Recurring Cystitis: How Can We Do Our Best to Help Patients Help Themselves?
Abstract
:1. Introduction
2. Method
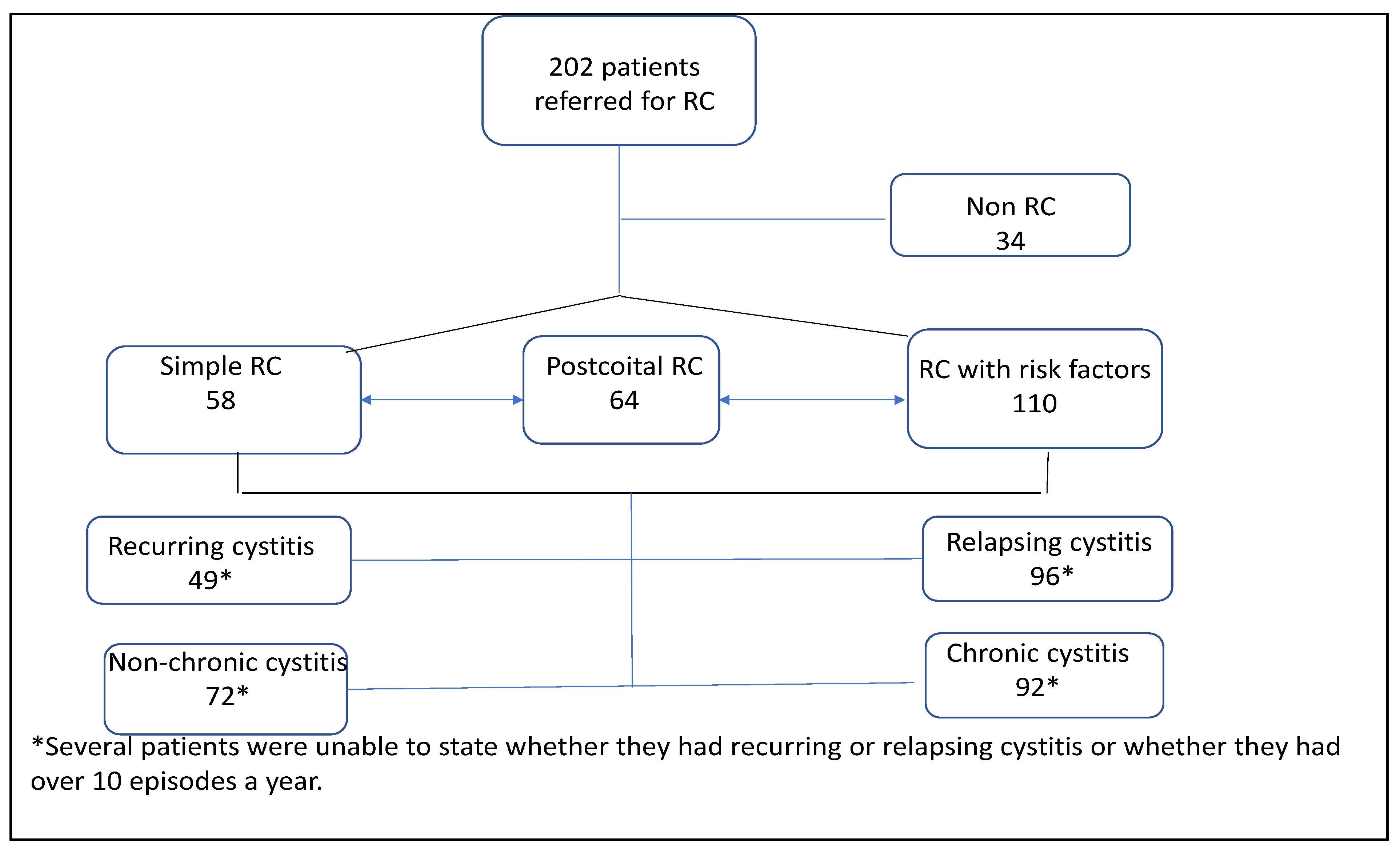
