Investigation of the Quality of the 12 Most-Used Antibiotics Available in Retail Private Pharmacies in Rwanda
Abstract
:1. Introduction
2. Results
2.1. Overview of Analyzed Antibiotics
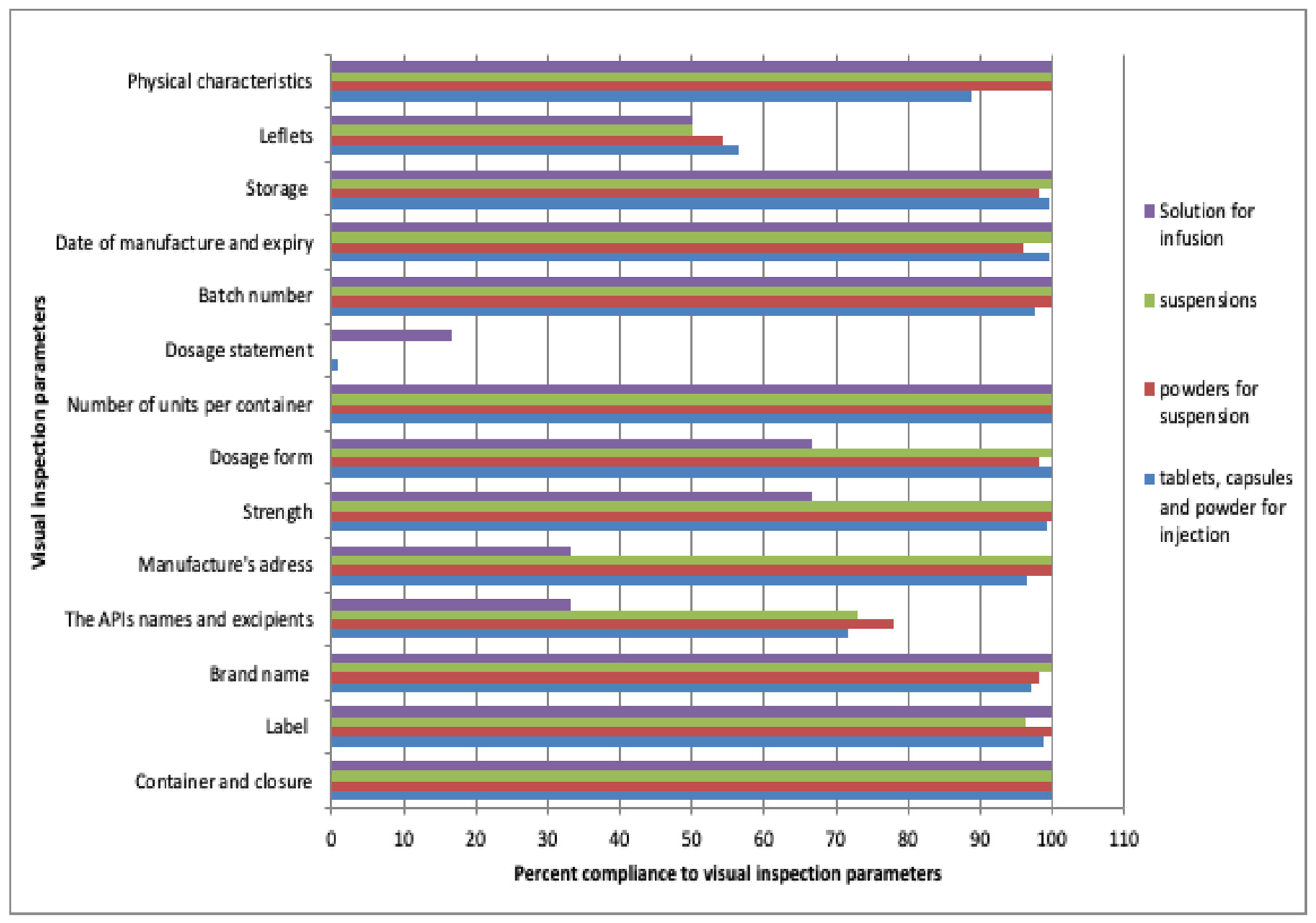
2.2. Compliance with Visual Inspection Parameters of 12 Antibiotics
2.2.1. Summary Percent Compliance of Pharmaceutical Dosage Forms to Visual Inspection Parameters
