Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes
Abstract
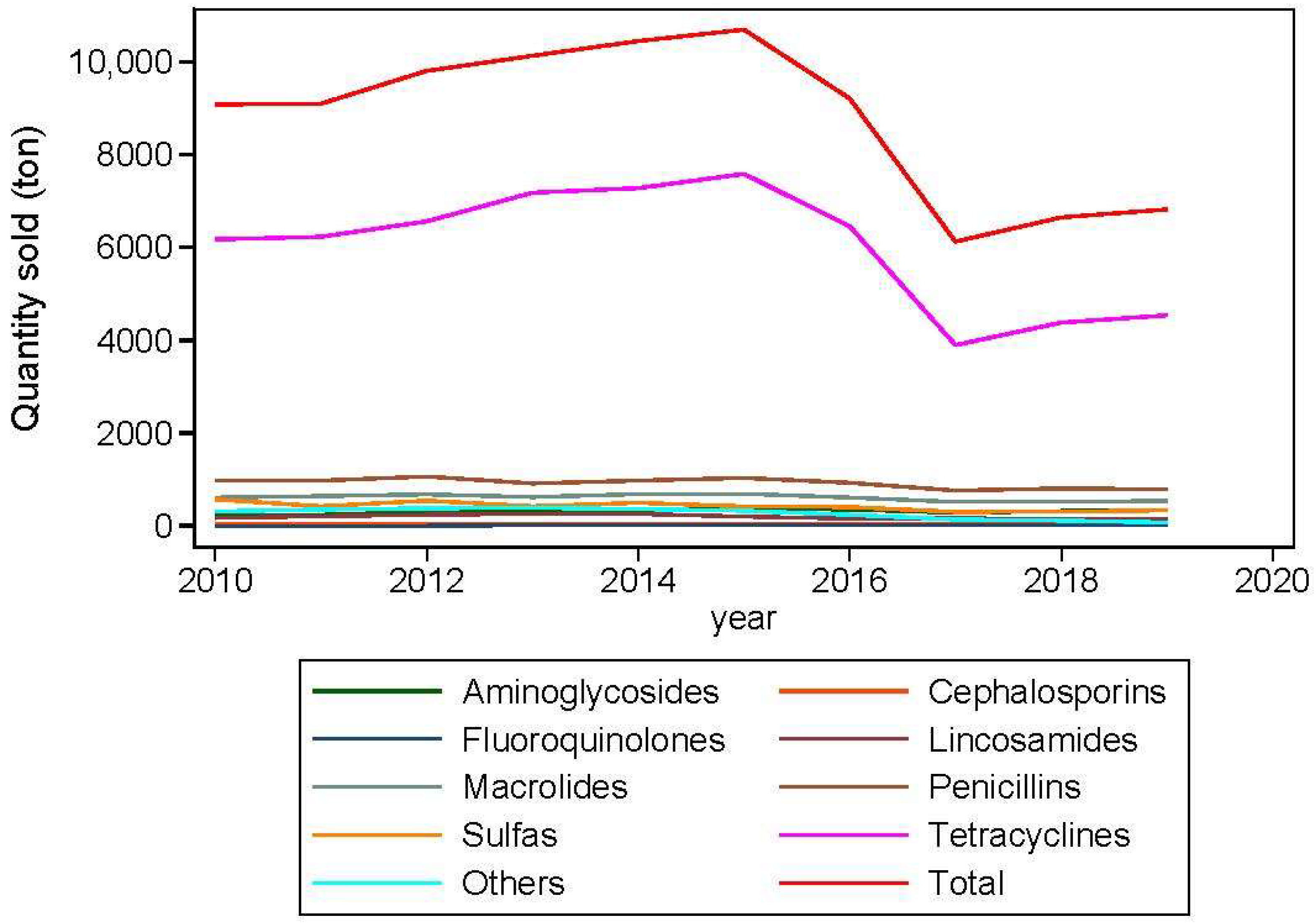
1. Introduction
2. Brief Description of Animal Manure Management Systems
2.1. Lagoons
2.2. Anaerobic Digestion
2.3. Composting
3. Effects of Animal Manure Treatments on the Removal of Tetracycline Resistance Genes
3.1. Impacts of Lagoons on Tetracycline Resistance Genes
3.2. Effect of Anaerobic Digestion on Tetracycline Resistance Genes
3.3. Impact of Composting on the Removal of tet Genes from Animal Manure
Composting as Post Anaerobic Digestion Treatment
4. Conclusions
5. Research Gaps and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henchion, M.; McCarthy, M.; Resconi, V.C.; Troy, D. Meat consumption: Trends and quality matters. Meat Sci. 2014, 98, 561–568. [Google Scholar] [CrossRef]
- Pagliari, P.H.; He, Z.; Waldrip, H.M. Preface; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2019. [Google Scholar]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- FAO. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Van Epps, A.; Blaney, L. Antibiotic residues in animal waste: Occurrence and degradation in conventional agricultural waste management practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Call, D.R.; Davis, M.A.; Sawant, A.A. Antimicrobial resistance in beef and dairy cattle production. Anim. Health Res. Rev. 2008, 9, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Kasumba, J.; Appala, K.; Agga, G.E.; Loughrin, J.H.; Conte, E.D. Anaerobic digestion of livestock and poultry manures spiked with tetracycline antibiotics. J. Environ. Sci. Health B 2020, 55, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.N.; Gaston, J.M.; Dai, C.L.; Zhao, S.; Poyet, M.; Groussin, M.; Yin, X.; Li, L.G.; van Loosdrecht, M.C.M.; Topp, E.; et al. An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat. Commun. 2021, 12, 4765. [Google Scholar] [CrossRef]
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and antibiotic resistance in agroecosystems: State of the science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef]
- Agga, G.E.; Kasumba, J.; Loughrin, J.H.; Conte, E.D. Anaerobic Digestion of Tetracycline Spiked Livestock Manure and Poultry Litter Increased the Abundances of Antibiotic and Heavy Metal Resistance Genes. Front. Microbiol. 2020, 11, 614424. [Google Scholar] [CrossRef]
- Keenum, I.; Williams, R.K.; Ray, P.; Garner, E.D.; Knowlton, K.F.; Pruden, A. Combined effects of composting and antibiotic administration on cattle manure–borne antibiotic resistance genes. Microbiome 2021, 9, 81. [Google Scholar] [CrossRef]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef]
- Seltenrich, N. Dust Emissions from Cattle Feed Yards: A Source of Antibiotic Resistance? Environ. Health Perspect. 2015, 123, A96. [Google Scholar] [CrossRef]