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
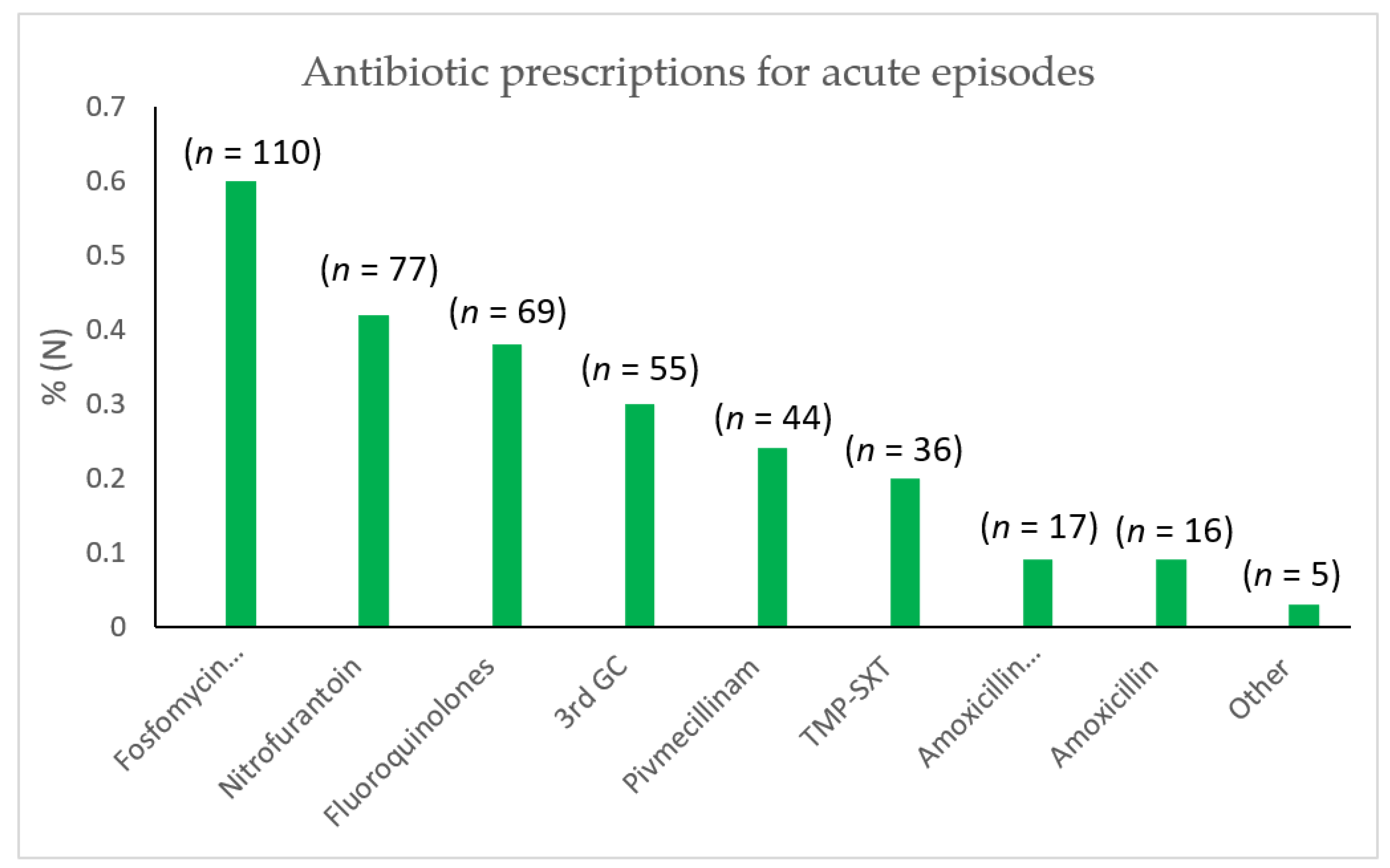
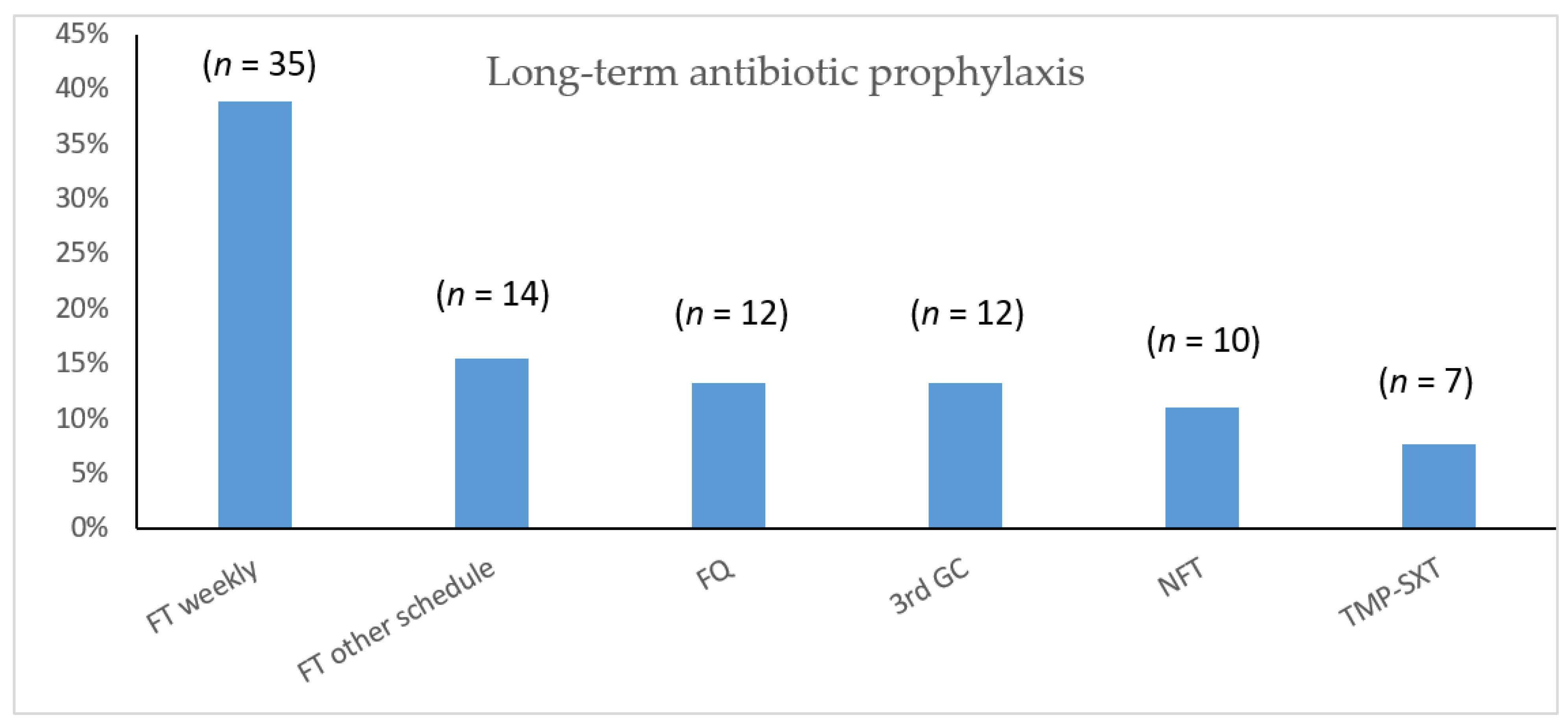
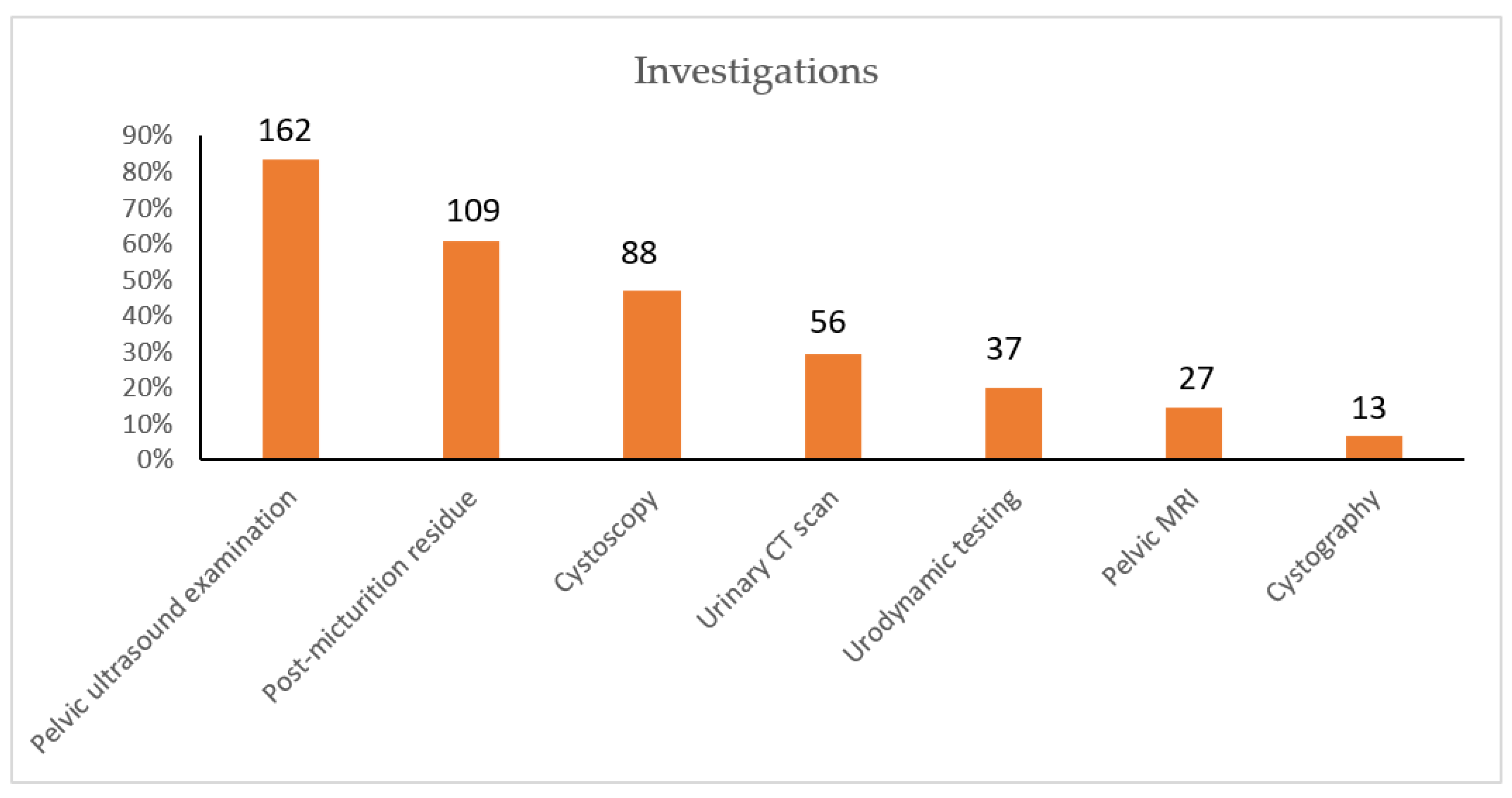
2.4. Results
2.5. Patient Categories
2.6. Differential and Associated Diagnoses
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ANSM—La Consommation d’antibiotiques en France en 2016–Décembre 2017. Available online: https://www.anses.fr/fr/content/surveillance-de-lusage-en-france. (accessed on 25 December 2021).