2.2.2. Percentage Compliance of Antibiotics to Visual Inspection Parameters
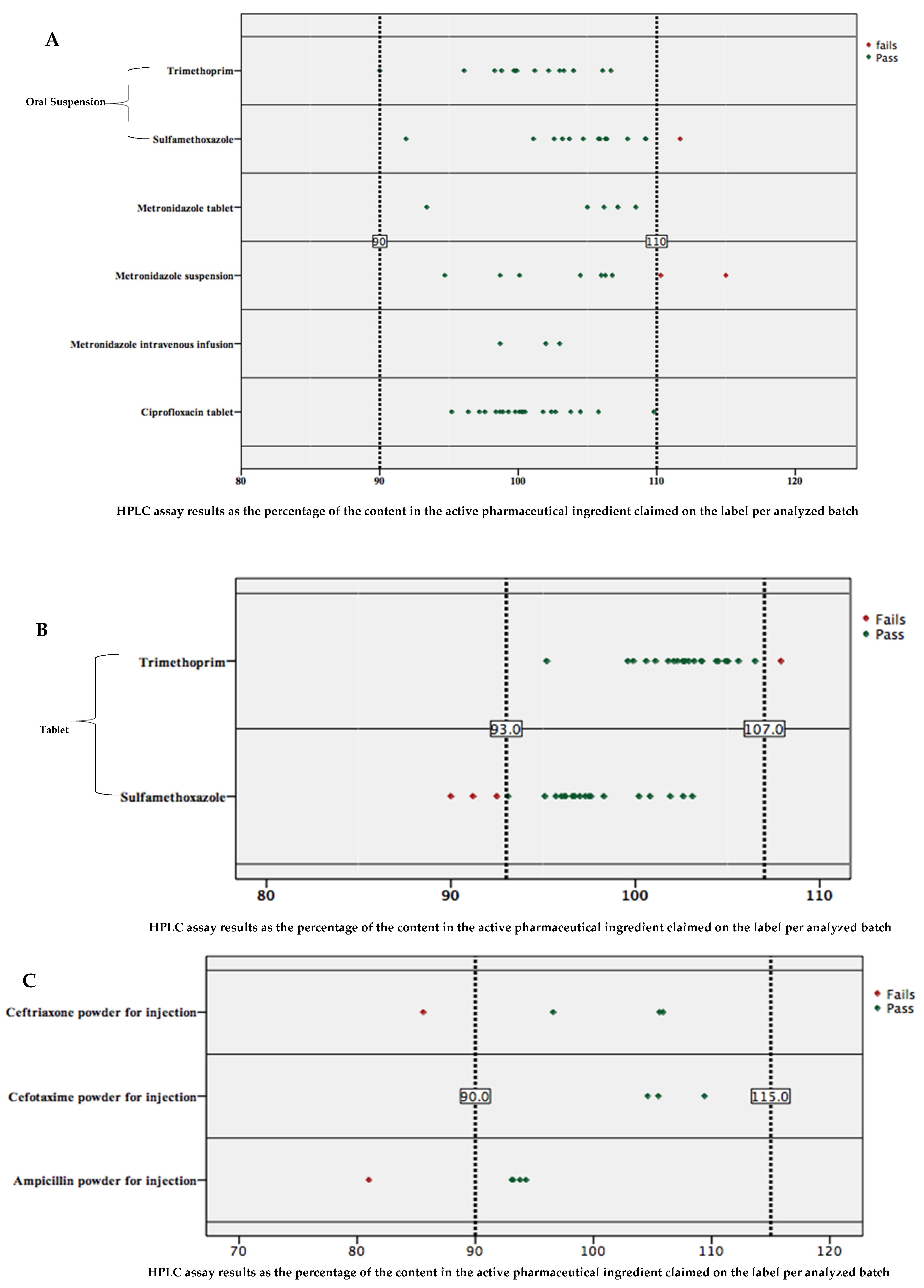
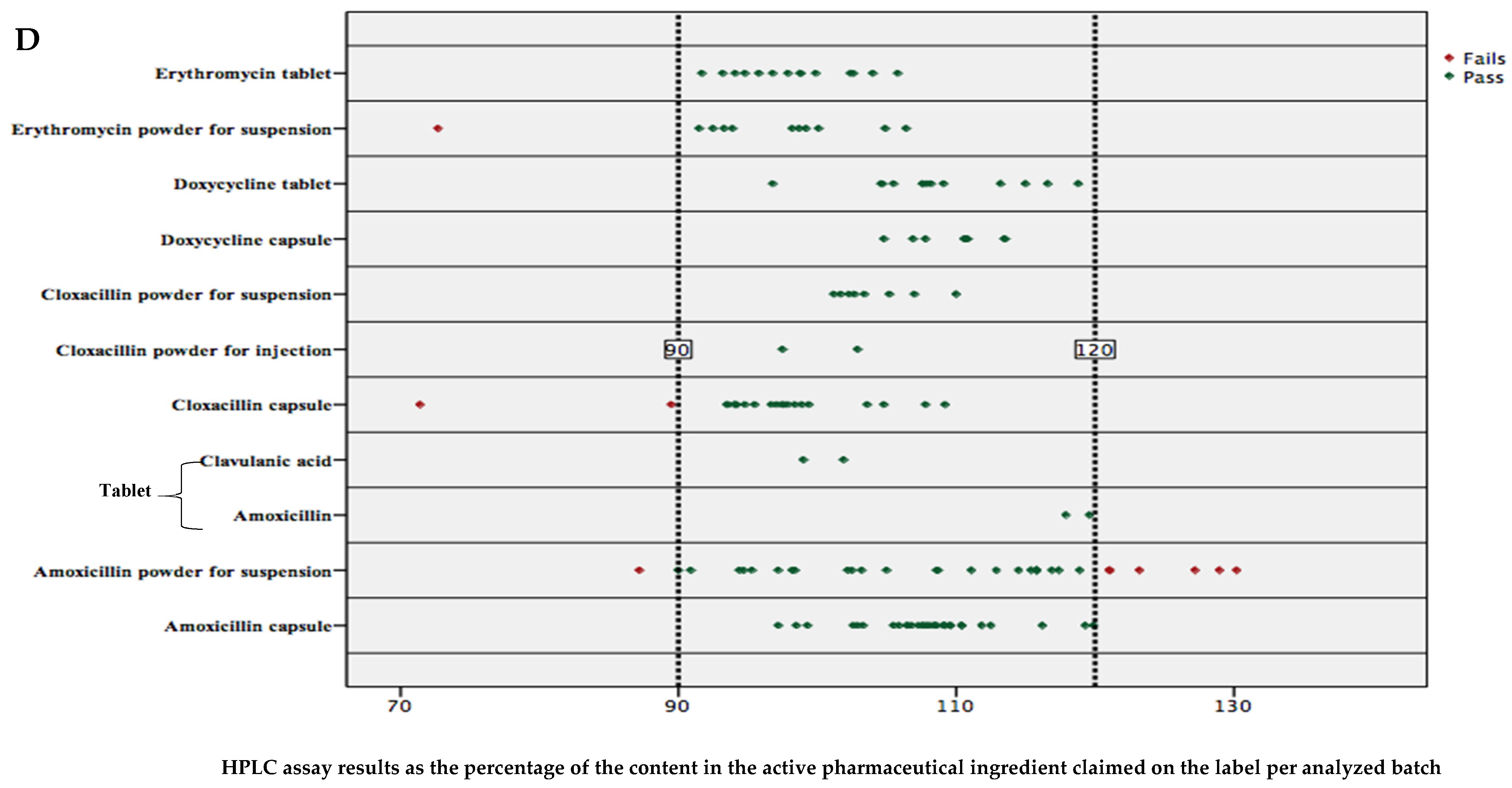
2.3. Percent Content in Active Pharmaceutical Ingredients of Analyzed Antibiotics
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Samples Collection
4.3. Visual Inspection
4.4. HPLC Analysis
4.4.1. Instrumentation and Chemicals
4.4.2. Samples Preparation and Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Message of the WHO Regional Director for Africa, Dr Matshidiso Moeti, on the Occasion of World Hearing Day 2017. 2017. Available online: https://www.afro.who.int/regional-director/speeches-messages/message-who-regional-director-africa-dr-matshidiso-moeti-3 (accessed on 17 December 2021).