- Koluman, A.; Dikici, A. Antimicrobial resistance of emerging foodborne pathogens: Status quo and global trends. Crit. Rev. Microbiol. 2013, 39, 57–69. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry #213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209; Center for Veterinary Medicine, Food and Drug Admisitration (FDA), U.S. Department of Health and Human Services: Rockville, MD, USA, 2013. [Google Scholar]
- FDA. Guidance for Industry # 152: Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern; U.S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Veterinary Medicine: Rockville, MD, USA, 2003. [Google Scholar]
- FDA. 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; Center for Veterinary Medicine, U.S. Food and Drug Adminstration (FDA), Department of Health and Human Services: Rockville, MD, USA, 2020. [Google Scholar]
- DANMAP. DANMAP 2019-Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; Statens Serum Institut, National Food Institute, Technical University of Denmark: Kongens Lyngby, Denmark, 2020; ISSN 1600-2032. [Google Scholar]
- Janni, K.; Cortus, E. Common animal production systems and manure storage methods. In Animal Manure; Waldrip, H., Pagliari, P., He, Z., Eds.; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 2020; pp. 27–43. [Google Scholar]
- Agga, G.E.; Arthur, T.M.; Durso, L.M.; Harhay, D.M.; Schmidt, J.W. Antimicrobial-resistant bacterial populations and antimicrobial resistance genes obtained from environments impacted by livestock and municipal waste. PLoS ONE 2015, 10, e0132586. [Google Scholar] [CrossRef]
- Gurmessa, B.; Ashworth, A.J.; Yang, Y.; Savin, M.; Moore, P.A., Jr.; Ricke, S.C.; Corti, G.; Pedretti, E.F.; Cocco, S. Variations in bacterial community structure and antimicrobial resistance gene abundance in cattle manure and poultry litter. Environ. Res. 2021, 197, 111011. [Google Scholar] [CrossRef]
- Staley, Z.R.; Woodbury, B.L.; Stromer, B.S.; Schmidt, A.M.; Snow, D.D.; Bartelt-Hunt, S.L.; Wang, B.; Li, X. Stockpiling versus Composting: Effectiveness in Reducing Antibiotic-Resistant Bacteria and Resistance Genes in Beef Cattle Manure. Appl. Environ. Microbiol. 2021, 87, e0075021. [Google Scholar] [CrossRef]
- Berry, E.D.; Woodbury, B.L.; Nienaber, J.A.; Eigenberg, R.A.; Thurston, J.A.; Wells, J.E. Incidence and Persistence of Zoonotic Bacterial and Protozoan Pathogens in a Beef Cattle Feedlot Runoff Control–Vegetative Treatment System. J. Environ. Qual. 2007, 36, 1873–1882. [Google Scholar] [CrossRef]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency); European Surveillance of Veterinary Antimicrobial Consumption. ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018’. (EMA/24309/2020); EMA: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef]
- Yin, X.; M’Ikanatha, N.M.; Nyirabahizi, E.; McDermott, P.F.; Tate, H. Antimicrobial resistance in non-Typhoidal Salmonella from retail poultry meat by antibiotic usage-related production claims—United States, 2008–2017. Int. J. Food Microbiol. 2021, 342, 109044. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.H.; Nyirabahizi, E.; Crarey, E.; Kabera, C.; Lam, C.; Rice-Trujillo, C.; McDermott, P.F.; Tate, H. Prevalence and antimicrobial resistance of Enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microbiol. 2018, 84, e01902-17. [Google Scholar] [CrossRef] [PubMed]