- François, M.; Hanslik, T.; Dervaux, B.; Le Strat, Y.; Souty, C.; Vaux, S.; Maugat, S.; Rondet, C.; Sarazin, M.; Heym, B.; et al. The economic burden of urinary tract infections in women visiting general practices in France: A cross-sectional survey. BMC Health Serv. Res. 2016, 16, 365. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Setodji, C.M.; Clemens, J.Q. Urologic Diseases of America Project. Incidence and Management of Uncomplicated Recurrent Urinary Tract Infections in a National Sample of Women in the United States. Urology 2016, 90, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haab, F.; Costa, P.; Colau, J.C.; Gérard, A.; Liard, F.; Bohbot, J.M.; Leng, J.J.; Lobel, B.; Soussy, C.J.; Boulanger, P. Management of urinary tract infections in women. Epidemiologic survey of 7916 women in general practice. Presse Med. 2006, 35 Pt 1, 1235–1240. [Google Scholar] [CrossRef]
- Haslund, J.M.; Dinesen, M.R.; Nielsen, A.B.S.; Llor, C.; Bjerrum, L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand. J. Prim. Health Care 2013, 31, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armelle, J. Cystites récidivantes: Des moyens de prévention non médicamenteux. Progrès Urol. 2017, 27, 823–830. [Google Scholar] [CrossRef]
- Lecky, D.M.; Howdle, J.; Butler, C.C.; McNulty, C.A. Optimising management of UTIs in primary care: A qualitative study of patient and GP perspectives to inform the development of an evidence-based, shared decision-making resource. Br. J. Gen. Pract. 2020, 70, e330–e338. [Google Scholar] [CrossRef]
- Available online: https://www.infectiologie.com/UserFiles/File/spilf/recos/infections-urinaires-spilf.pdf (accessed on 25 December 2021).
- Caron, F.; Galperine, T.; Flateau, C.; Azria, R.; Bonacorsi, S.; Bruyère, F.; Cariou, G.; Clouqueur, E.; Cohen, R.; Doco-Lecompte, T.; et al. Practice guidelines for the management of adult community-acquired urinary tract infections. Med. Mal. Infect. 2018, 48, 327–358. [Google Scholar] [CrossRef]
- Available online: https://www.sciencedirect.com/science/article/pii/S0399077X18304463 (accessed on 25 December 2021).
- Holm, S.E.; Stenlund, H.; Lundholm, R.; Monsen, T.J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo-controlled study. Scand. J. Infect. Dis. 2004, 36, 296–301. [Google Scholar]
- Wagenlehner, F.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. Pharm. Outcomes Res. 2018, 18, 107–117. [Google Scholar] [CrossRef]
- Rees, D.L.; Farhoumand, N. Psychiatric aspects of recurrent cystitis in women. Br. J. Urol. 1977, 49, 651–658. [Google Scholar] [CrossRef]
- Available online: https://www.cairn.info/revue-hegel-2021-4-page-332.htm (accessed on 25 December 2021).
- Available online: https://www.youtube.com/watch?v=Cjn1e8A1F6E (accessed on 25 December 2021).