- World Health Organisation. Antibiotic Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 17 December 2021).
- World Health Organization. Factors Contributing to the Emergence of Resistance—The Resistance Phenomenon in Microbes and Infectious Disease Vectors—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK97126/#ch5.s2 (accessed on 17 December 2021).
- World Health Organization. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 17 December 2021).
- Johnston, A.; Holt, D.W. Substandard drugs: A potential crisis for public health. Br. J. Clin. Pharmacol. 2014, 78, 218–243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. Geneva. 2017. Available online: https://www.who.int/medicines/regulation/ssffc/publications/SE-Study_EN_web.pdf (accessed on 17 December 2021).
- Ozawa, S.; Evans, D.R.; Bessias, S.; Haynie, D.G.; Yemeke, T.T.; Laing, S.K.; Herrington, J.E. Prevalence and Estimated Economic Burden of Substandard and Falsified Medicines in Low- And Middle-Income Countries: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e181662. [Google Scholar] [CrossRef] [PubMed]
- Nwokike, J.; Clark, A.; Nguyen, P.P. Medicines quality assurance to fight antimicrobial resistance. Bull. World Health Organ. 2018, 96, 135–137. [Google Scholar] [CrossRef]
- Nsimba, S.E.D. Problems associated with substandard and counterfeit drugs in developing countries: A review article on global implications of counterfeit drugs in the era of antiretroviral (ARVs) drugs in a free market economy. E. Afr. J. Public Health 2008, 5, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, P.; Swamidoss, I.; Mayxay, M.; Khanthavong, M.; Phonlavong, C.; Vilayhong, C.; Yeuchaixiong, S.; Sichanh, C.; Sengaloundeth, S.; Green, M.D.; et al. A random survey of the prevalence of falsified and substandard antibiotics in the Lao PDR. J. Antimicrob. Chemother. 2019, 74, 2417–2425. [Google Scholar] [CrossRef] [Green Version]
- Newton, P.N.; Tabernero, P.; Dwivedi, P.; Culzoni, M.J.; Monge, M.E.; Swamidoss, I.; Mildenhall, D.; Green, M.D.; Jähnke, R.; de Oliveira, M.D.; et al. Falsified medicines in Africa: All talk, no action. Lancet Glob. Health 2014, 2, e509–e510. [Google Scholar] [CrossRef] [Green Version]
- Glass, B. Counterfeit drugs and medical devices in developing countries. Res. Rep. Trop. Med. 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almuzaini, T.; Choonara, I.; Sammons, H. Substandard and counterfeit medicines: A systematic review of the literature. BMJ Open 2013, 3, e002923. [Google Scholar] [CrossRef]
- Delepierre, A.; Gayot, A.; Carpentier, A. Update on counterfeit antibiotics worldwide; Public health risks. Med. Mal. Infect. 2012, 42, 247–255. [Google Scholar] [CrossRef]
- Schäfermann, S.; Hauk, C.; Wemakor, E.; Neci, R.; Mutombo, G.; Ndze, E.N.; Cletus, T.; Nyaah, F.; Pattinora, M.; Wistuba, D.; et al. Substandard and falsified antibiotics and medicines against noncommunicable diseases in western Cameroon and northeastern Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2020, 103, 894–908. [Google Scholar] [CrossRef]
