Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance
Abstract
:1. Introduction
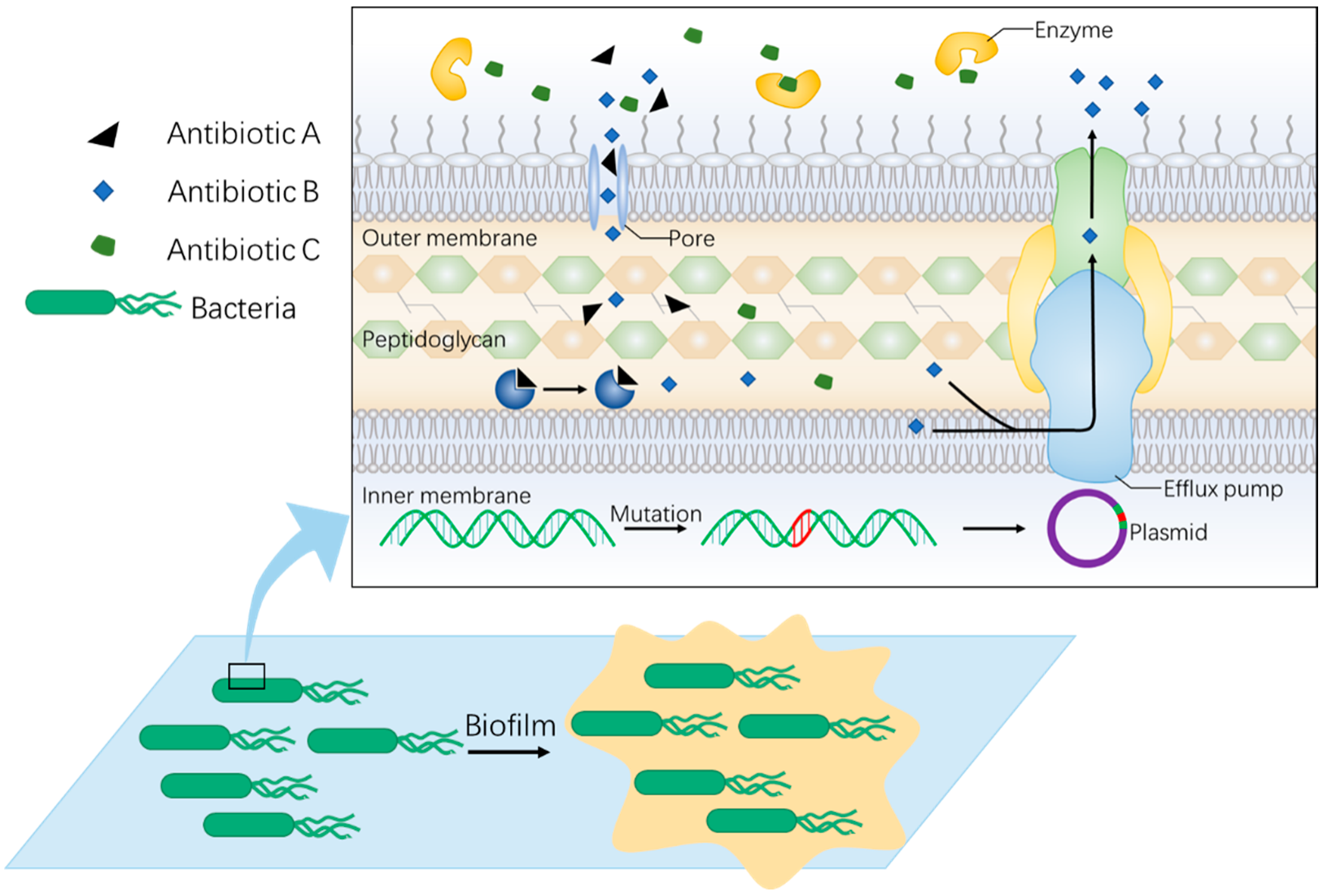
2. Non-Antibiotic Strategy Is Needed to Fight against Rapid Evolution of Bacterial Resistance
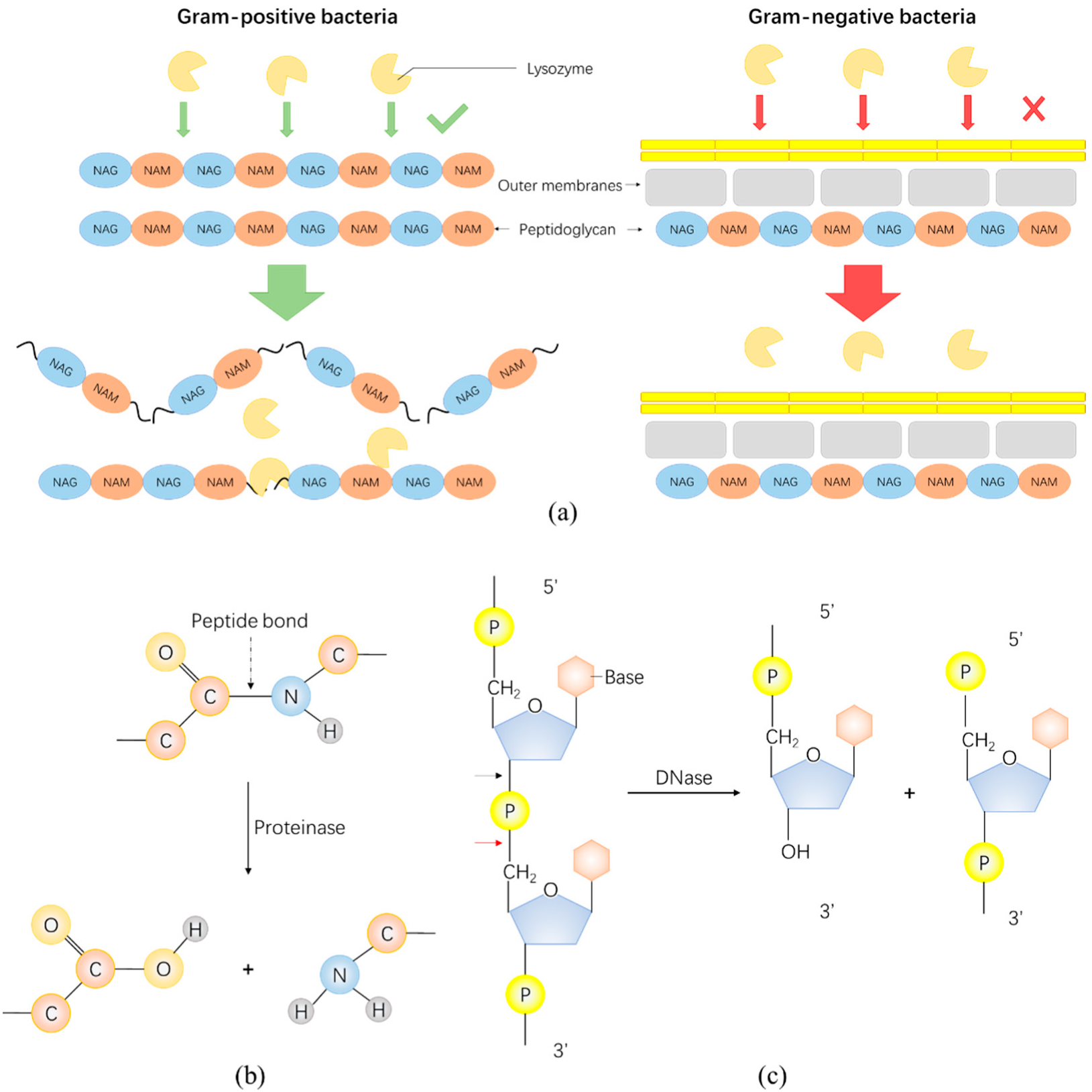
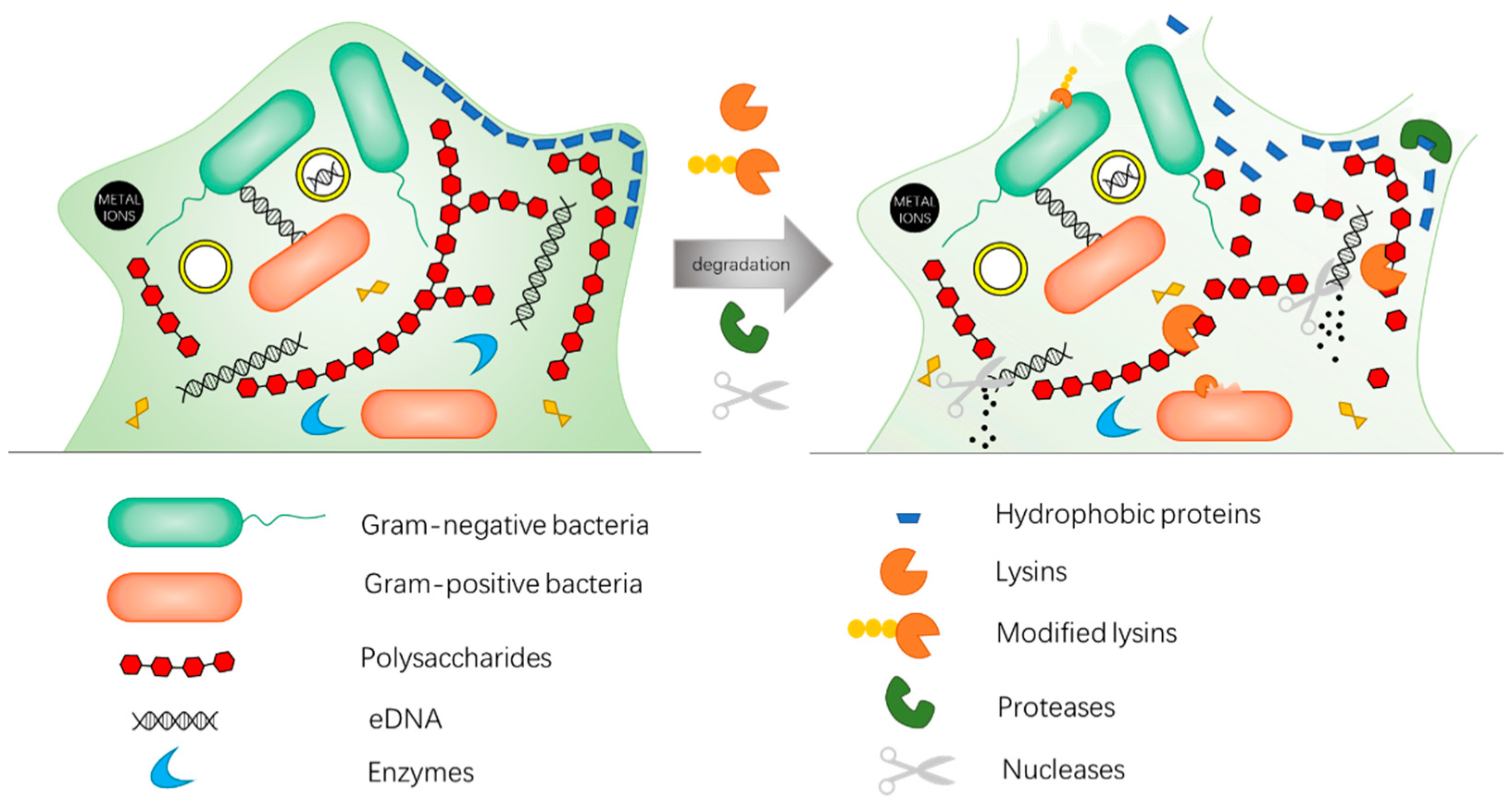
3. Enzybiotics Are Catalytic Antibacterials Based on Enzymes against Drug-Resistant Bacteria
4. Nanozybiotics Are Catalytic Antibacterials Based on Nanozymes with Enzyme-like Activities
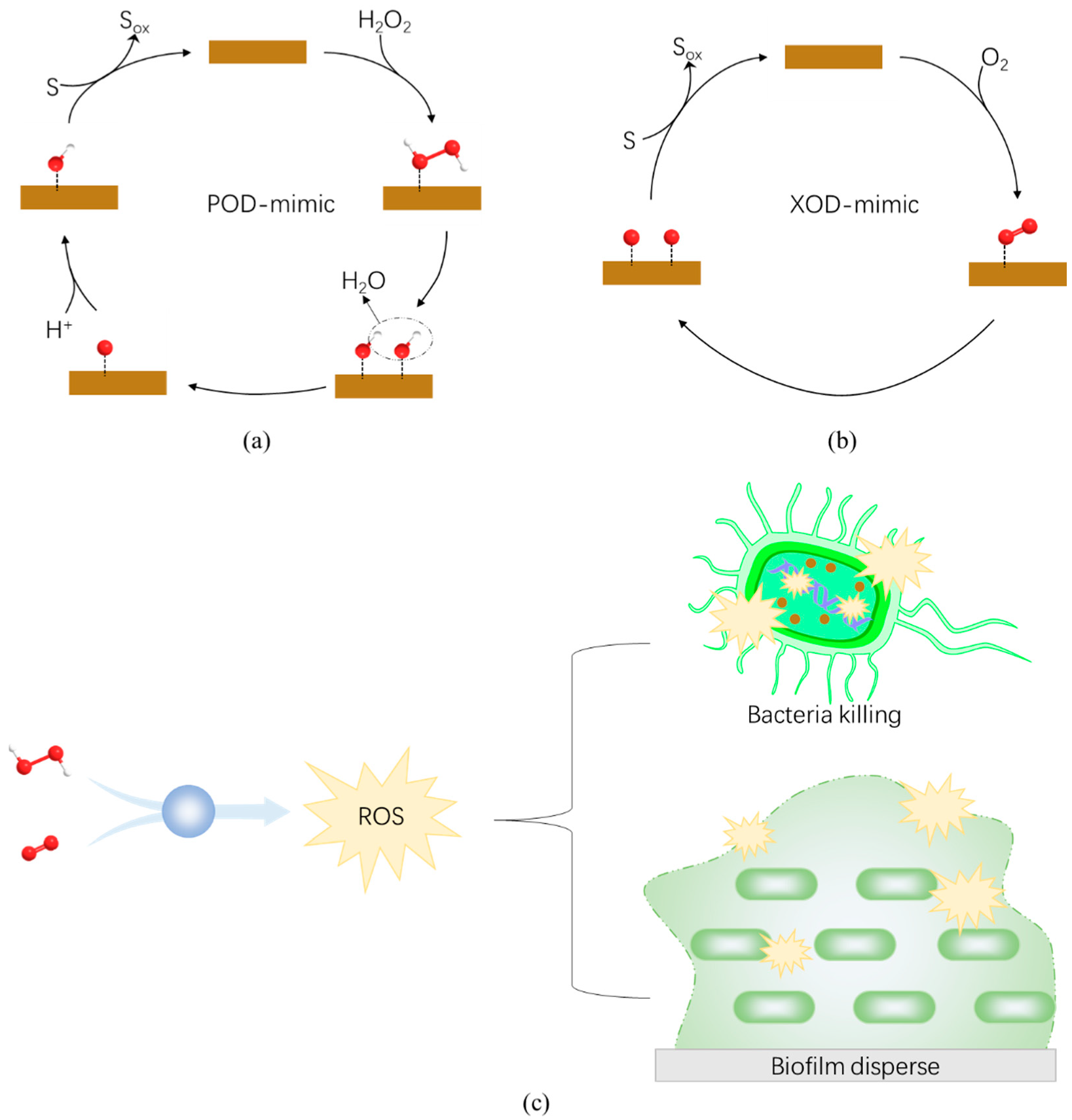
4.1. Peroxidase-like Nanozymes
4.2. Oxidase-like Nanozymes
4.3. Deoxyribonuclease-like Nanozymes
4.4. Combination Therapy
5. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-resistant bacterial infections in us hospitalized patients, 2012–2017. N. Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-Assisted Self-Assembled Polypeptide Nanogels to Selectively Combat Bacterial Infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Bacalum, M.; Radu, M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55. [Google Scholar] [CrossRef]
- Donlan, R.M. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009, 17, 66–72. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Rajnish, K.N.; Doss, C.G.P.; Samuel, S.M.; Selvarajan, E.; Zayed, H. Enzyme therapy: A forerunner in catalyzing a healthy society? Expert Opin. Biol. Ther. 2020, 20, 1151–1174. [Google Scholar] [CrossRef]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-Based Antibacterials as Therapeutics. In Therapeutic Enzymes: Function and Clinical Implications; Labrou, N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1148, pp. 233–253. [Google Scholar]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Yu, B.; Zhao, N.; Zhang, C.; Xu, F.-J. Rough Carbon–Iron Oxide Nanohybrids for Near-Infrared-II Light-Responsive Synergistic Antibacterial Therapy. ACS Nano 2021, 15, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Wen, J.; Yu, P.; Liu, J.; Li, J.; Chu, H. Chemically Grafted Nanozyme Composite Cryogels to Enhance Antibacterial and Biocompatible Performance for Bioliquid Regulation and Adaptive Bacteria Trapping. ACS Nano 2021, 15, 19672–19683. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Peng, M.; Chen, Y.; Cai, X.; Huang, F.; Yang, L.; Liu, X.; Li, T.; Wen, X.; Wang, N.; et al. Defect-rich graphene stabilized atomically dispersed Cu3 clusters with enhanced oxidase-like activity for antibacterial applications. Appl. Catal. B Environ. 2021, 301, 120826. [Google Scholar] [CrossRef]
- Li, Y.; Yu, P.; Wen, J.; Sun, H.; Wang, D.; Liu, J.; Li, J.; Chu, H. Nanozyme-Based Stretchable Hydrogel of Low Hysteresis with Antibacterial and Antioxidant Dual Functions for Closely Fitting and Wound Healing in Movable Parts. Adv. Funct. Mater. 2021, 2110720. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, H.; Liu, C.; Bing, W.; Wang, Z.; Qu, X. A Multinuclear Metal Complex Based DNase-Mimetic Artificial Enzyme: Matrix Cleavage for Combating Bacterial Biofilms. Angew. Chem. Int. Ed. 2016, 55, 10732–10736. [Google Scholar] [CrossRef]
- Luo, Z.; Cui, H.; Guo, J.; Yao, J.; Fang, X.; Yan, F.; Wang, B.; Mao, H. Poly(ionic liquid)/ce-based antimicrobial nanofibrous membrane for blocking drug-resistance dissemination from mrsa-infected wounds. Adv. Funct. Mater. 2021, 31, 2100336. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- HallR, M.; Collis, C.M. Mobile gene cassettes and integrons: Capture and spread of genes by site-specific recombination. Mol. Microbiol. 1995, 15, 593–600. [Google Scholar] [CrossRef]
- Hancock, R. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.-F.; Ji, J. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Górski, A. Enzybiotics and Their Potential Applications in Medicine. In Enzybiotics: Antibiotic Enzymes as Drugs and Therapeutics; Villa, T.G., Veiga-Crespo, P., Eds.; Wiley: New York, NY, USA, 2009; pp. 1–26. [Google Scholar]
- Heselpoth, R.D.; Swift, S.M.; Linden, S.B.; Mitchell, M.S.; Nelson, D.C. Enzybiotics: Endolysins and Bacteriocins. In Bacteriophages: Biology, Technology, Therapy; Harper, D., Abedon, S., Burrowes, B., McConville, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–42. [Google Scholar]
- King, B.F.; Biel, M.L.; Wilkinson, B.J. Facile penetration of the Staphylococcus aureus capsule by lysostaphin. Infect. Immun. 1980, 29, 892–896. [Google Scholar] [CrossRef] [PubMed]
- de Freire Bastos, M.d.C.; Coutinho, B.G.; Varella Coelho, M.L. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals 2010, 3, 1139–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo, E.D.; Otero, L.; Ruiz, F.; Klinke, S.; Giordano, W. The disruptive effect of lysozyme on the bacterial cell wall explored by an in-silico structural outlook. Biochem. Mol. Biol. Educ. 2018, 46, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H. On the novel catalytically-independent antimicrobial function of hen egg-white lysozyme: A conformation-dependent activity. Food/Nahrung 1998, 42, 187–193. [Google Scholar] [CrossRef]