- WHO. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 25 February 2022).
- Roberts, M.C.; Schwarz, S. Tetracycline and phenicol resistance genes and mechanisms: Importance for agriculture, the environment, and humans. J. Environ. Qual. 2016, 45, 576–592. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.W.; Loftin, K.A.; Meyer, M.T.; Davis, J.G.; Pruden, A. Tet and sul Antibiotic Resistance Genes in Livestock Lagoons of Various Operation Type, Configuration, and Antibiotic Occurrence. Environ. Sci. Technol. 2010, 44, 6102–6109. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. NPJ Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Roberts, M.C. Table 1. Mechanism of Resistance for Characterized Tet and Otr Genes. Modified 20 April 2021. Available online: http://faculty.washington.edu/marilynr/ (accessed on 3 March 2022).
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Couch, M.; Agga, G.E.; Kasumba, J.; Parekh, R.R.; Loughrin, J.H.; Conte, E.D. Abundances of Tetracycline Resistance Genes and Tetracycline Antibiotics during Anaerobic Digestion of Swine Waste. J. Environ. Qual. 2019, 48, 171–178. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting To Inactivate Foodborne Pathogens for Crop Soil Application: A Review. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef]
- Miller, J.H.; Novak, J.T.; Knocke, W.R.; Pruden, A. Survival of Antibiotic Resistant Bacteria and Horizontal Gene Transfer Control Antibiotic Resistance Gene Content in Anaerobic Digesters. Front. Microbiol. 2016, 7, 263. [Google Scholar] [CrossRef]
- Diehl, D.L.; LaPara, T.M. Effect of temperature on the fate of genes encoding tetracycline resistance and the integrase of class 1 integrons within anaerobic and aerobic digesters treating municipal wastewater solids. Environ. Sci. Technol. 2010, 44, 9128–9133. [Google Scholar] [CrossRef]
- Ma, Y.; Wilson, C.A.; Novak, J.T.; Riffat, R.; Aynur, S.; Murthy, S.; Pruden, A. Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons. Environ. Sci. Technol. 2011, 45, 7855–7861. [Google Scholar] [CrossRef]
- O’Connor, S.; Ehimen, E.; Pillai, S.C.; Black, A.; Tormey, D.; Bartlett, J. Biogas production from small-scale anaerobic digestion plants on European farms. Renew. Sustain. Energy Rev. 2021, 139, 110580. [Google Scholar] [CrossRef]
- Lovanh, N.; Loughrin, J.H.; Cook, K.; Rothrock, M.; Sistani, K. The effect of stratification and seasonal variability on the profile of an anaerobic swine waste treatment lagoon. Bioresour. Technol. 2009, 100, 3706–3712. [Google Scholar] [CrossRef]
- Heber, A.; Ni, J.Q.; Lim, T. Odor flux measurements at a facultative swine lagoon stratified by surface aeration. Appl. Eng. Agric. 2002, 18, 593. [Google Scholar] [CrossRef][Green Version]
