Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis
Abstract
:1. Introduction
2. Results
2.1. Absence of Hgt1 or C3 Increases the Virulence of Candida albicans in a Murine Model of Systemic Candidiasis
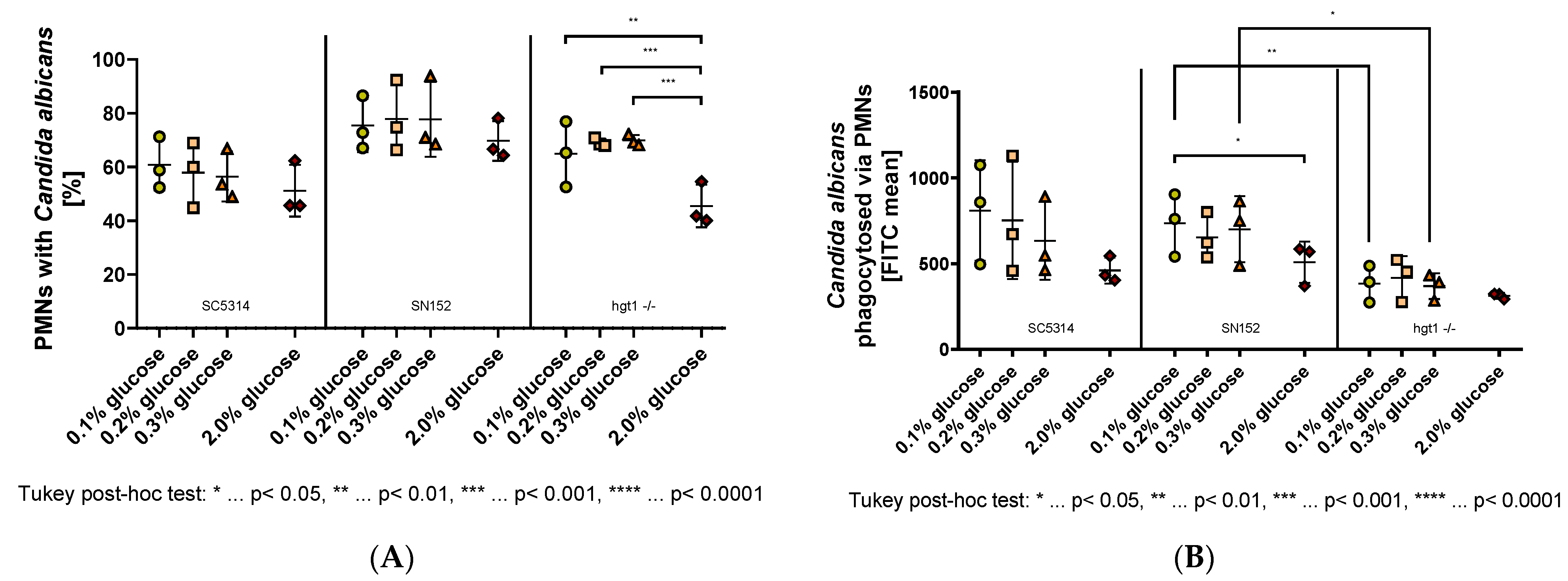
2.2. Effect of Glucose on PMN-Mediated Phagocytosis
2.3. Effect of Glucose on the Deposition of C3b/iC3b on C. albicans
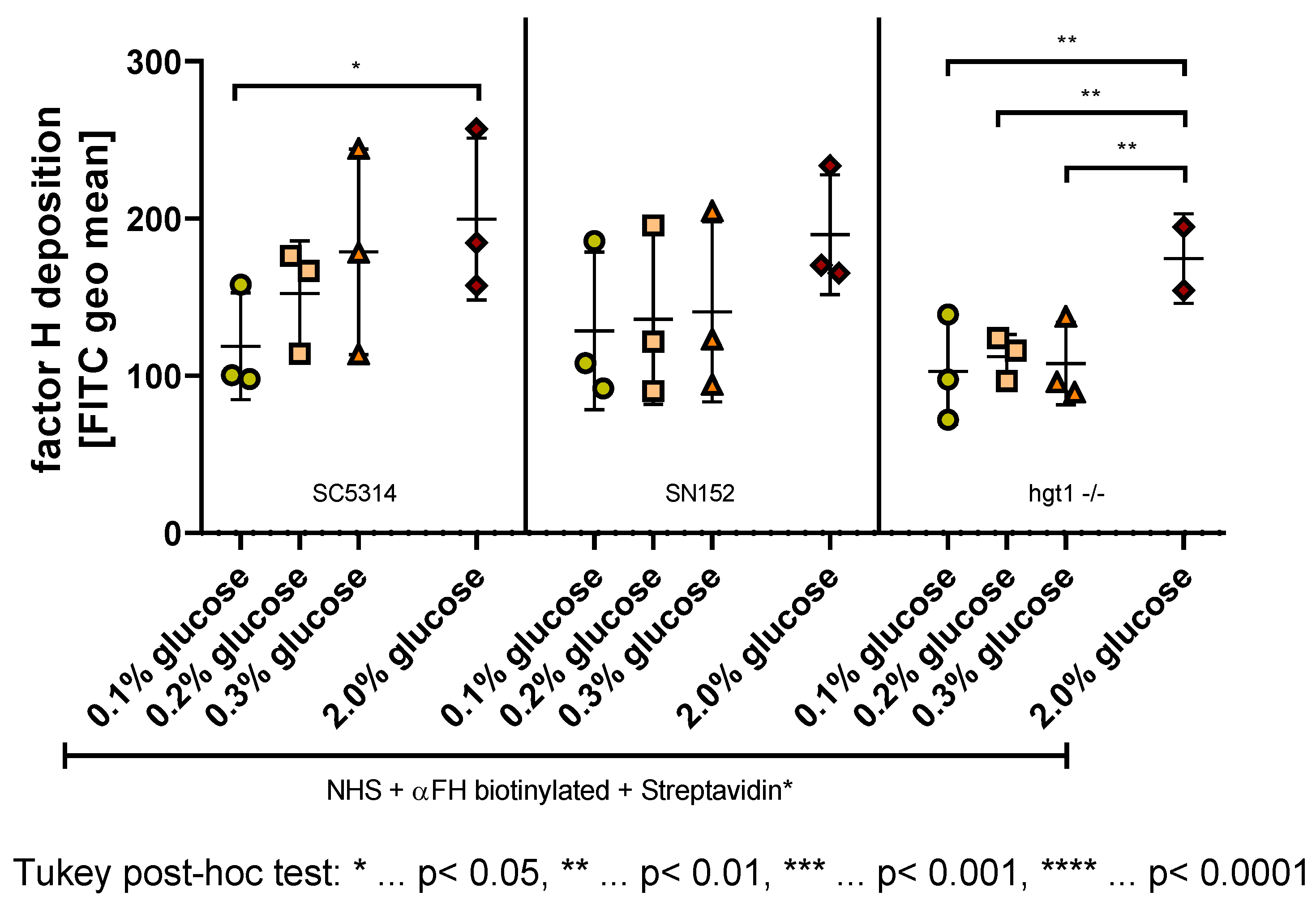
2.4. Effect of Glucose on the Deposition of FH on C. albicans
3. Discussion
4. Materials and Methods
4.1. Reagents and Media
4.2. Mice
4.3. Candida albicans Strains
4.4. Murine Model of Disseminated Systemic Candidiasis
4.5. Isolation of Polymorphonuclear Leukocytes (PMNs)
4.6. PMN-Mediated Phagocytosis of Candida albicans Ex Vivo
4.7. FH and C3b/iC3b Deposition on C. albicans Surface by Fluorescence-Activated Cell Sampling (FACS)
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rambach, G.; Wurzner, R.; Speth, C. Complement: An efficient sword of innate immunity. Contrib. Microbiol. 2008, 15, 78–100. [Google Scholar]
- Kopp, A.; Hebecker, M.; Svobodova, E.; Jozsi, M. Factor h: A complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2012, 2, 46–75. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S. Immunochemical study on mannans of genus candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top Med. Mycol. 1997, 8, 57–70. [Google Scholar]
- Gropp, K.; Schild, L.; Schindler, S.; Hube, B.; Zipfel, P.F.; Skerka, C. The yeast candida albicans evades human complement attack by secretion of aspartic proteases. Mol. Immunol. 2009, 47, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Poltermann, S.; Kunert, A.; von der Heide, M.; Eck, R.; Hartmann, A.; Zipfel, P.F. Gpm1p is a factor h-, fhl-1-, and plasminogen-binding surface protein of candida albicans. J. Biol. Chem. 2007, 282, 37537–37544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Poltermann, S.; Kunert, A.; Rupp, S.; Zipfel, P.F. Immune evasion of the human pathogenic yeast candida albicans: Pra1 is a factor h, fhl-1 and plasminogen binding surface protein. Mol. Immunol. 