- Arima, H.; Ibrahim, H.R.; Kinoshita, T.; Kato, A. Bactericidal action of lysozymes attached with various sizes of hydrophobic peptides to the C-terminal using genetic modification. FEBS Lett. 1997, 415, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Saperas, N.; Fonfría-Subirós, E. Proteolytic Enzymes in Detergents: Evidence of Their Presence through Activity Measurements Based on Electrophoresis. J. Chem. Educ. 2011, 88, 1702–1706. [Google Scholar] [CrossRef] [Green Version]
- Eshamah, H.; Han, I.; Naas, H.; Acton, J.; Dawson, P. Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef. Meat Sci. 2014, 96, 1494–1500. [Google Scholar] [CrossRef]
- Praveen, N.C.; Rajesh, A.; Madan, M.; Chaurasia, V.R.; Hiremath, N.V.; Sharma, A.M. In vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. J. Int. Oral Health 2014, 6, 96–98. [Google Scholar]
- Eller, C.H.; Raines, R.T. Antimicrobial Synergy of a Ribonuclease and a Peptide Secreted by Human Cells. ACS Infect. Dis. 2020, 6, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.F.; Thamotharan, S.; Gowrishankar, S.; Pandian, S.K.; Nithyanand, P. Marine bacterial DNase curtails virulence and disrupts biofilms of Candida albicans and non-albicans Candida species. Biofouling 2019, 35, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060. [Google Scholar] [CrossRef]
- Ragg, R.; Tahir, M.N.; Tremel, W. Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics. Eur. J. Inorg. Chem. 2015, 2016, 1906–1915. [Google Scholar] [CrossRef]
- Gao, L.; Yan, X. Nanozymes: An emerging field bridging nanotechnology and biology. Sci. China Life Sci. 2016, 59, 400–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Ren, J.; Qu, X. Catalytically Active Nanomaterials: A Promising Candidate for Artificial Enzymes. Accounts Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Accounts Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Laurents, D.; Baldwin, R.L. Characterization of the Unfolding Pathway of Hen Egg White Lysozyme. Biochemistry 1997, 36, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Liu, X.P.; Yan, Z.Q.; Zhang, Y.; Liu, Z.W.; Sun, Y.H.; Ren, J.S.; Qu, X.G. Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and m self-activated cascade reagent for in vivo wound healing. Acs Nano 2019, 13, 5222–5230. [Google Scholar] [CrossRef]
- Hwang, G.; Paula, A.J.; Hunter, E.E.; Liu, Y.; Babeer, A.; Karabucak, B.; Stebe, K.; Kumar, V.; Steager, E.; Koo, H. Catalytic antimicrobial robots for biofilm eradication. Sci. Robot. 2019, 4, eaaw2388. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, A.; Liu, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Enzyme-Responsive Mesoporous Ruthenium for Combined Chemo-Photothermal Therapy of Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26590–26606. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of Single-Iron-Atom Nanocatalysts for Highly Efficient Catalytic Antibiotics. Small 2019, 15, e1901834. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Liu, Z.; Liu, X.; Dong, K.; Liu, C.; Ren, J.; Qu, X. Hydrogel-based artificial enzyme for combating bacteria and accelerating wound healing. Nano Res. 2020, 13, 496–502. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Ma, S.; Guo, Q.; Zhang, W.; Cheng, L.; Ding, L.; Xu, Z.; Jiang, J.; Gao, L. Oral biofilm elimination by combining iron-based nanozymes and hydrogen peroxide-producing bacteria. Biomater. Sci. 2020, 8, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, W.; Sun, J.; Lin, M.; Niu, Y.; Yang, X.; Xu, Y. Electrochemical generation of Fe3C/N-doped graphitic carbon nanozyme for efficient wound healing in vivo. Carbon 2020, 159, 149–160. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Mei, L.; Ma, D.; Liao, Y.; Zu, Y.; Xu, P.; Yin, W.; Gu, Z. A two-step gas/liquid strategy for the production of N-doped defect-rich transition metal dichalcogenide nanosheets and their antibacterial applications. Nanoscale 2020, 12, 8415–8424. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wei, G.; Wu, Q.; Xu, Z.; Liu, Y.; Han, J.; Fan, L.; Gao, L. Light-enhanced sponge-like carbon nanozyme used for synergetic antibacterial therapy. Biomater. Sci. 2019, 7, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C.; Younis, M.R.; Zhou, Y.; Wang, C.; Xia, X.H. In situ fabrication of ultrasmall gold nanoparticles/2d mofs hybrid as nanozyme for antibacterial therapy. Small 2020, 16, e2000553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Chen, Z.T.; Kong, J.L.; Liang, Y.L.; Chen, K.; Chang, Y.A.; Yuan, H.; Wang, Y.J.; Liang, H.J.; Li, J.C.; et al. Fullerenol nanoparticles eradicate helicobacter pylori via ph-responsive peroxidase activity. ACS Appl. Mater. Interfaces 2020, 12, 29013–29023. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Y.; Zhang, Y.; Sun, X.; Li, F.; Bu, T.; Wang, Q.; Wang, L. A bifunctional nanoplatform based on copper manganate nanoflakes for bacterial elimination via a catalytic and photothermal synergistic effect. Biomater. Sci. 2020, 8, 4266–4274. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, X.; Zhang, T.; Ghosal, A.; Zhang, G.; Fan, H.M.; Zhao, L. Iron nanoparticles augmented chemodynamic effect by alternative magnetic field for wound disinfection and healing. J. Control. Release 2020, 324, 598–609. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, X.; Shi, S.; Li, M.; Liu, L.; Han, X.; Zhu, W.; Yue, T.; Sun, J.; Wang, J. Ionic silver-infused peroxidase-like metal–organic frameworks as versatile “antibiotic” for enhanced bacterial elimination. Nanoscale 2020, 12, 16330–16338. [Google Scholar] [CrossRef]
