–473. [Google Scholar] [CrossRef]
- Lowe, G.; Twaddle, S. The Scottish Intercollegiate Guidelines Network (SIGN): An update. Scott. Med. J. 2005, 50, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.R.; Chwals, W.J.; Doski, J.J.; Suescun, E.A.; Cheu, H.W.; Lally, K.P. Pediatric wound infections: A prospective multicenter study. Ann. Surg. 1998, 227, 553–558. [Google Scholar] [CrossRef]
- Casanova, J.F.; Herruzo, R.; Diez, J. Risk factors for surgical site infection in children. Infect. Control. Hosp. Epidemiol. 2006, 27, 709–715. [Google Scholar] [CrossRef]
- Raval, M.V.; Dillon, P.W.; Bruny, J.L.; Ko, C.Y.; Hall, B.L.; Moss, R.L.; Oldham, K.T.; Richards, K.E.; Vinocur, C.D.; Ziegler, M.M.; et al. American college of surgeons national surgical quality improvement program pediatric: A phase 1 report. J. Am. Coll. Surg. 2011, 212, 1–11. [Google Scholar] [CrossRef]
- National Health System Greater Glasgow and Clyde. Antibiotic Prophylaxis for Pediatric Surgery. Available online: https://www.clinicalguidelines.scot.nhs.uk/nhsggc-guidelines/nhsggc-guidelines/haematologyoncology/antibiotic-prophylaxis-for-paediatric-surgery/ (accessed on 3 January 2022).
- Aydin, N.; Uraloğlu, M.; Burhanoğlu, A.D.Y.; Sensöz, Ö. A prospective trial on the use of antibiotics in hand surgery. Plast. Reconstr. Surg. 2010, 126, 1617–1623. [Google Scholar] [CrossRef]
- Roberts, A.H.; Teddy, P.J. A prospective trial of prophylactic antibiotics in hand lacerations. Br. J. Surg. 1977, 64, 394–396. [Google Scholar] [CrossRef]
- Sloan, J.P.; Dove, A.F.; Maheson, M.; Cope, A.N.; Welsh, K.R. Antibiotics in open fractures of the distal phalanx? J. Hand Surg. Br. 1987, 12, 123–124. [Google Scholar] [CrossRef]
- Stevenson, J.; McNaughton, G.; Riley, J. The use of Prophylactic Flucloxacillin in Treatment of Open Fractures of the Distal Phalanx within an Accident and Emergency Department: A Double-Blind Randomized Placebo-Controlled Trial. J. Hand Surg. 2003, 28, 388–394. [Google Scholar] [CrossRef]
- Beesley, J.R.; Bowden, G.; Hardy, R.H.; Reynolds, T.D. Prophylactic antibiotics in minor hand injuries. Injury 1975, 6, 366. [Google Scholar] [CrossRef]
- Cassell, O.C.; Ion, L. Are antibiotics necessary in the surgical management of upper limb lacerations? Br. J. Plast. Surg. 1997, 50, 523–529. [Google Scholar] [CrossRef]
- Grossman, J.A.; Adams, J.P.; Kunec, J. Prophylactic antibiotics in simple hand lacerations. JAMA 1981, 245, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Haughey, R.E.; Lammers, R.L.; Wagner, D.K. Use of antibiotics in the initial management of soft tissue hand wounds. Ann. Emerg. Med. 1981, 10, 187–192. [Google Scholar] [CrossRef]
- Stone, J.F.; Davidson, J.S. The role of antibiotics and timing of repair in flexor tendon injuries of the hand. Ann. Plast. Surg. 1998, 40, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Suprock, M.D.; Hood, J.M.; Lubahn, J.D. Role of antibiotics in open fractures of the finger. J. Hand Surg. Am. 1990, 15, 761–764. [Google Scholar] [CrossRef]
- Thirlby, R.C.; Blair, A.J., III; Thal, E.R. The value of prophylactic antibiotics for simple lacerations. Surg. Gynecol. Obstet. 1983, 156, 212–216. [Google Scholar]