2009, 47, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Lesiak-Markowicz, I.; Vogl, G.; Schwarzmuller, T.; Speth, C.; Lass-Florl, C.; Dierich, M.P.; Kuchler, K.; Wurzner, R. Candida albicans hgt1p, a multifunctional evasion molecule: Complement inhibitor, cr3 analogue, and human immunodeficiency virus-binding molecule. J. Infect. Dis. 2011, 204, 802–809. [Google Scholar] [CrossRef]
- Luo, S.; Hoffmann, R.; Skerka, C.; Zipfel, P.F. Glycerol-3-phosphate dehydrogenase 2 is a novel factor h-, factor h-like protein 1-, and plasminogen-binding surface protein of candida albicans. J. Infect. Dis. 2013, 207, 594–603. [Google Scholar] [CrossRef]
- Harpf, V.; Rambach, G.; Wurzner, R.; Lass-Florl, C.; Speth, C. Candida and complement: New aspects in an old battle. Front. Immunol. 2020, 11, 1471. [Google Scholar] [CrossRef]
- Speth, C.; Rambach, G.; Lass-Florl, C.; Dierich, M.P.; Wurzner, R. The role of complement in invasive fungal infections. Mycoses 2004, 47, 93–103. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvet, H.M.; Yoshikawa, T.T. Infections in diabetes. Infect Dis. Clin. N. Am. 2001, 15, 407–421. [Google Scholar] [CrossRef]
- Kenno, S.; Speth, C.; Rambach, G.; Binder, U.; Chatterjee, S.; Caramalho, R.; Haas, H.; Lass-Florl, C.; Shaughnessy, J.; Ram, S.; et al. Candida albicans factor h binding molecule hgt1p—A low glucose-induced transmembrane protein is trafficked to the cell wall and impairs phagocytosis and killing by human neutrophils. Front. Microbiol. 2018, 9, 3319. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Elahi, S.; Pang, G.; Gotjamanos, T.; Seymour, G.J.; Clancy, R.L.; Ashman, R.B. T cells augment monocyte and neutrophil function in host resistance against oropharyngeal candidiasis. Infect Immun. 2001, 69, 6110–6118. [Google Scholar] [CrossRef] [Green Version]
- Fulurija, A.; Ashman, R.B.; Papadimitriou, J.M. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 1996, 142 Pt 12, 3487–3496. [Google Scholar] [CrossRef] [Green Version]