- Yim, G.; Kim, C.Y.; Kang, S.; Min, D.-H.; Kang, K.; Jang, H. Intrinsic Peroxidase-Mimicking Ir Nanoplates for Nanozymatic Anticancer and Antibacterial Treatment. ACS Appl. Mater. Interfaces 2020, 12, 41062–41070. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, Y.; Ma, K.; Yu, S.; Chen, Y.; Deng, Z.; Liu, Y.; Wang, F. Engineering Inorganic Nanoflares with Elaborate Enzymatic Specificity and Efficiency for Versatile Biofilm Eradication. Small 2020, 16, 2002348. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cui, X.; Wang, Z.; Dong, C.; Li, J.; Han, X. Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem. Eng. J. 2021, 408, 127240. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.R.; Aloui, H.; Kim, B.S. In situ growth of gold and silver nanoparticles onto phyto-functionalized boron nitride nanosheets: Catalytic, peroxidase mimicking, and antimicrobial activity. J. Clean. Prod. 2020, 270, 122339. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Bao, X.; Ni, Y.; Teng, Y.; Liu, J.; Sun, X.; Sun, Y.; Li, H.; Zhou, Y. Nanodiamond as efficient peroxidase mimic against periodontal bacterial infection. Carbon 2020, 169, 370–381. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Shah, S.; Kim, D.; Simon-Soro, A.; Ito, T.; Hajfathalian, M.; Li, Y.; Hsu, J.C.; Nieves, L.M.; et al. Precision targeting of bacterial pathogen via bi-functional nanozyme activated by biofilm microenvironment. Biomaterials 2021, 268, 120581. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Tan, J.; Chang, Z.; Liu, X.; Ma, W.; Xu, Y. Near-Infrared Regulated Nanozymatic/Photothermal/Photodynamic Triple-Therapy for Combating Multidrug-Resistant Bacterial Infections via Oxygen-Vacancy Molybdenum Trioxide Nanodots. Small 2021, 17, e2005739. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, S.; Karmacharya, M.; Dubbu, S.; Kwon, T.; Singh, V.; Chae, K.H.; Kumar, A.; Cho, Y.-K.; Lee, I.S. Surface-Textured Mixed-Metal-Oxide Nanocrystals as Efficient Catalysts for ROS Production and Biofilm Eradication. Nano Lett. 2020, 21, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Mahadevegowda, S.H.; Lu, D.; Zhang, K.; Chan-Park, M.B.; Duan, H. Metabolic Labeling Mediated Targeting and Thermal Killing of Gram-Positive Bacteria by Self-Reporting Janus Magnetic Nanoparticles. Small 2021, 17, e2006357. [Google Scholar] [CrossRef]
- Yan, L.; Mu, J.; Ma, P.; Li, Q.; Yin, P.; Liu, X.; Cai, Y.; Yu, H.; Liu, J.; Wang, G.; et al. Gold nanoplates with superb photothermal efficiency and peroxidase-like activity for rapid and synergistic antibacterial therapy. Chem. Commun. 2020, 57, 1133–1136. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, N.; Tang, H.; Zhang, C.; Chen, K.; Wang, W.; Liu, J. Effective Antibacterial Activity of Degradable Copper-Doped Phosphate-Based Glass Nanozymes. ACS Appl. Mater. Interfaces 2021, 13, 11631–11645. [Google Scholar] [CrossRef]
- Wang, T.; Bai, Q.; Zhu, Z.; Xiao, H.; Jiang, F.; Du, F.; Yu, W.W.; Liu, M.; Sui, N. Graphdiyne-supported palladium-iron nanosheets: A dual-functional peroxidase mimetic nanozyme for glutathione detection and antibacterial application. Chem. Eng. J. 2021, 413, 127537. [Google Scholar] [CrossRef]
- Jia, Z.; Lv, X.; Hou, Y.; Wang, K.; Ren, F.; Xu, D.; Wang, Q.; Fan, K.; Xie, C.; Lu, X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioact. Mater. 2021, 6, 2676–2687. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, J.; Ding, F.; Ren, X. Nanocatalyst doped bacterial cellulose-based thermosensitive nanogel with biocatalytic function for antibacterial application. Int. J. Biol. Macromol. 2021, 195, 294–301. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Bu, T.; Wang, Q.; Jia, P.; Dong, M.; Wang, L. In situ fabrication of metal-organic framework derived hybrid nanozymes for enhanced nanozyme-photothermal therapy of bacteria-infected wounds. Compos. Part B Eng. 2021, 229, 109465. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, J.; Zhou, Y.; Yue, Q.; Wei, J. Spherical mesoporous Fe-N-C single-atom nanozyme for photothermal and catalytic synergistic antibacterial therapy. J. Colloid Interface Sci. 2021, 606, 826–836. [Google Scholar] [CrossRef]
- Fan, Y.; Gan, X.; Zhao, H.; Zeng, Z.; You, W.; Quan, X. Multiple application of SAzyme based on carbon nitride nanorod-supported Pt single-atom for H2O2 detection, antibiotic detection and antibacterial therapy. Chem. Eng. J. 2021, 427, 131572. [Google Scholar] [CrossRef]
- Xiao, J.; Hai, L.; Li, Y.; Li, H.; Gong, M.; Wang, Z.; Tang, Z.; Deng, L.; He, D. An Ultrasmall Fe 3 O 4 -Decorated Polydopamine Hybrid Nanozyme Enables Continuous Conversion of Oxygen into Toxic Hydroxyl Radical via GSH-Depleted Cascade Redox Reactions for Intensive Wound Disinfection. Small 2021, 281, 2105465. [Google Scholar] [CrossRef]