- Johnson, J.T.; Yu, V.L.; Myers, E.N.; Muder, R.R.; Thearle, P.B.; Diven, W.F. Efficacy of two third-generation cephalosporins in prophylaxis for head and neck surgery. Arch. Otolaryngol. 1984, 110, 224–227. [Google Scholar] [CrossRef]
- Ketcham, A.S.; Bloch, J.H.; Crawford, D.T.; Lieberman, J.E.; Smith, R.R. The role of prophylactic antibiotic therapy in control of staphylococcal infections following cancer surgery. Surg. Gynecol. Obstet. 1962, 114, 345–352. [Google Scholar] [CrossRef]
- Saginur, R.; Odell, P.F.; Poliquin, J.F. Antibiotic prophylaxis in head and neck cancer surgery. J. Otolaryngol. 1988, 17, 78–80. [Google Scholar]
- Seagle, M.B.; Duberstein, L.E.; Gross, C.W.; Fletcher, J.L. Efficacy of cefazolin as a prophylactic antibiotic in head and neck surgery. Otolaryngology 1978, 86, 568–571. [Google Scholar] [CrossRef]
- Baran, C.N.; Sensöz, O.; Ulusoy, M.G. Prophylactic antibiotics in plastic and reconstructive surgery. Plast. Reconstr. Surg. 1999, 103, 1561–1566. [Google Scholar] [CrossRef]
- Roodsari, G.S.; Zahedi, F.; Zehtabchi, S. The risk of wound infection after simple hand laceration. World J. Emerg Med. 2015, 6, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Sonesson, A.; Persson, B.; Riesbeck, K.; Schmidtchen, A. A descriptive study of bacterial load of full-thickness surgical wounds in dermatologic surgery. Dermatol. Surg. 2011, 37, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.; Bachini, L.; Bagnoni, G.; Biondi, A.; Cardinali, C.; Eberle, O.; Pomponi, A.; Vitolo, M. Antibiotic prophylaxis in day surgery in dermatology. Minerva Chir. 2005, 60, 293–298. (In Italian) [Google Scholar] [PubMed]
- Rogues, A.M.; Lasheras, A.; Amici, J.M.; Guillot, P.; Beylot, C.; Taïeb, A.; Gachie, J.P. Infection control practices and infectious complications in dermatological surgery. J. Hosp. Infect. 2007, 65, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Dixon, M.P.; Askew, D.A.; Wilkinson, D. Prospective study of wound infections in dermatologic surgery in the absence of prophylactic antibiotics. Dermatol. Surg. 2006, 32, 819–827. [Google Scholar] [PubMed]
- Rosengren, H.; Dixon, A. Antibacterial prophylaxis in dermatologic surgery: An evidence- based review. Am. J. Clin. Dermatol. 2010, 11, 35–44. [Google Scholar] [CrossRef]
- Degli Atti, M.L.C.; Serino, L.; Piga, S.; Tozzi, A.E.; Raponi, M. Incidence of surgical site infections in children: Active surveillance in an Italian academic Children’s hospital. Ann. Ig. 2017, 29, 46–53. [Google Scholar]
- Haley, R.W.; Culver, D.H.; Morgan, W.M.; White, J.W.; Emori, T.G.; Hooton, T.M. Identifying patients at high risk of surgical wound infection. A simple multivariate index of patient susceptibility and wound contamination. Am. J. Epidemiol. 1985, 121, 206–215. [Google Scholar] [CrossRef]
- Garibaldi, R.A.; Cushing, D.; Lerer, T. Risk factors for postoperative infection. Am. J. Med. 1991, 91, 158S–163S. [Google Scholar] [CrossRef]