- de Souza Ferreira, C.; Araujo, T.H.; Angelo, M.L.; Pennacchi, P.C.; Okada, S.S.; de Araujo Paula, F.B.; Migliorini, S.; Rodrigues, M.R. Neutrophil dysfunction induced by hyperglycemia: Modulation of myeloperoxidase activity. Cell Biochem. Funct. 2012, 30, 604–610. [Google Scholar] [CrossRef]
- Meri, T.; Hartmann, A.; Lenk, D.; Eck, R.; Wurzner, R.; Hellwage, J.; Meri, S.; Zipfel, P.F. The yeast candida albicans binds complement regulators factor h and fhl-1. Infect Immun. 2002, 70, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Jafar, N.; Edriss, H.; Nugent, K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Kjersem, H.; Hilsted, J.; Madsbad, S.; Wandall, J.H.; Johansen, K.S.; Borregaard, N. Polymorphonuclear leucocyte dysfunction during short term metabolic changes from normo- to hyperglycemia in type 1 (insulin dependent) diabetic patients. Infection 1988, 16, 215–221. [Google Scholar] [CrossRef] [PubMed]
- van Oss, C.J.; Border, J.R. Influence of intermittent hyperglycemic glucose levels on the phagocytosis of microorganisms by human granulocytes in vitro. Immunol. Commun. 1978, 7, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Polage, C.; Devaraj, S. Severe hyperglycemia down regulates toll-like receptors on neutrophils: Implications for propensity to infections in diabetics. FASEB J. 2013, 27, 648.11. [Google Scholar] [CrossRef]
- Bassyouni, R.H.; Wegdan, A.A.; Abdelmoneim, A.; Said, W.; AboElnaga, F. Phospholipase and aspartyl proteinase activities of candida species causing vulvovaginal candidiasis in patients with type 2 diabetes mellitus. J. Microbiol. Biotechnol. 2015, 25, 1734–1741. [Google Scholar] [CrossRef]
- Rodaki, A.; Bohovych, I.M.; Enjalbert, B.; Young, T.; Odds, F.C.; Gow, N.A.; Brown, A.J. Glucose promotes stress resistance in the fungal pathogen candida albicans. Mol. Biol. Cell 2009, 20, 4845–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetter, M.K. Handicaps to host defense. Effects of hyperglycemia on c3 and candida albicans. Diabetes 1990, 39, 271–275. [Google Scholar] [CrossRef]