- Hou, X.; Zeng, H.; Chi, X.; Hu, X. Pathogen Receptor Membrane-Coating Facet Structures Boost Nanomaterial Immune Escape and Antibacterial Performance. Nano Lett. 2021, 21, 9966–9975. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, J.; Li, H.; Hai, L.; Yang, K.; Li, J.; Wang, Z.; Deng, L.; He, D. Magnetically retained and glucose-fueled hydroxyl radical nanogenerators for H2O2-self-supplying chemodynamic therapy of wound infections. Mater. Sci. Eng. C 2021, 131, 112522. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Jia, Z.; Huo, D.; Liu, Y.; Liu, J. Cationic chitosan@Ruthenium dioxide hybrid nanozymes for photothermal therapy enhancing ROS-mediated eradicating multidrug resistant bacterial infection. J. Colloid Interface Sci. 2021, 603, 615–632. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Kim, D.; Ren, Z.; Oh, M.J.; Cormode, D.P.; Hara, A.T.; Zero, D.T.; Koo, H. Ferumoxytol Nanoparticles Target Biofilms Causing Tooth Decay in the Human Mouth. Nano Lett. 2021, 21, 9442–9449. [Google Scholar] [CrossRef]
- Fuentes, K.M.; Onna, D.; Rioual, T.; Huvelle, M.A.L.; Britto, F.; Simian, M.; Sánchez-Domínguez, M.; Soler-Illia, G.J.; Bilmes, S.A. Copper upcycling by hierarchical porous silica spheres functionalized with branched polyethylenimine: Antimicrobial and catalytic applications. Microporous Mesoporous Mater. 2021, 327, 111391. [Google Scholar] [CrossRef]
- Niu, J.; Zhao, C.; Liu, C.; Ren, J.; Qu, X. Bio-Inspired Bimetallic Enzyme Mimics as Bio-Orthogonal Catalysts for Enhanced Bacterial Capture and Inhibition. Chem. Mater. 2021, 33, 8052–8058. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Q.; Shen, A.-G.; Fan, B.; Hu, J.-M. Nano Au@Cu2-xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chem. Eng. J. 2021, 422, 130090. [Google Scholar] [CrossRef]
- Shan, J.; Li, X.; Yang, K.; Xiu, W.; Wen, Q.; Zhang, Y.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Efficient Bacteria Killing by Cu2WS4 Nanocrystals with Enzyme-like Properties and Bacteria-Binding Ability. ACS Nano 2019, 13, 13797–13808. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Zhang, D.; Wang, J. Bifunctionalized novel co-v mmo nanowires: Intrinsic oxidase and peroxidase like catalytic activities for antibacterial application. Appl. Catal. B-Environ. 2020, 261, 118256. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, T.; Li, R.; Niu, Y.; Yang, X.; Liu, J.; Xu, Y.; Li, C.M. Bienzymatic synergism of vanadium oxide nanodots to efficiently eradicate drug-resistant bacteria during wound healing in vivo. J. Colloid Interface Sci. 2020, 559, 313–323. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, H.; Lin, Z.; Chen, L.; Li, Z.; Ge, J.; Zhang, T.; Xia, H.; Li, L.; et al. Efficient elimination of multidrug-resistant bacteria using copper sulfide nanozymes anchored to graphene oxide nanosheets. Nano Res. 2020, 13, 2156–2164. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, D.; Xu, C.J.; Xing, R.E. Dual response mimetic enzyme of novel co4s3/co3o4 composite nanotube for antibacterial application. J. Hazard. Mater. 2020, 392, 122278. [Google Scholar] [CrossRef]
- Shan, J.; Yang, K.; Xiu, W.; Qiu, Q.; Dai, S.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Cu 2 MoS 4 Nanozyme with NIR-II Light Enhanced Catalytic Activity for Efficient Eradication of Multidrug-Resistant Bacteria. Small 2020, 16, 2001099. [Google Scholar] [CrossRef]
- Xu, M.; Hu, Y.; Xiao, Y.; Zhang, Y.; Sun, K.; Wu, T.; Lv, N.; Wang, W.; Ding, W.; Li, F.; et al. Near-Infrared-Controlled Nanoplatform Exploiting Photothermal Promotion of Peroxidase-like and OXD-like Activities for Potent Antibacterial and Anti-biofilm Therapies. ACS Appl. Mater. Interfaces 2020, 12, 50260–50274. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Huang, D.; Huang, L.; Zhang, H.; Li, N.; Wang, M. Through quorum sensing, Pseudomonas aeruginosa resists noble metal-based nanomaterials toxicity. Environ. Pollut. 2021, 269, 116138. [Google Scholar] [CrossRef]
- Mu, Q.; Sun, Y.; Guo, A.; Xu, X.; Qin, B.; Cai, A. A bifunctionalized NiCo2O4-Au composite: Intrinsic peroxidase and oxidase catalytic activities for killing bacteria and disinfecting wound. J. Hazard. Mater. 2021, 402, 123939. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Guo, Z.; Zhang, H.; Wang, J.; Jia, P.; Bu, T.; Liu, Y.; Li, L.; Wang, L. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int. J. Biol. Macromol. 2021, 167, 10–22. [Google Scholar] [CrossRef]
- Yan, L.X.; Wang, B.B.; Zhao, X.; Chen, L.J.; Yan, X.P. A ph-responsive persistent luminescence nanozyme for selective imaging and killing of helicobacter pylori and common resistant bacteria. ACS Appl. Mater. Interfaces 2021, 13, 60955–60965. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Zeng, S.; Wang, C.; Li, W.; Wang, M.; Wang, X.; Zhou, X.; Zhao, X.; Ren, L. Optimization of Nanostructured Copper Sulfide to Achieve Enhanced Enzyme-Mimic Activities for Improving Anti-Infection Performance. ACS Appl. Mater. Interfaces 2021, 13, 53659–53670. [Google Scholar] [CrossRef]