- Toia, F.; D’Arpa, S.; Massenti, M.F.; Amodio, E.; Pirrello, R.; Moschella, F. Perioperative antibiotic prophylaxis in plastic surgery: A prospective study of 1100 adult patients. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 601–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Qiao, Y.; He, J.; Wang, T.; Ma, S. Efficacy and safety profile of antibiotic prophylaxis usage in clean and clean-contaminated plastic and reconstructive surgery: A meta-analysis of randomized controlled trials. Ann. Plast. Surg. 2014, 72, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Porras-Hernández, J.D.; Vilar-Compte, D.; Cashat-Cruz, M.; Ordorica-Flores, R.M.; Bracho-Blanchet, E.; Avila-Figueroa, C. A prospective study of surgical site infections in a pediatric hospital in Mexico City. Am. J. Infect. Control 2003, 31, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Ergün, O.; Celik, A.; Ergün, G.; Ozok, G. Prophylactic antibiotic use in pediatric burn units. Eur. J. Pediatr. Surg. 2004, 14, 422–426. [Google Scholar] [CrossRef]
- Sheridan, R.L.; Weber, J.M.; Pasternack, M.S.; Tompkins, R.G. Antibiotic prophylaxis for group A streptococcal burn wound infection is not necessary. J. Trauma 2001, 51, 352–355. [Google Scholar] [CrossRef]
- Mulgrew, S.; Khoo, A.; Cartwright, R.; Reynolds, N. Morbidity in pediatric burns, toxic shock syndrome, and antibiotic prophylaxis: A retrospective comparative study. Ann. Plast. Surg. 2014, 72, 34–37. [Google Scholar] [CrossRef]
- Lee, F.; Wong, P.; Hill, F.; Burgner, D.; Taylor, R. Evidence behind the WHO guidelines: Hospital care for children: What is the role of prophylactic antibiotics in the management of burns? J. Trop. Pediatr. 2009, 55, 73–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ISBI Practice Guidelines Committee; Advisory Subcommittee; Steering Subcommittee. ISBI Practice Guidelines for Burn Care, Part 2. Burns 2018, 44, 1617–1706. [Google Scholar] [CrossRef]
- Cummings, P.; Del Beccaro, M.A. Antibiotics to prevent infection of simple wounds: A meta-analysis of randomized studies. Am. J. Emerg. Med. 1995, 13, 396–400. [Google Scholar] [CrossRef]
- Nakamura, Y.; Daya, M. Use of appropriate antimicrobials in wound management. Emerg. Med. Clin. N. Am. 2007, 25, 159–176. [Google Scholar] [CrossRef]
- Wedmore, I. Wound care: Modern evidence in the treatment of man’s age-old injuries. Emerg. Med. Pract. 2005, 7, 1–24. [Google Scholar]
- Sirijatuphat, R.; Siritongtaworn, P.; Sripojtham, V.; Boonyasiri, A.; Thamlikitkul, V. Bacterial contamination of fresh traumatic wounds at Trauma Center, Siriraj Hospital, Bangkok, Thailand. J. Med. Assoc. Thail. 2014, 97 (Suppl. S3), S20–S25. [Google Scholar] [CrossRef]
- Berwald, N.; Khan, F.; Zehtabchi, S. Antibiotic prophylaxis for ED patients with simple hand lacerations: A feasibility randomized controlled trial. Am. J. Emerg. Med. 2014, 32, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Prevaldi, C.; Paolillo, C.; Locatelli, C.; Ricci, G.; Catena, F.; Ansaloni, L.; Cervellin, G. Management of traumatic wounds in the Emergency Department: Position paper from the Academy of Emergency Medicine and Care (AcEMC) and the World Society of Emergency Surgery (WSES). World J. Emerg. Surg. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Sandy-Hodgetts, K.; Andersen, C.A.; Al-Jalodi, O.; Serena, L.; Teimouri, C.; Serena, T.E. Uncovering the high prevalence of bacterial burden in surgical site wounds with point-of-care fluorescence imaging. Int. Wound J. 2021. [Google Scholar] [CrossRef] [PubMed]
- García-Gubern, C.F.; Colon-Rolon, L.; Bond, M.C. Essential concepts of wound management. Emerg. Med. Clin. N. Am. 2010, 28, 951–967. [Google Scholar] [CrossRef]