- Fan, Y.H.; Lin, A.T.; Wu, H.M.; Hong, C.J.; Chen, K.K. Psychological profile of female patients with dysfunctional voiding. Urology 2008, 71, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.C.; Waller, G. Psychological factors in recurrent uncomplicated urinary tract infection. Br. J. Urol. 1992, 69, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, S.; Halldorsdottir, S. Screaming Body and Silent Healthcare Providers: A Case Study with a Childhood Sexual Abuse Survivor. Int. J. Environ. Res. Public Health 2018, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Panicker, J.N.; Anding, R.; Arlandis, S.; Blok, B.; Dorrepaal, C.; Harding, C.; Marcelissen, T.; Rademakers, K.; Abrams, P.; Apostolidis, A. Do we understand voiding dysfunction in women? Current understanding and future perspectives: ICI-RS 2017. Neurourol. Urodyn. 2018, 37, S75–S85. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, S.L.; Ashe, J.F. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int. 2012, 110, E830-6. [Google Scholar] [CrossRef] [PubMed]
- Flower, A.; Bishop, F.L.; Lewith, G. How women manage recurrent urinary tract infections: An analysis of postings on a popular web forum. BMC Fam. Pract. 2014, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- Ciani, O.; Grassi, D.; Tarricone, R. An economic perspective on urinary tract infection: The “costs of resignation”. Clin. Drug Investig. 2013, 33, 255–261. [Google Scholar] [CrossRef]
- Salinas-Casado, J.; Méndez-Rubio, S.; Esteban-Fuertes, M.; Gómez-Rodríguez, A.; Vírseda-Chamorro, M.; Luján-Galán, M.; Rituman, G. Efficacy and safety of D-mannose (2 g), 24 h prolonged release, associated with Proanthocyanidin (PAC), versus isolate PAC, in the management of a series of women with recurrent urinary infections. Arch. Esp. Urol. 2018, 71, 169–177. [Google Scholar]
- Flower, A.; Wang, L.-Q.; Lewith, G.; Liu, J.P.; Li, Q. Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database Syst. Rev. 2015, 6, CD010446. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, A.; Alraek, T.; LiHua, H.; Baerheim, A. Acupuncture in the Prophylaxis of Recurrent Lower Urinary Tract Infection in Adult Women. Scand. J. Prim. Health Care 1998, 16, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Sundsbak, J.L.; Mulvey, M.A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 2006, 8, 704–717. [Google Scholar] [CrossRef]
- Scott, V.C.; Haake, D.A.; Churchill, B.M.; Justice, S.S.; Kim, J.H. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology 2015, 86, 425–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellens, A.; Garofalo, C.; Nguyen, H.; Van Gerven, N.; Slättegård, R.; Hernalsteens, J.P.; Wyns, L.; Oscarson, S.; De Greve, H.; Hultgren, S.; et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Hawn, T.R.; Scholes, D.; Li, S.S.; Wang, H.; Yang, Y.; Roberts, P.L.; Stapleton, A.E.; Janer, M.; Aderem, A.; Stamm, W.E.; et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS ONE 2009, 4, e5990. [Google Scholar] [CrossRef]
- Martin, D.; Fougnot, S.; Grobost, F.; Thibaut-Jovelin, S.; Ballereau, F.; Gueudet, T.; de Mouy, D.; Robert, J. ONERBA-ville network. Prevalence of extended-spectrum beta-lactamase producing Escherichia coli in community-onset urinary tract infections in France in 2013. J. Infect. 2016, 72, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Matthews, S.J.; Lancaster, J.W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Gandhi, J.; Chen, A.; Dagur, G.; Suh, Y.; Smith, N.; Cali, B.; Khan, S.A. Genitourinary syndrome of menopause: An overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am. J. Obstet. Gynecol. 2016, 215, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portman, D.J.; Gass, M.L. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 79, 349–354. [Google Scholar]
- Fritel, X.; Campagne-Loiseau, S.; Cosson, M.; Ferry, P.; Saussine, C.; Lucot, J.P.; Salet-Lizee, D.; Barussaud, M.L.; Boisramé, T.; Carlier-Guérin, C.; et al. Complications after pelvic floor repair surgery (with and without mesh): Short-term incidence after 1873 inclusions in the French VIGI-MESH registry. BJOG 2020, 127, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, J. Designing a Mobile Health Application Prototype for the Management of Interstitial Cystitis/Painful Bladder Syndrome. Stud. Health Technol. Inform. 2017, 245, 94–97. [Google Scholar]
- Available online: https://medqualville.antibioresistance.fr/resistances/synthese?accommodation=VILLE&age=all&analyze_type1=S&analyze_type2=S&antibiotic1&antibiotic2&bacterium_code=Ecoli&departement&level=reseau&phenotype=all®ion&sample_type=URINES&sex=all&year=2021#year-detail (accessed on 25 December 2021).