- Zhuang, Q.-Q.; Deng, Q.; He, S.-B.; Chen, Q.-Q.; Peng, H.-P.; Deng, H.-H.; Xia, X.-H.; Chen, W. Bifunctional cupric oxide nanoparticle-catalyzed self-cascade oxidation reactions of ascorbic acid for bacterial killing and wound disinfection. Compos. Part B Eng. 2021, 222, 109074. [Google Scholar] [CrossRef]
- Wang, P.; Peng, L.; Lin, J.; Li, Y.; Luo, Q.; Jiang, S.; Tian, H.; Zhang, Y.; Liu, X.; Liu, J. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose-activated cascade reaction to efficiently promote diabetic wound healing. Chem. Eng. J. 2021, 415, 128901. [Google Scholar] [CrossRef]
- Xu, Q.; Hua, Y.; Zhang, Y.; Lv, M.; Wang, H.; Pi, Y.; Xie, J.; Wang, C.; Yong, Y. A Biofilm Microenvironment-Activated Single-Atom Iron Nanozyme with NIR-Controllable Nanocatalytic Activities for Synergetic Bacteria-Infected Wound Therapy. Adv. Heal. Mater. 2021, 10, 2101374. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Wang, T.T.; Lao, Z.T.; Lu, M.L.; Liang, S.; Cui, X.P.; Li, Q.L.; Zhao, S.Q. Au-au/iro2@cu(paba) reactor with tandem enzyme-mimicking catalytic activity for organic dye degradation and antibacterial application. ACS Appl. Mater. Interfaces 2021, 13, 21680–21692. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, X.; Ma, D.; Wang, Y.; Peng, L.; Shi, J.; Sun, J.; Gan, X.; Deng, Y.; Yang, W. Growth Factor-Decorated Ti 3 C 2 MXene/MoS 2 2D Bio-Heterojunctions with Quad-Channel Photonic Disinfection for Effective Regeneration of Bacteria-Invaded Cutaneous Tissue. Small 2021, 17, 2103993. [Google Scholar] [CrossRef]
- Wang, L.; Gao, F.; Wang, A.; Chen, X.; Li, H.; Zhang, X.; Zheng, H.; Ji, R.; Li, B.; Yu, X.; et al. Defect-Rich Adhesive Molybdenum Disulfide/rGO Vertical Heterostructures with Enhanced Nanozyme Activity for Smart Bacterial Killing Application. Adv. Mater. 2020, 32, 2005423. [Google Scholar] [CrossRef]
- Xi, J.Q.; Wei, G.; An, L.F.; Xu, Z.B.; Xu, Z.L.; Fan, L.; Gao, L.Z. Copper/carbon hybrid nanozyme: Tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 2019, 19, 7645–7654. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Adaptive Hydrogels Based on Nanozyme with Dual-Enhanced Triple Enzyme-Like Activities for Wound Disinfection and Mimicking Antioxidant Defense System. Adv. Heal. Mater. 2021, 11, 2101849. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Li, X.; Fan, S.; Liu, Y.; Jiang, X.; Boudreau, M.D.; Pan, Y.; Tian, X.; Yin, J.-J. Bactericidal effects and accelerated wound healing using Tb4O7 nanoparticles with intrinsic oxidase-like activity. J. Nanobiotechnol. 2019, 17, 54. [Google Scholar] [CrossRef]
- Cai, T.; Fang, G.; Tian, X.; Yin, J.-J.; Chen, C.; Ge, C. Optimization of Antibacterial Efficacy of Noble-Metal-Based Core-Shell Nanostructures and Effect of Natural Organic Matter. ACS Nano 2019, 13, 12694–12702. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, D.; Chen, C. Intrinsic oxidase-like nanoenzyme co4s3/co(oh)(2) hybrid nanotubes with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 29614–29624. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Wang, W.; Peng, J.; Li, Y.; Shangguan, Y.; Ouyang, G.; Xu, M.; Wang, S.; Wei, J.; et al. Colloidal Surface Engineering: Growth of Layered Double Hydroxides with Intrinsic Oxidase-Mimicking Activities to Fight against Bacterial Infection in Wound Healing. Adv. Heal. Mater. 2020, 9, 2000092. [Google Scholar] [CrossRef]
- Sharma, S.; Chakraborty, N.; Jha, D.; Gautam, H.K.; Roy, I. Robust dual modality antibacterial action using silver-Prussian blue nanoscale coordination polymer. Mater. Sci. Eng. C 2020, 113, 110982. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 745. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhang, L.; Deng, H.; Li, H.; Tang, W.T.; Guan, L.Y.; Qiu, Y.; Donovan, M.J.; Chen, Z.; Tan, W.H. In vivo activation of ph-responsive oxidase-like graphitic nanozymes for selective killing of helicobacter pylori. Nat. Commun. 2021, 12, 2002. [Google Scholar] [CrossRef]
- Dong, M.; Sun, X.; Bu, T.; Zhang, H.; Wang, J.; He, K.; Li, L.; Li, Z.; Wang, L. 3D/2D TMSs/TiO2 nanofibers heterojunctions for photodynamic-photothermal and oxidase-like synergistic antibacterial therapy co-driven by VIS and NIR biowindows. Compos. Part B Eng. 2021, 230, 109498. [Google Scholar] [CrossRef]
- Sun, D.; Pang, X.; Cheng, Y.; Ming, J.; Xiang, S.; Zhang, C.; Lv, P.; Chu, C.; Chen, X.; Liu, G.; et al. Ultrasound-Switchable Nanozyme Augments Sonodynamic Therapy against Multidrug-Resistant Bacterial Infection. ACS Nano 2020, 14, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tian, F.; Chang, J.; Bai, X.; Yuan, C.; Wang, C.; Neville, A. Haloperoxidase Mimicry by CeO2–x Nanorods of Different Aspect Ratios for Antibacterial Performance. ACS Sustain. Chem. Eng. 2020, 8, 6744–6752. [Google Scholar] [CrossRef]