- Jean-Baptiste, T.; Carpenter, J.F.; Dahl, K.; Derameau, W.; Veillard, R.; Jacquet, J.R.; Osselyn, P.L.; Figueras, A. Substandard quality of the antimicrobials sold in the street markets in haiti. Antibiotics 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Hobeika, E.; Farhat, J.; Saab, J.; Hleihel, W.; Azzi-Achkouty, S.; Sili, G.; Hallit, S.; Salameh, P. Are antibiotics substandard in Lebanon? Quantification of active pharmaceutical ingredients between brand and generics of selected antibiotics. BMC Pharmacol. Toxicol. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Bekoe, S.O.; Ahiabu, M.A.; Orman, E.; Tersbøl, B.P.; Adosraku, R.K.; Hansen, M.; Frimodt-Moller, N.; Styrishave, B. Exposure of consumers to substandard antibiotics from selected authorised and unauthorised medicine sales outlets in Ghana. Trop. Med. Int. Heal. 2020, 25, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.N.; Lee, S.J.; Goodman, C.; Fernández, F.M.; Yeung, S.; Phanouvong, S.; Kaur, H.; Amin, A.A.; Whitty, C.J.; Kokwaro, G.O.; et al. Guidelines for field surveys of the quality of medicines: A proposal. PLoS Med. 2009, 6, e1000052. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia. 2018. Available online: https://online.uspnf.com/uspnf/document/1_GUID-0111DA94-EAAF-4FAE-905E-0C25EE2DDB16_3_en-US (accessed on 10 December 2021).
- Nasr, M.M.; Stanley, C.M. High Performance Liquid Chromatographic Assay of Erythromycin Salts and Esters in Bulk and Pharmaceutical Dosage Forms. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 1147–1160. [Google Scholar] [CrossRef]
- Schäfermann, S.; Wemakor, E.; Hauk, C.; Heide, L. Quality of medicines in southern Togo: Investigation of antibiotics and of medicines for non-communicable diseases from pharmacies and informal vendors. PLoS ONE 2018, 13, e0207911. [Google Scholar] [CrossRef] [PubMed]
- Seitzer, M.; Klapper, S.; Mazigo, H.D.; Holzgrabe, U.; Mueller, A.; Garba, A. Quality and composition of albendazole, mebendazole and praziquantel available in Burkina Faso, Côte d’Ivoire, Ghana and Tanzania. PLoS Negl. Trop. Dis. 2021, 15, e0009038. [Google Scholar] [CrossRef] [PubMed]
- Belew, S.; Suleman, S.; Wynendaele, E.; D’Hondt, M.; Kosgei, A.; Duchateau, L.; De Spiegeleer, B. Quality of Anthelminthic Medicines Available in Jimma Ethiopia. Acta Trop. 2018, 177, 157–163. Available online: https://www.sciencedirect.com/science/article/pii/S0001706X17307751 (accessed on 11 November 2021). [CrossRef]
- Robertson, S.G.; Hehonah, N.T.; Mayaune, R.D.; Glass, B.D. Prevalence of substandard amoxicillin oral dosage forms in the National Capital District of Papua New Guinea. Am. J. Trop. Med. Hyg. 2021, 105, 238–244. [Google Scholar] [CrossRef]
- Fadeyi, I.; Lalani, M.; Mailk, N.; Van Wyk, A.; Kaur, H. Quality of the antibiotics-amoxicillin and co-trimoxazole from Ghana, Nigeria, and the United Kingdom. Am. J. Trop. Med. Hyg. 2015, 92, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Khuluza, F.; Kigera, S.; Heide, L. Low prevalence of substandard and falsified antimalarial and antibiotic medicines in public and faith-based health facilities of southern Malawi. Am. J. Trop. Med. Hyg. 2017, 96, 1124–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, A.; Held, N.; Heide, L. Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab. PLoS ONE 2017, e0184165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuda, M.; Yoshida, N.; Takaoka, T.; Sanada, T.; Rahman, M.S.; Tanimoto, T.; Zin, T.; Kimura, K.; Tsuboi, H. Substandard and falsified medicines in Myanmar. Pharmacy 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Products Regulatory Authority. Guide to Labels and Leaflets of Human Medicines. 2020. Available online: https://www.hpra.ie/docs/default-source/publications-forms/guidance-documents/aut-g0034-guide-to-labels-and-leaflets-of-human-medicines-v21.pdf?sfvrsn=60 (accessed on 11 November 2021).