- Westerman, P.; Zhang, R. Aeration of livestock manure slurry and lagoon liquid for odor control: A review. Appl. Eng. Agric. 1997, 13, 245–249. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 2000, 3, 252–256. [Google Scholar] [CrossRef]
- Hamilton, W.D.; Fathepure, B.; Fulhage, D.C.; Clarkson, W.; Lalman, J. Treatment Lagoons for Animal Agriculture; ASABE: St. Joseph, MI, USA, 2006. [Google Scholar]
- USDA-NRCS. Agricultural waste management system component design. In Agricultural Waste Management Field Book; United States Departmnet of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2009; Volume 651, Chapter 10, Part 651. [Google Scholar]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- Koike, S.; Aminov, R.I.; Yannarell, A.C.; Gans, H.D.; Krapac, I.G.; Chee-Sanford, J.C.; Mackie, R.I. Molecular ecology of macrolide-lincosamide-streptogramin B methylases in waste lagoons and subsurface waters associated with swine production. Microb. Ecol. 2010, 59, 487–498. [Google Scholar] [CrossRef]
- Koike, S.; Krapac, I.G.; Oliver, H.D.; Yannarell, A.C.; Chee-Sanford, J.C.; Aminov, R.I.; Mackie, R.I. Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period. Appl. Environ. Microbiol. 2007, 73, 4813–4823. [Google Scholar] [CrossRef]
- Do, Y.S.; Schmidt, T.M.; Zahn, J.A.; Boyd, E.S.; Mora, A.D.L.; DiSpirito, A.A. Role of Rhodobacter sp. Strain PS9, a Purple Non-Sulfur Photosynthetic Bacterium Isolated from an Anaerobic Swine Waste Lagoon, in Odor Remediation. Appl. Environ. Microbiol. 2003, 69, 1710–1720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kyselková, M.; Jirout, J.; Vrchotová, N.; Schmitt, H.; Elhottová, D. Spread of tetracycline resistance genes at a conventional dairy farm. Front. Microbiol. 2015, 6, 536. [Google Scholar] [CrossRef] [PubMed]
- Kyselková, M.; Kotrbová, L.; Bhumibhamon, G.; Chroňáková, A.; Jirout, J.; Vrchotová, N.; Schmitt, H.; Elhottová, D. Tetracycline resistance genes persist in soil amended with cattle feces independently from chlortetracycline selection pressure. Soil Biol. Biochem. 2015, 81, 259–265. [Google Scholar] [CrossRef]
- Congilosi, J.L.; Aga, D.S. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J. Hazard. Mater. 2021, 405, 123634. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.; Antle, S.; Bryant, M.; Berry, Z.; Lovanh, N. Evaluation of Microaeration and Sound to Increase Biogas Production from Poultry Litter. Environments 2020, 7, 62. [Google Scholar] [CrossRef]
- Wilkie, A.C. Anaerobic digestion of dairy manure: Design and process considerations. Dairy Manure Manag. Treat. Handl. Community Relat. 2005, 301, 301–312. [Google Scholar]
- Harrison, J.H.; Ndegwa, P.M. Anaerobic digestion of dairy and swine waste. In Animal Manure; Waldrip, H., Pagliari, P., He, Z., Eds.; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 2020; pp. 115–127. [Google Scholar]
- AgSTAR. Livestock Anaerobic Digester Database. Available online: https://www.epa.gov/agstar/livestock-anaerobic-digester-database (accessed on 4 November 2021).