- Hickling, D.R. Recurrent urinary tract infection: Rates and prescription patterns in the antibiotic stewardship era. Can. Urol. Assoc. J. 2021, 15, 405–406. [Google Scholar] [CrossRef]
| Favouring Factors | N | % |
|---|---|---|
| Trigger Factors: | ||
| Sexual intercourse | 73 | 36% |
| Stress | 71 | 35% |
| Diarrhoea | 38 | 19% |
| Behavioural factors | ||
| Anxiety | 125 | 62% |
| Hydration < 1.5 L/day | 65 | 32% |
| Bladder irritants: | ||
| Excess intake of tea, coffee, alcohol | 38 | 19% |
| Tobacco | 25 | 13% |
| Withheld or non-seated micturition | 55 | 28.5% |
| Excessive intimate hygiene ≥2x/day | 78 | 40.5% |
| Inadequate drying | 5 | 2.5% |
| Aggravating sports (cycling, horse-riding…) | 16 | 9% |
| Obesity | 23 | 12% |
| Non-behavioural factors | ||
| Family history of cystitis | 56 | 28% |
| Irritable bowel syndrome | 105 | 52% |
| Constipation | 50 | 25% |
| Diarrhoea | 27 | 14% |
| Alternating diarrhoea/constipation | 30 | 15% |
| Perceived vaginal dryness | 121 | 60% |
| Non-perceived vaginal dryness | 23 | 12% |
| Risk Factors for Complications | N | % |
|---|---|---|
| Age > 75 years | 49 | 24% |
| Organic abnormalities of the urinary tract | 42 | 22% |
| Hymenal adhesions | 13 | 7.5% |
| Urolithiasis | 25 | 13% |
| Bladder diverticula | 4 | 2% |
| Dilated urethral stenosis | 20 | 10% |
| Non-operated pelvic floor dysfunction | 18 | 10% |
| Sling surgery—Prolapse cure | 49 | 24% |
| Among which ineffective and/or with complications (34/49) | 34 | 69% |
| Other urological surgery | 25 | 12% |
| Functional abnormalities of the urinary tract | ||
| Residual urine > 100 mL | 32 | 16% |
| Neurogenic bladder | 7 | 3% |
| Hyperactive bladder | 10 | 6% |
| Dystonic urethral sphincter | 8 | 5% |
| Medical treatment favouring post-micturition bladder residue | 48 | 24% |
| Among which those with proven bladder residue (8/48) | 8 | 16% |
| Iatrogenic immune depression: | ||
| methotrexate (8), steroids (9), immune suppressors (11), immune modulators (1), monoclonal antibodies (7) | 27 | 13% |
| Non-iatrogenic immune depression: | ||
| amyloidosis, hypogammaglobulinemia, HIV | 3 | 1% |
| Pelvic radiotherapy | 3 | 1% |
| Urine Culture and Urinalysis | N | % |
|---|---|---|
| Never | 6 | 3% |
| Sometimes | 65 | 32% |
| Always | 131 | 65% |
| Post-treatment urine culture and urinalysis | ||
| Never | 161 | 80% |
| Sometimes | 17 | 8% |
| Always | 12 | 12% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hadj Messaoud, S.; Demonchy, E.; Mondain, V. Recurring Cystitis: How Can We Do Our Best to Help Patients Help Themselves? Antibiotics 2022, 11, 269. https://doi.org/10.3390/antibiotics11020269
Ben Hadj Messaoud S, Demonchy E, Mondain V. Recurring Cystitis: How Can We Do Our Best to Help Patients Help Themselves? Antibiotics. 2022; 11(2):269. https://doi.org/10.3390/antibiotics11020269
Chicago/Turabian StyleBen Hadj Messaoud, Sarah, Elisa Demonchy, and Véronique Mondain. 2022. "Recurring Cystitis: How Can We Do Our Best to Help Patients Help Themselves?" Antibiotics 11, no. 2: 269. https://doi.org/10.3390/antibiotics11020269
APA StyleBen Hadj Messaoud, S., Demonchy, E., & Mondain, V. (2022). Recurring Cystitis: How Can We Do Our Best to Help Patients Help Themselves? Antibiotics, 11(2), 269. https://doi.org/10.3390/antibiotics11020269