- Gnamey, J.; Gambogou, B.; Dossou, M.; Anani, K.; Ameyapoh, Y. Contribution of storage conditions of antibiotics in pharmacies on efficacy loss of Amoxicillin and Tetracycline against strains of Escherichia coli and Staphylococcus aureus in the city of Lome. Am. J. Physiol. Biochem. Pharmacol. 2019, 9, 52. [Google Scholar] [CrossRef]
- McManus, D.; Naughton, B.D. A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: A focus on context, prevalence, and quality. BMJ Glob. Health 2020, 5, e002393. [Google Scholar] [CrossRef] [PubMed]
- Government of Rwanda. Law Establishing Rwanda Food and Drug Authority. Off Gaz. 2018, 56–92. Available online: http://www.rwandafda.gov.rw/web/fileadmin/law_rwanda_fda.pdf (accessed on 11 November 2021).
- Rwanda Food and Drug Authority. Rwanda FDA Registered Human Medicinal Products-September 2021. 2021. Available online: https://www.rwandafda.gov.rw/web/products/RWANDAFDA_REGISTERED_HUMAN_MEDICINAL_PRODUCTS_SEPTEMBER_2021.pdf (accessed on 17 December 2021).
- Rwanda Food and Drug Authority. Rwanda Fda Authorized Human Medicinal Product List June 2021. Volume 4. Kigali. 2021. Available online: https://www.rwandafda.gov.rw/web/products/eRWANDA_FDA_AUTHORIZED_HUMAN_MEDICINAL_PRODUCT_LIST-DECEMBER-2021.pdf (accessed on 17 December 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizimana, T.; Kagisha, V.; Nyandwi, J.B.; Nyirimigabo, A.K.; Muganga, R.; Mukanyangezi, M.F.; Kayitare, E. Investigation of the Quality of the 12 Most-Used Antibiotics Available in Retail Private Pharmacies in Rwanda. Antibiotics 2022, 11, 329. https://doi.org/10.3390/antibiotics11030329
Bizimana T, Kagisha V, Nyandwi JB, Nyirimigabo AK, Muganga R, Mukanyangezi MF, Kayitare E. Investigation of the Quality of the 12 Most-Used Antibiotics Available in Retail Private Pharmacies in Rwanda. Antibiotics. 2022; 11(3):329. https://doi.org/10.3390/antibiotics11030329
Chicago/Turabian StyleBizimana, Thomas, Védaste Kagisha, Jean Baptiste Nyandwi, Alain Katembezi Nyirimigabo, Raymond Muganga, Marie Françoise Mukanyangezi, and Egide Kayitare. 2022. "Investigation of the Quality of the 12 Most-Used Antibiotics Available in Retail Private Pharmacies in Rwanda" Antibiotics 11, no. 3: 329. https://doi.org/10.3390/antibiotics11030329
APA StyleBizimana, T., Kagisha, V., Nyandwi, J. B., Nyirimigabo, A. K., Muganga, R., Mukanyangezi, M. F., & Kayitare, E. (2022). Investigation of the Quality of the 12 Most-Used Antibiotics Available in Retail Private Pharmacies in Rwanda. Antibiotics, 11(3), 329. https://doi.org/10.3390/antibiotics11030329