- Moran, G.J.; Talan, D.A.; Abrahamian, F.M. Antimicrobial prophylaxis for wounds and procedures in the emergency department. Infect. Dis. Clin. N. Am. 2008, 22, 117–143. [Google Scholar] [CrossRef]
- Laurens, M.B. Common indications for pediatric antibiotic prophylaxis. Emerg. Med. Clin. N. Am. 2013, 31, 875–894. [Google Scholar] [CrossRef]
- Cardany, C.R.; Rodeheaver, G.; Thacker, J.; Edgerton, M.T.; Edlich, R.F. The crush injury: A high risk wound. J. Am. Coll. Emerg. Physicians 1976, 5, 965–970. [Google Scholar] [CrossRef]
- Fitzgerald, R.H.; Cowan, J.D. Puncture wounds of the foot. Orthop. Clin. N. Am. 1975, 6, 965–972. [Google Scholar] [CrossRef]
- Abubaker, A.O. Use of prophylactic antibiotics in preventing infection of traumatic injuries. Dent. Clin. N. Am. 2009, 53, 707–715. [Google Scholar] [CrossRef]
- Singer, A.J.; Hollander, J.E.; Quinn, J.V. Evaluation and management of traumatic lacerations. N. Engl. J. Med. 1997, 337, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Opri, F.; Bianchini, S.; Nicoletti, L.; Monaco, S.; Opri, R.; Di Pietro, M.; Carrara, E.; Rigotti, E.; Auriti, C.; Caminiti, C.; et al. Surgical Antimicrobial Prophylaxis in Patients of Neonatal and Pediatric Age Undergoing Orthopedic and Hand Surgery: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.; Brasch, L.; Getson, P. Antibiotic prophylaxis in intraoral wounds. Am. J. Emerg. Med. 1986, 4, 507–510. [Google Scholar] [CrossRef]
- Tintinalli, J.E.; Kelen, G.D.; Stapczynski, J.S. (Eds.) Emergency Medicine: A Comprehensive Study Guide, 9th ed.; McGraw-Hill: New York, NY, USA, 2020. [Google Scholar]
- Mankowitz, S.L. Laceration Management. J. Emerg. Med. 2017, 53, 369–382. [Google Scholar] [CrossRef]
- Mark, D.G.; Granquist, E.J. Are prophylactic oral antibiotics indicated for the treatment of intraoral wounds? Ann. Emerg. Med. 2008, 52, 368–372. [Google Scholar] [CrossRef]
- Medeiros, I.; Saconato, H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst. Rev. 2001, 2, CD001738. [Google Scholar] [CrossRef]
- Taplitz, R.A. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad. Med. 2004, 116, 49–59. [Google Scholar] [CrossRef]
- Bula-Rudas, F.J.; Olcott, J.L. Human and Animal Bites. Pediatr. Rev. 2018, 39, 490–500. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Zangari, A.; Cerigioni, E.; Nino, F.; Guidi, R.; Gulia, C.; Piergentili, R.; Ilari, M.; Mazzoni, N.; Cobellis, G. Dog bite injuries in a tertiary care children’s hospital: A seven-year review. Pediatr. Int. 2021, 63, 575–580. [Google Scholar] [CrossRef]
- Jenkins, G.W.; Isaac, R.; Mustafa, S. Human bite injuries to the head and neck: Current trends and management protocols in England and Wales. Oral Maxillofac. Surg. 2018, 22, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Management of human and animal bite wounds: An overview. Adv. Skin Wound Care 2005, 18, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Beres, A.; Tashjian, D.B.; Moriarty, K.P. Primary repair of facial dog bite injuries in children. Pediatr. Emerg. Care 2011, 27, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Jaindl, M.; Oberleitner, G.; Endler, G.; Thallinger, C.; Kovar, F.M. Management of bite wounds in children and adults-an analysis of over 5000 cases at a level I trauma centre. Wien. Klin. Wochenschr. 2016, 128, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Palmer, J. Dog bites. BMJ 2007, 334, 413–417. [Google Scholar] [CrossRef]