- Frerichs, H.; Putz, E.; Pfitzner, F.; Reich, T.; Gazanis, A.; Panthofer, M.; Hartmann, J.; Jegel, O.; Heermann, R.; Tremel, W. Nanocomposite antimicrobials prevent bacterial growth through the enzyme-like activity of bi-doped cerium dioxide (ce1-xbixo2-delta). Nanoscale 2020, 12, 21344–21358. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; An, L.; Wei, G.; Huang, Y.; Li, D.; Fan, L.; Gao, L. Photolysis of methicillin-resistant Staphylococcus aureus using Cu-doped carbon spheres. Biomater. Sci. 2020, 8, 6225–6234. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Giglio, K.M.; Nelson, J.L.; Sondermann, H.; Travis, A.J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 2014, 6, 2588–2593. [Google Scholar] [CrossRef] [Green Version]
- Loo, A.E.K.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef] [Green Version]
- Natalio, F.; André, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Shi, S.; Wu, S.; Shen, Y.; Zhang, S.; Xiao, Y.; He, X.; Gong, J.; Farnell, Y.; Tang, Y.; Huang, Y.; et al. Iron oxide nanozyme suppresses intracellular Salmonella Enteritidis growth and alleviates infection in vivo. Theranostics 2018, 8, 6149–6162. [Google Scholar] [CrossRef]
- Karim, N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S.; et al. Visible-Light-Triggered Reactive-Oxygen-Species-Mediated Antibacterial Activity of Peroxidase-Mimic CuO Nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Roudbaneh, S.Z.K.; Kahbasi, S.; Sohrabi, M.J.; Hasan, A.; Salihi, A.; Mirzaie, A.; Niyazmand, A.; Nanakali, N.M.Q.; Shekha, M.S.; Aziz, F.M.; et al. Albumin binding, antioxidant and antibacterial effects of cerium oxide nanoparticles. J. Mol. Liq. 2019, 296, 111839. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Sun, M.; Qian, H.; Liu, J.; Li, Y.; Pang, S.; Xu, M.; Zhang, J. A flexible conductive film prepared by the oriented stacking of Ag and Au/Ag alloy nanoplates and its chemically roughened surface for explosive SERS detection and cell adhesion. RSC Adv. 2017, 7, 7073–7078. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zhang, G.; Li, P.; Lu, H.; Tang, W.; Yang, X.; Huang, R.; Yu, F.; Wu, W.; Xiao, Y.; et al. Acid-activated ROS generator with folic acid targeting for bacterial biofilm elimination. Mater. Sci. Eng. C 2021, 127, 112225. [Google Scholar] [CrossRef]
- Vallabani, N.S.; Vinu, A.; Singh, S.; Karakoti, A. Tuning the ATP-triggered pro-oxidant activity of iron oxide-based nanozyme towards an efficient antibacterial strategy. J. Colloid Interface Sci. 2020, 567, 154–164. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and ph-activated biofilm disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Bifunctionalized Mesoporous Silica-Supported Gold Nanoparticles: Intrinsic Oxidase and Peroxidase Catalytic Activities for Antibacterial Applications. Adv. Mater. 2015, 27, 1097–1104. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Huang, J.; Zhang, Q.; Zhao, W.; Xu, Z.; Zhang, W. Bamboo-Like Nanozyme Based on Nitrogen-Doped Carbon Nanotubes Encapsulating Cobalt Nanoparticles for Wound Antibacterial Applications. Adv. Funct. Mater. 2021, 31, 2105198. [Google Scholar] [CrossRef]
- Allentoft, M.E.; Collins, M.; Harker, D.; Haile, J.; Oskam, C.; Hale, M.L.; Campos, P.; Samaniego, J.A.; Gilbert, M.; Willerslev, E.; et al. The Half-Life of DNA in bone: Measuring Decay Kinetics in 158 Dated Fossils. In Proceedings of the Royal Society of London; Series B: Biological Sciences; The Royal Society: London, UK, 2012; Volume 279, pp. 4724–4733. [Google Scholar]
- Luong, T.K.N.; Govaerts, I.; Robben, J.; Shestakova, P.; Parac-Vogt, T.N. Polyoxometalates as artificial nucleases: Hydrolytic cleavage of DNA promoted by a highly negatively charged ZrIV-substituted Keggin polyanion. Chem. Commun. 2017, 53, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, S.; Lönnberg, T.; Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 2018, 14, 803–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.; Jikumaru, A.; Ueno, T.; Sei, K. Inactivation Effect of Antibiotic-Resistant Gene Using Chlorine Disinfection. Water 2017, 9, 547. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Karaolia, P.; Fatta-Kassinos, D. The role of operating parameters and oxidative damage mechanisms of advanced chemical oxidation processes in the combat against antibiotic-resistant bacteria and resistance genes present in urban wastewater. Water Res. 2018, 129, 208–230. [Google Scholar] [CrossRef]
- Giannakis, S.; Le, T.-T.M.; Entenza, J.M.; Pulgarin, C. Solar photo-Fenton disinfection of 11 antibiotic-resistant bacteria (ARB) and elimination of representative AR genes. Evidence that antibiotic resistance does not imply resistance to oxidative treatment. Water Res. 2018, 143, 334–345. [Google Scholar] [CrossRef]
- Massoud, S.S.; Perkins, R.S.; Louka, F.R.; Xu, W.; Le Roux, A.; Dutercq, Q.; Fischer, R.C.; Mautner, F.A.; Handa, M.; Hiraoka, Y.; et al. Efficient hydrolytic cleavage of plasmid DNA by chloro-cobalt(ii) complexes based on sterically hindered pyridyl tripod tetraamine ligands: Synthesis, crystal structure and DNA cleavage. Dalton Trans. 2014, 43, 10086–10103. [Google Scholar] [CrossRef] [Green Version]
- Soler, M.; Figueras, E.; Serrano-Plana, J.; González-Bártulos, M.; Massaguer, A.; Company, A.; Martínez, M.Á.; Malina, J.; Brabec, V.; Feliu, L.; et al. Design, Preparation, and Characterization of Zn and Cu Metallopeptides Based on Tetradentate Aminopyridine Ligands Showing Enhanced DNA Cleavage Activity. Inorg. Chem. 2015, 54, 10542–10558. [Google Scholar] [CrossRef]