- Lansing, S.; Víquez, J.; Martínez, H.; Botero, R.; Martin, J. Quantifying electricity generation and waste transformations in a low-cost, plug-flow anaerobic digestion system. Ecol. Eng. 2008, 34, 332–348. [Google Scholar] [CrossRef]
- Chen, L.; Neibling, H. Anaerobic Digestion Basics; University of Idaho Extension: Moscow, ID, USA, 2014; Volume 6. [Google Scholar]
- Bernal, M.P.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Youngquist, C.P.; Mitchell, S.M.; Cogger, C.G. Fate of antibiotics and antibiotic resistance during digestion and composting: A review. J. Environ. Qual. 2016, 45, 537–545. [Google Scholar] [CrossRef]
- FDA. Produce Safety Rule. 21 CFR §§112; US Food and Drug Administration: Rockville, MD, USA, 2020. [Google Scholar]
- Jindal, A.; Kocherginskaya, S.; Mehboob, A.; Robert, M.; Mackie, R.I.; Raskin, L.; Zilles, J.L. Antimicrobial use and resistance in swine waste treatment systems. Appl. Environ. Microbiol. 2006, 72, 7813–7820. [Google Scholar] [CrossRef]
- Wallace, J.S.; Garner, E.; Pruden, A.; Aga, D.S. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ. Pollut. 2018, 236, 764–772. [Google Scholar] [CrossRef]
- Barkovskii, A.; Manoylov, K.; Bridges, C. Positive and negative selection towards tetracycline resistance genes in manure treatment lagoons. J. Appl. Microbiol. 2012, 112, 907–919. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Parker, D.B.; Snow, D.D.; Zhou, Z.; Li, X. Occurrence of antimicrobials and antimicrobial resistance genes in beef cattle storage ponds and swine treatment lagoons. Sci. Total Environ. 2013, 463, 631–638. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Aminov, R.I.; Krapac, I.J.; Garrigues-Jeanjean, N.; Mackie, R.I. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 2001, 67, 1494–1502. [Google Scholar] [CrossRef]
- Chen, J.; Michel, F.C.; Sreevatsan, S.; Morrison, M.; Yu, Z. Occurrence and Persistence of Erythromycin Resistance Genes (erm) and Tetracycline Resistance Genes (tet) in Waste Treatment Systems on Swine Farms. Microb. Ecol. 2010, 60, 479–486. [Google Scholar] [CrossRef]
- Smith, M.S.; Yang, R.K.; Knapp, C.W.; Niu, Y.; Peak, N.; Hanfelt, M.M.; Galland, J.C.; Graham, D.W. Quantification of tetracycline resistance genes in feedlot lagoons by real-time PCR. Appl. Environ. Microbiol. 2004, 70, 7372–7377. [Google Scholar] [CrossRef]
- Peak, N.; Knapp, C.W.; Yang, R.K.; Hanfelt, M.M.; Smith, M.S.; Aga, D.S.; Graham, D.W. Abundance of six tetracycline resistance genes in wastewater lagoons at cattle feedlots with different antibiotic use strategies. Environ. Microbiol. 2007, 9, 143–151. [Google Scholar] [CrossRef]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure anaerobic digestion effects and the role of pre-and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef]
- Burch, T.R.; Firnstahl, A.D.; Spencer, S.K.; Larson, R.A.; Borchardt, M.A. Fate and Seasonality of Antimicrobial Resistance Genes During Full-scale Anaerobic Digestion of Manure across Seven Livestock Production Facilities. J. Environ. Qual. 2022; in press. [Google Scholar]