- Esposito, S.; Picciolli, I.; Semino, M.; Principi, N. Dog and cat bite-associated infections in children. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 971–976. [Google Scholar] [CrossRef]
- Ellis, R.; Ellis, C. Dog and cat bites. Am. Fam. Phys. 2014, 90, 239–243. [Google Scholar]
- Alizadeh, K.; Shayesteh, A.; Xu, M.L. An Algorithmic Approach to Operative Management of Complex Pediatric Dog Bites: 3-Year Review of a Level I Regional Referral Pediatric Trauma Hospital. Plastic and reconstructive surgery. Plast. Reconstr. Surg. Glob. Open. 2017, 5, e1431. [Google Scholar] [CrossRef]
- Wang, H.D.; Cho, A.; Quan, A.; Ibrahim, Z.; Yang, R.; Steinberg, J.P.; Redett, R.J. What Is the Impact of Postoperative Antibiotic Prophylaxis on Tissue Expander Infection Rates in Pediatric Patients? Plast. Reconstr. Surg. 2021, 148, 236e–242e. [Google Scholar] [CrossRef]
- Dotan, L.; Icekson, M.; Yanko-Arzi, R.; Ofek, A.; Neuman, R.; Margulis, A. Pediatric tissue expansion: Our experience with 103 expanded flap reconstructive procedures in 41 children. Isr. Med. Assoc. J. 2009, 11, 474–479. [Google Scholar]
- Hurvitz, K.A.; Rosen, H.; Meara, J.G. Pediatric cervicofacial tissue expansion. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Dorafshar, A.H.; Bauer, B.S.; Hoadley, S.; Tournell, M. Tissue expander infections in pediatric patients: Management and outcomes. Plast. Reconstr. Surg. 2009, 124, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.K.; Turin, S.Y.; Chim, H.; LoGiudice, J.A. Salvaging the Unavoidable: A Review of Complications in Pediatric Tissue Expansion. Plast. Reconstr. Surg. 2018, 142, 759–768. [Google Scholar] [CrossRef]
- Wang, H.D.; Ibrahim, Z.; Quan, A.; Bai, J.; Ostrander, B.T.; Redett, R.J. Pediatric Tissue Expansion: Predictors of Premature Expander Removal in a Single Surgeon’s Experience with 472 Expanders. Plast. Reconstr. Surg. 2020, 145, 755–762. [Google Scholar] [CrossRef]
- Azadgoli, B.; Fahradyan, A.; Wolfswinkel, E.M.; Tsuha, M.; Magee, W., 3rd; Hammoudeh, J.A.; Urata, M.M.; Howell, L.K. External Port Tissue Expansion in the Pediatric Population: Confirming Its Safety and Efficacy. Plast. Reconstr. Surg. 2018, 141, 883e–890e. [Google Scholar] [CrossRef]
- Phillips, B.T.; Halvorson, E.G. Antibiotic Prophylaxis following Implant-Based Breast Reconstruction: What Is the Evidence? Plast. Reconstr. Surg. 2016, 138, 751–757. [Google Scholar] [CrossRef]
- Bianchini, S.; Rigotti, E.; Nicoletti, L.; Monaco, S.; Auriti, C.; Castagnola, E.; Castelli Gattinara, G.; De Luca, M.; Galli, L.; Garazzino, S.; et al. Surgical Antimicrobial Prophylaxis in Neonates and Children with Special High-Risk Conditions: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics 2022, 11, 246. [Google Scholar] [CrossRef]
- Adler, N.; Elia, J.; Billig, A.; Margulis, A. Complications of nonbreast tissue expansion: 9 Years experience with 44 adult patients and 119 pediatric patients. J. Pediatr. Surg. 2015, 50, 1513–1516. [Google Scholar] [CrossRef]
- Centers for Diseases Control and Prevention. Tetanus. Available online: https://www.cdc.gov/tetanus/clinicians.html (accessed on 1 April 2022).