- Salvio, R.; Volpi, S.; Cacciapaglia, R.; Sansone, F.; Mandolini, L.; Casnati, A. Upper Rim Bifunctional cone-Calix[4 ]arenes Based on a Ligated Metal Ion and a Guanidinium Unit as DNAase and RNAase Mimics. J. Org. Chem. 2016, 81, 4728–4735. [Google Scholar] [CrossRef]
- Piovezan, C.; Jovito, R.; Bortoluzzi, A.J.; Terenzi, H.; Fischer, F.L.; Severino, P.C.; Pich, C.T.; Azzolini, G.G.; Peralta, R.A.; Rossi, L.M.; et al. Heterodinuclear (feznii)-zn-iii-bioinspired complex supported on 3-aminopropyl silica. Efficient hydrolysis of phosphate diester bonds. Inorg. Chem. 2010, 49, 2580–2582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Xin, Z.; Xu, S.; Shi, H.; Yang, H.; Song, L.; Yan, S.; Luan, S.; Yin, J.; Khan, A.F.; et al. Enzyme-mimicking polymer brush-functionalized surface for combating biomaterial-associated infections. Appl. Surf. Sci. 2017, 423, 869–880. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials 2019, 208, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Hua, X.-W.; Wu, F.-G.; Li, B.; Liu, P.; Gu, N.; Wang, Z.; Chen, Z. Synthesis of Ultrastable Copper Sulfide Nanoclusters via Trapping the Reaction Intermediate: Potential Anticancer and Antibacterial Applications. ACS Appl. Mater. Interfaces 2015, 7, 7082–7092. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Deokar, A.R.; Liao, J.-H.; Shih, P.-Y.; Ling, Y.-C. Graphene-Based Photothermal Agent for Rapid and Effective Killing of Bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Natan, M.; Edin, F.; Perkas, N.; Yacobi, G.; Perelshtein, I.; Segal, E.; Homsy, A.; Laux, E.; Keppner, H.; Rask-Andersen, H.; et al. Two are better than one: Combining zno and mgf2 nanoparticles reduces streptococcus pneumoniae and staphylococcus aureus biofilm formation on cochlear implants. Adv. Funct. Mater. 2016, 26, 2473–2481. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Ju, E.; Wang, Z.; Dong, K.; Ren, J.; Qu, X. Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy. Chem. Commun. 2014, 50, 10488–10490. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wang, M.; Mikhailovsky, A.; Wang, S.; Bazan, G.C. A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity. Angew. Chem. Int. Ed. 2017, 56, 5031–5034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, S.; Wang, Y.; Yu, S.; Zhu, W.; Zhang, X.; Zhang, D.; Yang, B.; Wang, X.; Wang, J. Versatile molybdenum disulfide based antibacterial composites for in vitro enhanced sterilization and in vivo focal infection therapy. Nanoscale 2016, 8, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
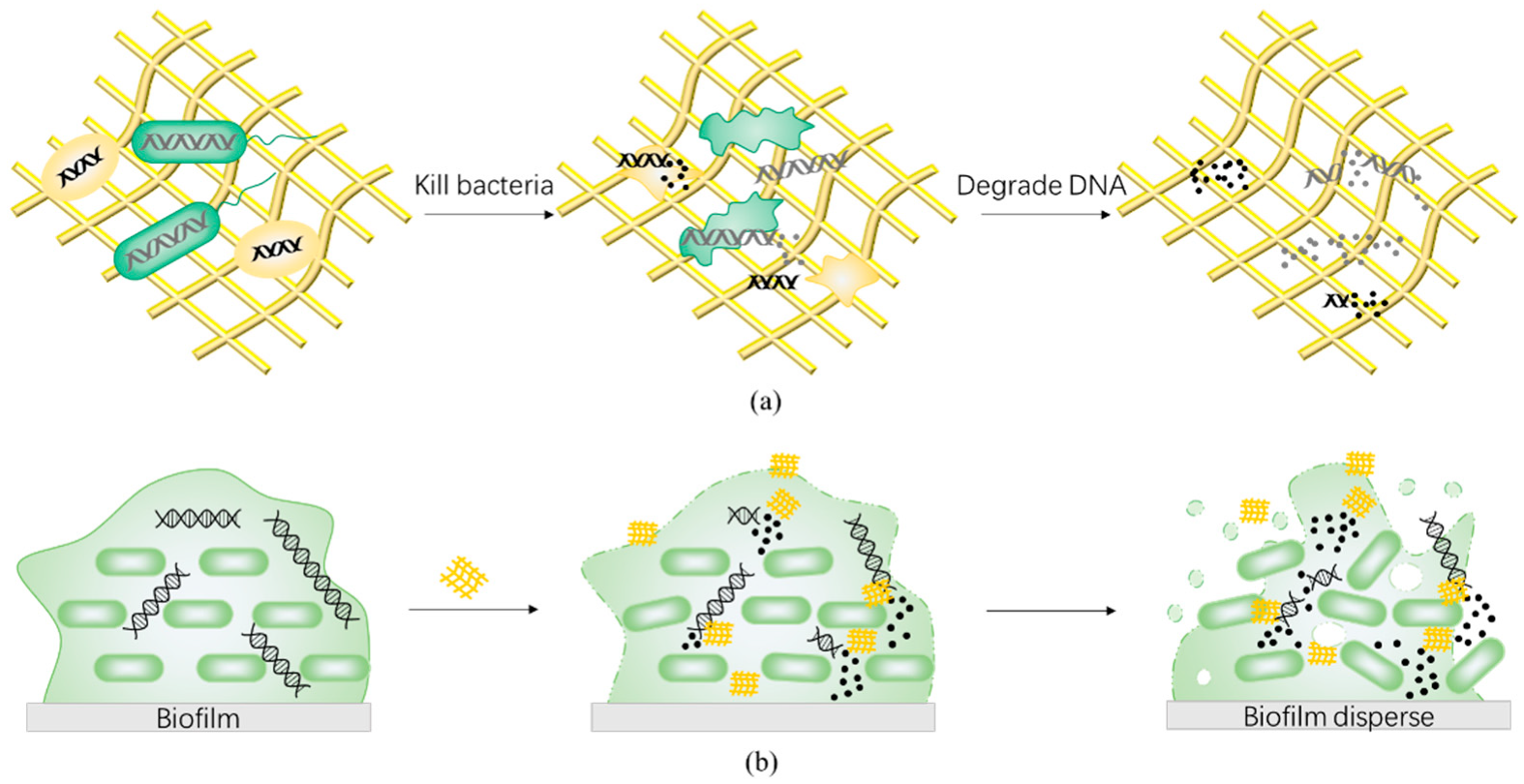
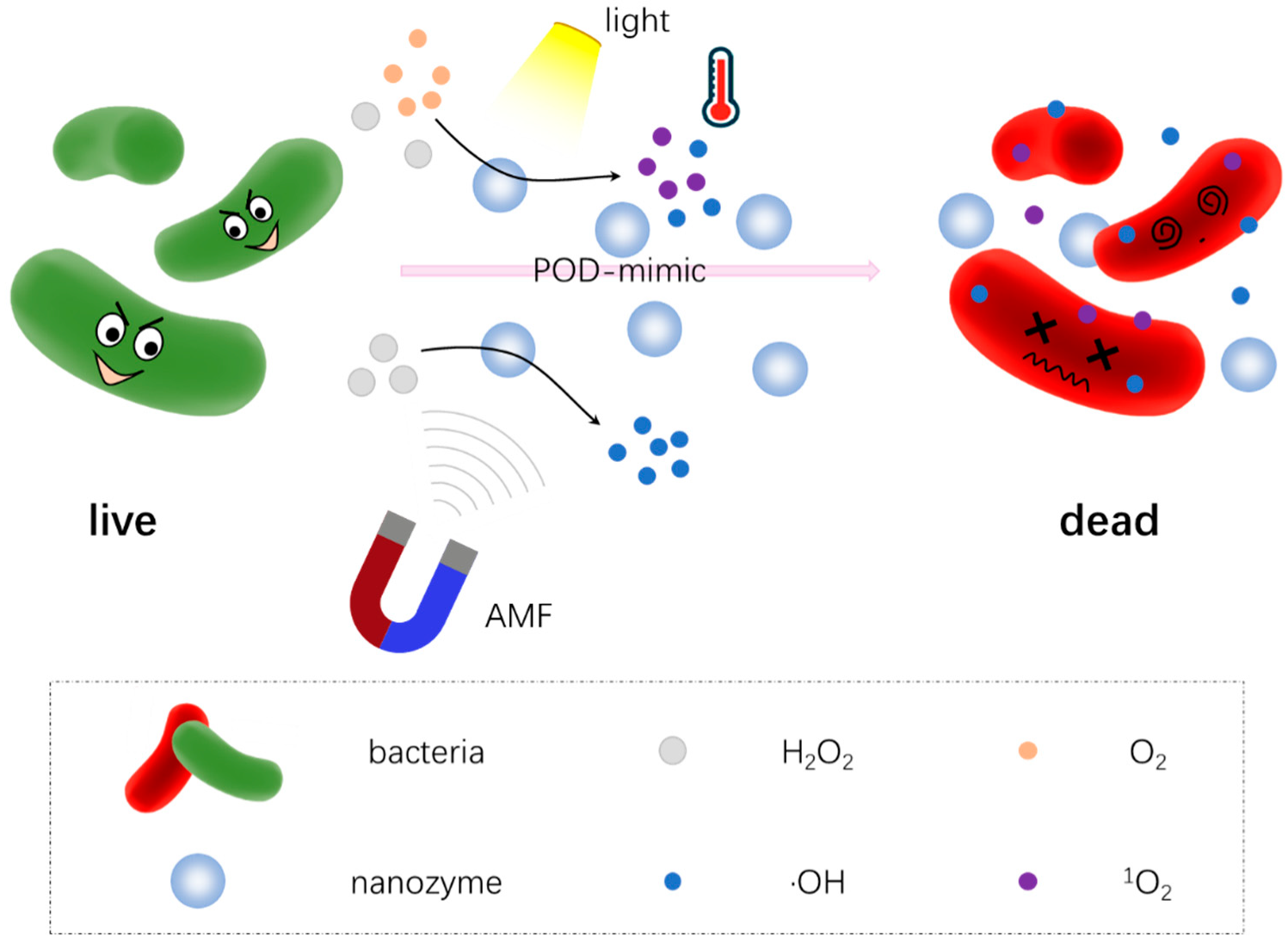
| Nanozybiotics | Enzyme Like Activity | Pathogens | Ref |
|---|---|---|---|
| PMCS | Peroxidase | Pseudomonas aeruginosa (P. aeruginosa) | [48] |
| 2D Cu-TCPP(Fe) nanosheets | Peroxidase | E. coli, S. aureus | [49] |
| biohybrid CARs | Peroxidase | Streptococcus mutans UA159 | [50] |
| MoS2-hydrogel | Peroxidase | E. coli, S. aureus | [51] |
| AA@Ru@HA-MoS2 | Peroxidase | MDR S. aureus, P. aeruginosa | [52] |
| SAF NCs | Peroxidase | E. coli, S. aureus | [53] |
| hydrogel-based artificial enzyme | Peroxidase | Drug-resistant (DR) S. aureus, DR-E. coli | [54] |
| IONzymes | Peroxidase | Streptococcus mutans (S. mutans) | [55] |
| Fe3C/N-C | Peroxidase | E. coli, S. aureus | [56] |
| N-MoS2, N-WS2 NSs | Peroxidase | ampicillin resistant Escherichia coli (AmprE. coli), endospore-forming Bacillus subtilis (B. subtilis) | [57] |
| N-SCSs | Peroxidase | MDR S. aureus, E. coli | [58] |
| UsAuNPs/MOFs | Peroxidase | E. coli, S. aureus | [59] |
| FNPs | Peroxidase | Helicobacter pylori (H. pylori) | [60] |
| CuMnO2 NFs | Peroxidase | E. coli, S. aureus | [61] |
| AIronNPs | Peroxidase | E. coli, S. aureus | [62] |
| NH2-MIL-88B(Fe)-Ag | Peroxidase | E. coli, S. aureus | [63] |
| IrNPs | Peroxidase | E. coli | [64] |
| CDs@PtNPs | Peroxidase | MRSA | [65] |
| Fe3O4@MoS2-Ag | Peroxidase | E. coli, S. aureus, Bacillus subtilis (B. subtili), MRSA, Candida albicans (C. albicans) | [66] |
| Au-BNNs, Ag-BNNs | Peroxidase | E. coli, S. aureus | [67] |
| oxygenated nanodiamonds (O-NDs) | Peroxidase | Fusobacterium nucleatum (F. nucleatum), Porphyromonas gingivalis (P. gingivalis), S. sanguis | [68] |
| Dex-IONP | Peroxidase | S. mutans | [69] |
| MoO3−x NDs | Peroxidase | MRSA, ESBL-producing E.coli | [70] |
| MTex | Peroxidase | E. coli, S. aureus | [71] |
| Au/MnFe2O4 | Peroxidase | S. aureus, B. subtilis, E. faecalis, S. pyogenes | [72] |
| AuNPTs | Peroxidase | MRSA, E. coli, S. aureus | [73] |
| rough C–Fe3O4 | Peroxidase | MRSA, E. coli, S. aureus | [12] |
| Cu-PBG | Peroxidase | E. coli, S. aureus | [74] |
| PdFe/GDY | Peroxidase | E. coli, S. aureus | [75] |
| ultrasmall TA-Ag nanozyme | Peroxidase | E. coli, Staphylococcus epidermidis (S. epidermidis) | [76] |
| Cu-SA@BCNW/PNI | Peroxidase | E. coli, S. aureus | [77] |
| PEG@Zn/Pt–CN | Peroxidase | E. coli, S. aureus | [78] |
| Fe-N-C SAzyme | Peroxidase | E. coli, S. aureus | [79] |