- Ghosh, S.; Ramsden, S.J.; LaPara, T.M. The role of anaerobic digestion in controlling the release of tetracycline resistance genes and class 1 integrons from municipal wastewater treatment plants. Appl. Microbiol. Biotechnol. 2009, 84, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gu, J.; Wang, X.; Qian, X.; Peng, H. Solid-state anaerobic digestion facilitates the removal of antibiotic resistance genes and mobile genetic elements from cattle manure. Bioresour. Technol. 2019, 274, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Scott, A.; Tien, Y.C.; Murray, R.; Boerlin, P.; Pearl, D.L.; Liu, K.; Robertson, J.; Nash, J.H.E.; Topp, E. On-Farm Anaerobic Digestion of Dairy Manure Reduces the Abundance of Antibiotic Resistance-Associated Gene Targets and the Potential for Plasmid Transfer. Appl. Environ. Microbiol. 2021, 87, e0298020. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.S.; Aga, D.S. Enhancing Extraction and Detection of Veterinary Antibiotics in Solid and Liquid Fractions of Manure. J. Environ. Qual. 2016, 45, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Esperón, F.; Albero, B.; Ugarte-Ruíz, M.; Domínguez, L.; Carballo, M.; Tadeo, J.L.; del Mar Delgado, M.; Moreno, M.Á.; de la Torre, A. Assessing the benefits of composting poultry manure in reducing antimicrobial residues, pathogenic bacteria, and antimicrobial resistance genes: A field-scale study. Environ. Sci. Pollut. Res. 2020, 27, 27738–27749. [Google Scholar] [CrossRef] [PubMed]
- Gurmessa, B.; Milanovic, V.; Pedretti, E.F.; Corti, G.; Ashworth, A.J.; Aquilanti, L.; Ferrocino, I.; Corvaglia, M.R.; Cocco, S. Post-digestate composting shifts microbial composition and degrades antimicrobial resistance genes. Bioresour. Technol. 2021, 340, 125662. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A. Quantitative human health risk assessments of antimicrobial use in animals and selection of resistance: A review of publicly available reports. Rev. Sci. Tech. 2012, 31, 261–276. [Google Scholar] [CrossRef] [PubMed]
| Efflux (36) | Ribosomal Protection (13) | Enzymatic Degradation (13) | Mosaic Ribosomal Protection (11) | Unknown |
|---|---|---|---|---|
| tet(A), tet(B), tet(C), tet(D), tet(E), tet(59) tet(G), tet(H), tet(J), tet(V), tet(Y) tet(Z), tet(30), tet(31), tet(33), tet(57) tet(35) tet(39), tet(41) tet(K), tet(L), tet(38), tet(45), tet(58), tet(63) tetA(P), tet(40) otr(B), otr(C) tcr3 tet(42) tet(43) tetAB(46) tetAB(60) tet(62) tet(64) | tet(M), tet(O), tet(S), tet(W), tet(32) tet(Q), tet(T), tet(36), tet(61) otr(A), tetB(P), tet tet(44) | tet(X) tet(37) tet(34) tet(47), tet(48), tet(49), tet(50) tet(51), tet(52), tet(53), tet(54) tet(55), tet(56) | tet(O/32/O), tet(O/W/32/O), tet(O/32/O) tet(O/W/32/O/W/O), tet(W/32/O), tet(O/W) tet(W/32/O/W/O), tet(O/W/O), tet(O/W/32/O) tet(S/M), tet(W/N/W) | tet(U) |
| Reference | tet Genes Targeted | Animal Operation | Conclusions |
|---|---|---|---|
| [68] | M, O, Q, W, A, C, H, Z | Swine | No substantial difference. M, O, Q, and W were detected at 100% from all building and lagoon samples tested at all farms. tet(A) was detected only from one building sample at one farm. tet(C) was detected from building samples at two farms, and lagoon samples at one farm; lagoon samples showed a 20% lower prevalence than building samples for tet(C). H and Z were detected from all tested samples; H showed a 20% lower prevalence in one farm, while Z showed a 30% higher prevalence. |
| [52] | M, O, Q, W, C, H, Z | Swine | All seven genes were detected at 100% prevalence from all lagoon samples and six sampling dates at the two swine farms. Concentrations fluctuated during the three-year monitoring period, with an average concentration of 1.42 × 104 copies per 106 16S rRNA copies. |
| [22] | A, B | Swine | Occurred at 92% (tet(A)) and 75% (tet(B)) prevalence from pooled samples |