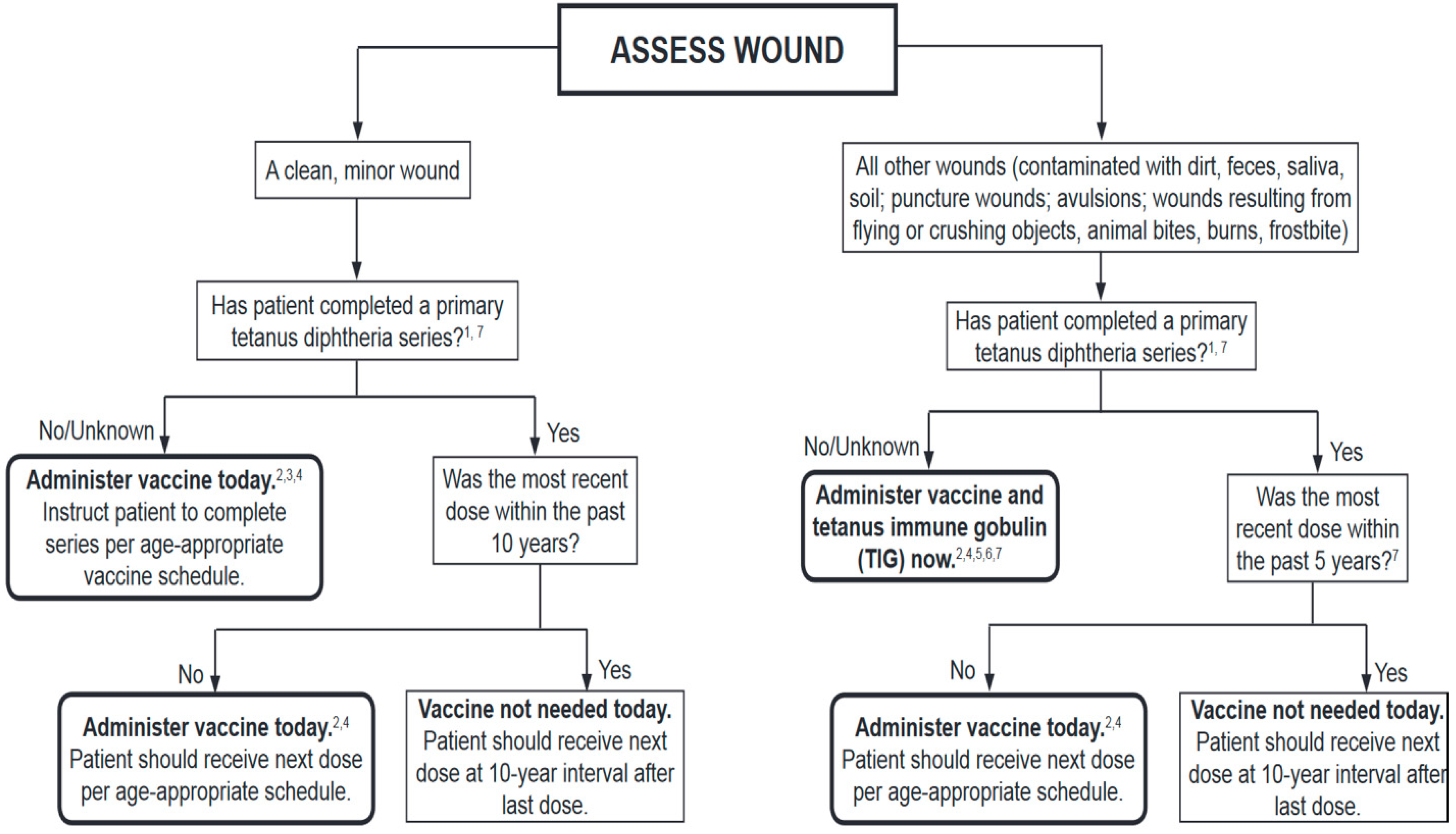
| 1. Clean Elective Procedures without Flaps or Grafts |
|---|
| Congenital skin lesions or vascular lesions excision Otoplasty |
| 2. Clean-Contaminated/Contaminated Elective Procedures without Flaps or Grafts |
| Cystic lesions excision Ingrown toenail correction Skin lesions of any kind with fistula to respiratory or alimentary tract |
| 3. Elective Procedures with Local Flaps |
| Head and neck, hand and limb, urinary malformations (for example, cleft lip and palate, syndactyly) Scar contractures release—scar revision Chronic wounds (pressure sores) |
| 4. Elective Procedures with Grafts |
| Skin grafts/bone grafts/nerve grafts/lipofilling Malformations (for example, bone graft in the alveolar process in complete cleft lip and palate) Scar contractures release—scar revision |
| 5. Prolonged Elective Procedures (More than 2 h) |
| Complex malformations (for example, craniosynostosis, rare clefts) Oncologic surgery and reconstruction with free flaps |
| 6. Acute Burns |
| Escarectomy and skin graft or flap |
| 7. Clean Contused Lacerated Wounds without Bone Exposure |
| 8. High-Risk Contused Lacerated Wounds or with Bone Exposure |
| 9. Contused Lacerated Wound Involving the oral Mucosa |
| 10. Human Bite |
| 11. Animal Bite |
| 12. Elective Procedure with Skin Expander Insertion |
| Congenital skin lesion (giant congenital nevus) Scar revision—excision |
| Type of Plastic Surgical Procedure | Recommendation |
|---|---|
| Clean plastic surgery in elective procedures | Not recommended |
| Clean-contaminated/contaminated plastic surgery in elective procedures | Cefazoline 30 mg/kg (maximum dose 2 g) IV within 30 min before surgery |
| Elective plastic surgery with the use of flaps | Not recommended In cases involving at-risk sites (i.e., leg below the knee, nose, ear, armpit, lip, groin) cefazolin 30 mg/kg (maximum dose 2 g) IV within the 30 min before surgery |
| Elective plastic surgery with the use of graft | Amoxicillin–clavulanic acid (50 mg/kg as amoxicillin) oral or IV or ampicillin–sulbactam (50 mg/kg as ampicillin) IV when the procedure involves the oral or nasal mucosa. Cefazolin 30 mg/kg (maximum dose 2 g) IV in the other cases within the 30 min before surgery and in the first 24 h after the procedure |