| SA-Pt/g-C3N4-K | Peroxidase | E. coli, S. aureus, Bacillus cereus, P. aeruginosa | [80] |
| PDA/Fe3O4 | Peroxidase | E. coli, S. aureus | [81] |
| CuFeSe2 | Peroxidase | S. aureus | [82] |
| pFe3O4@GOx | Peroxidase | E. coli, S. aureus | [83] |
| Cu SASs/NPC | Peroxidase | E. coli, MRSA | [84] |
| QCS-RuO2@RBT | Peroxidase | P. aeruginosa | [85] |
| FerIONP | Peroxidase | S. mutans | [86] |
| w-SiO2/CuO | Peroxidase | E. coli | [87] |
| PdCu-Urchin | Peroxidase | E. coli, S. aureus | [88] |
| Au@Cu2−xS NPs | Peroxidase | E. faecalis, Fusobacterium nucleus | [89] |
| Cu2WS4 nanocrystals (CWS NCs) | Peroxidase, oxidase | MDR S. aureus, E. coli | [90] |
| 3CoV-400 | Peroxidase, oxidase | E. coli, Bacillus algicola, Staphylococcus sciuri (S. sciuri), Vibrio harveyi, Pseudoalteromonas | [91] |
| VOxNDs | Peroxidase, oxidase | E. coli, S. aureus | [92] |
| GO NSs, CuS/GO NC | Peroxidase, oxidase | E. coli, S. aureus, MRSA | [93] |
| Co4S3/Co3O4 NTs | Peroxidase, oxidase | E. coli, S. sciuri | [94] |
| Cu2MoS4 NPs | Peroxidase, oxidase | MDR E. coli, MDR S. aureus | [95] |
| WS2QDs | Peroxidase, oxidase | Mu50 (a vancomycin-intermediate Staphylococcus aureus reference strain), E.coli | [96] |
| Pd@NPs | Peroxidase, oxidase | S. aureus, P. aeruginosa | [97] |
| NiCo2O4-Au | Peroxidase, oxidase | E. coli, S. aureus | [98] |
| CS-Cu-GA NCs | Peroxidase, oxidase | E. coli, S. aureus | [99] |
| MSPLNP-Au-CB | Peroxidase, oxidase | Helicobacter pylori (H. pylori), MRSA | [100] |
| CSG-MX | Peroxidase, oxidase | E. coli, S. aureus | [13] |
| Cu2−xS | Peroxidase, oxidase | AmprE. coli 1, S. aureus | [101] |
| CuO NPs/AA | Peroxidase, oxidase | E. coli, S. aureus | [102] |
| HvCuO@GOx | Peroxidase, catalase | E. coli, S. aureus, E. coli with streptomycin resistance (SR-E. coli) | [103] |
| FePN SAzyme | Peroxidase, catalase | E. coli, S. aureus | [104] |
| Au-Au/IrO2@Cu (PABA) | Peroxidase, glucose oxidase (GOx) | E. coli, S. aureus | [105] |
| Ti3C2 MXene/MoS2 (MM) 2D bio-heterojunctions | Peroxidase, glutathione oxidase | E. coli, S. aureus | [106] |
| MoS2/rGO VHS | Peroxidase, oxidase, catalase | E. coli, S. aureus | [107] |
| Cu-HCSs, CuO-HCSs | Peroxidase, catalase, superoxide dismutase | Cu-HCSs: Gram-positive and negative bacteria (S. aureus, S. typhimurium, E. coli, P. aeruginosa) CuO-HCSs: Gram-negative bacteria (S. typhimurium, E. coli, P. aeruginosa) | [108] |
| CNT@MoS2 NSs | Peroxidase, superoxide, catalase | E. coli, S. aureus | [109] |
| MoS2-PDA nanozyme composite hydrogel (MPH) | Peroxidase, catalase, superoxide dismutase | E. coli, S. aureus | [15] |
| Tb4O7 NPs | Oxidase | S. aureus, E. coli | [110] |
| Pd@Ir octahedra (or cubes) | Oxidase | E. coli, S. aureus, Bacillus subtilis, Salmonella enteritidis | [111] |
| Co4S3/Co(OH)2 HNTs | Oxidase | E. coli, P. aeruginosa, S. sciuri, Bacillus | [112] |
| Mn/Ni(OH)x LDHs | Oxidase | E. coli, S. aureus | [113] |
| SPB NCPs | Oxidase | S. aureus, P. aeruginosa | [114] |
| AgPd0.38 | Oxidase | S. aureus, B. subtilis, E. coli, P. aeruginosa, MRSA | [115] |
| PtCo@Graphene | Oxidase | H. pylori | [116] |
| MoS2/TiO2 NFs | Oxidase | E. coli, S. aureus | [117] |
| Cu3/ND@G | Oxidase | E. coli | [14] |
| Pd@Pt-T790 | Catalase | MRSA | [118] |
| DMAE | DNase | S. aureus | [16] |
| PIL-Ce | DNase | E. coli, S. aureus, MRSA | [17] |
| CeO2−x nanorods | Haloperoxidase | E. coli | [119] |
| Ce1−xBixO2−δ | Haloperoxidase | P. aeruginosa, Phaeobacter gallaeciensis | [120] |
| Cu-HCSs | Nuclease/protease | MRSA | [121] |
| Enzybiotics | Nanozybiotics | |
|---|---|---|
| Derivation | natural enzymes | nanozymes (nanomaterials) |
| Catalytic activity | peptidoglycan hydrolases, proteases, and nuclease | peroxidase, oxidase, catalase, deoxyribonuclease |
| Main antibacterial mechanism | destroy bacterial cell structure | catalyze the production of ROS |
| Application advantages | rapid and unique mode of action, high specificity of kill pathogens, low probability for bacterial resistance development and a proteinaceous nature | economical, stable, with catalytic function without additional modification, easy to integrate a variety of antibacterial strategies |
| Application disadvantages | environmentally sensitive and unstable, high cost, short half-life and immunogenicity of proteins | low enzyme activity, limited types of enzyme catalysis and complicated toxicological profile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Wang, Q.; Jiang, J.; Gao, L. Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics 2022, 11, 390. https://doi.org/10.3390/antibiotics11030390
Zhou C, Wang Q, Jiang J, Gao L. Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics. 2022; 11(3):390. https://doi.org/10.3390/antibiotics11030390
Chicago/Turabian StyleZhou, Caiyu, Qian Wang, Jing Jiang, and Lizeng Gao. 2022. "Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance" Antibiotics 11, no. 3: 390. https://doi.org/10.3390/antibiotics11030390
APA StyleZhou, C., Wang, Q., Jiang, J., & Gao, L. (2022). Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics, 11(3), 390. https://doi.org/10.3390/antibiotics11030390