| [33] | O, W | Chicken layer, dairy cattle, beef cattle, swine | The tet genes tended to decrease in concentration as the animal waste effluents passed through multiple treatment lagoons, ranging from 0–1 log reduction depending on animal spp. However, no complete removal. |
| [69] | O, W | Dairy cattle | 8.3 and 8.9 log10 copies/mL, respectively |
| [70] | 16 genes | Swine | Three genes (G, M, X) persisted and amplified ~100–1000 fold; two genes (B, L) were attenuated in the lagoons. Others were similar between feces and lagoon samples. |
| [75] | M, O, Q, W, B, L | Cattle feedlot | 2.8 × 106 copies/mL high use lagoons; 7.3 × 105 copies/mL in mixed use lagoons; 5.1 × 103 copies/mL in no-use lagoons |
| [73] | G | Swine | 1.1 log reduction in the covered lagoon; post-treatment resulted in 3.4 log reduction |
| [71] | O, Q, X | Feedlot cattle, swine | The average relative abundance of ARGs ranged from 5.5 × 10−6 to 6.3 × 10−1 copies per 16S rRNA gene. |
| [72] | W, O, Q, M, S, T, B, otr(A) | Swine | All genes were detected from the lagoon samples |
| [74] | O, W, Q | Feedlot cattle | Concentration ranged from 2.8–4.3 logs/50 µL |
| Genes | Change in Abundance | Type of Digestion | Manure Type | Reference |
|---|---|---|---|---|
| tet(G) | No change | Mesophilic | Swine | [73] |
| tet(O) | No change | Advanced mesophilic after pre-digestion pasteurization | Dairy cattle | [69] |
| tet(W) | ||||
| tet(A) | Decreased by 0.7 log10 | Mesophilic | Dairy cattle | [77] |
| tet(W) | No change |
| Gene | Manure Type | Bulking Agent | Compost Type | Composting Duration | Change | Reference |
|---|---|---|---|---|---|---|
| tet(A), tet(B), tet(K), tet(M), tet(Q), tet(S), tet(W) | Poultry litter | Barley straw | Windrow, turned once/week | 10 weeks | Decreased (by 2.5 logs on average) | [82] |
| tet(Y) | Increased (by 0.7 logs) | |||||
| tet(W) | Dairy manure | Alfalfa hay, pine bark mulch, and sawdust | Static composting | 42 days | Decreased (1–2 log reduction) | [13] |
| Feedlot cattle | Turned composting, turned daily | |||||
| tet(O), tet(Q) | Feedlot cattle | Ground corn stalks | Turned after days 49 and 112 for the winter-spring cycle; no turning for the summer-fall cycle | 140 days | Up to 2 log reduction | [24] |
| tet(K), tet(M), tet(O) and tet(S) | Solid poultry litter digestate | Alone or with co- composting materials | Turned weekly, biweekly, and trice weekly at each composting stage | 90 days | >80% reduction | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agga, G.E.; Couch, M.; Parekh, R.R.; Mahmoudi, F.; Appala, K.; Kasumba, J.; Loughrin, J.H.; Conte, E.D. Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes. Antibiotics 2022, 11, 391. https://doi.org/10.3390/antibiotics11030391
Agga GE, Couch M, Parekh RR, Mahmoudi F, Appala K, Kasumba J, Loughrin JH, Conte ED. Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes. Antibiotics. 2022; 11(3):391. https://doi.org/10.3390/antibiotics11030391
Chicago/Turabian StyleAgga, Getahun E., Melanie Couch, Rohan R. Parekh, Faranak Mahmoudi, Keerthi Appala, John Kasumba, John H. Loughrin, and Eric D. Conte. 2022. "Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes" Antibiotics 11, no. 3: 391. https://doi.org/10.3390/antibiotics11030391
APA StyleAgga, G. E., Couch, M., Parekh, R. R., Mahmoudi, F., Appala, K., Kasumba, J., Loughrin, J. H., & Conte, E. D. (2022). Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes. Antibiotics, 11(3), 391. https://doi.org/10.3390/antibiotics11030391