| Prolonged elective plastic surgery (lasting more than 2 h) | Cefazolin 30 mg/kg (maximum dose 2 g) IV within the 30 min before surgery, repeatable in case of surgery lasting more than 4 h |
| Plastic surgery following acute burns | When the surgery includes insertion or flaps or graft, cefazolin 30 mg/kg (maximum dose 2 g) IV given within the 30 min before surgery and every 4 h during the first 24 h after the procedure |
| Plastic surgery following clean contused lacerated wounds without bone exposure | Not recommended. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; Sgarzani, R.; Bianchini, S.; Monaco, S.; Nicoletti, L.; Rigotti, E.; Di Pietro, M.; Opri, R.; Caminiti, C.; Ciccia, M.; et al. Surgical Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics 2022, 11, 506. https://doi.org/10.3390/antibiotics11040506
Esposito S, Sgarzani R, Bianchini S, Monaco S, Nicoletti L, Rigotti E, Di Pietro M, Opri R, Caminiti C, Ciccia M, et al. Surgical Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics. 2022; 11(4):506. https://doi.org/10.3390/antibiotics11040506
Chicago/Turabian StyleEsposito, Susanna, Rossella Sgarzani, Sonia Bianchini, Sara Monaco, Laura Nicoletti, Erika Rigotti, Marilia Di Pietro, Roberta Opri, Caterina Caminiti, Matilde Ciccia, and et al. 2022. "Surgical Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery: A RAND/UCLA Appropriateness Method Consensus Study" Antibiotics 11, no. 4: 506. https://doi.org/10.3390/antibiotics11040506
APA StyleEsposito, S., Sgarzani, R., Bianchini, S., Monaco, S., Nicoletti, L., Rigotti, E., Di Pietro, M., Opri, R., Caminiti, C., Ciccia, M., Conti, G., Donà, D., Giuffré, M., La Grutta, S., Lancella, L., Lima, M., Lo Vecchio, A., Pelizzo, G., Piacentini, G., ... on behalf of the Peri-Operative Prophylaxis in Neonatal and Paediatric Age (POP-NeoPed) Study Group. (2022). Surgical Antimicrobial Prophylaxis in Pediatric Patients Undergoing Plastic Surgery: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics, 11(4), 506. https://doi.org/10.3390/antibiotics11040506