- Hair, P.S.; Echague, C.G.; Rohn, R.D.; Krishna, N.K.; Nyalwidhe, J.O.; Cunnion, K.M. Hyperglycemic conditions inhibit c3-mediated immunologic control of staphylococcus aureus. J. Transl. Med. 2012, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Blom, A.M.; Hallstrom, T.; Riesbeck, K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol. Immunol. 2009, 46, 2808–2817. [Google Scholar] [CrossRef]
- Ermert, D.; Niemiec, M.J.; Rohm, M.; Glenthoj, A.; Borregaard, N.; Urban, C.F. Candida albicans escapes from mouse neutrophils. J. Leukoc. Biol. 2013, 94, 223–236. [Google Scholar] [CrossRef]
- Mencacci, A.; Romani, L.; Mosci, P.; Cenci, E.; Tonnetti, L.; Vecchiarelli, A.; Bistoni, F. Low-dose streptozotocin-induced diabetes in mice. Ii. Susceptibility to candida albicans infection correlates with the induction of a biased th2-like antifungal response. Cell Immunol. 1993, 150, 36–44. [Google Scholar] [CrossRef]
- Venturini, J.; Fraga-Silva, T.F.; Marchetti, C.M.; Mimura, L.A.; Conti, B.J.; Golim Mde, A.; Mendes, R.P.; de Arruda, M.S. Imbalanced macrophage and dendritic cell activations in response to candida albicans in a murine model of diabetes mellitus. Immunol. Investig. 2016, 45, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Aldebasi, Y.H.; Alsuhaibani, S.A.; AlSahli, M.A.; Alzohairy, M.A.; Khan, A.; Younus, H. Therapeutic potential of thymoquinone liposomes against the systemic infection of candida albicans in diabetic mice. PLoS ONE 2018, 13, e0208951. [Google Scholar]
- Sasada, M.; Johnston, R.B., Jr. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of candida by macrophages. J. Exp. Med. 1980, 152, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing activity of neutrophils is mediated through activation of proteases by k+ flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Tsoni, S.V.; Kerrigan, A.M.; Marakalala, M.J.; Srinivasan, N.; Duffield, M.; Taylor, P.R.; Botto, M.; Steele, C.; Brown, G.D. Complement c3 plays an essential role in the control of opportunistic fungal infections. Infect. Immun. 2009, 77, 3679–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of c5a in the absence of c3: A new complement activation pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.H.; Johnson, C.; Quach, Q.H.; Macpherson, A.; Durrant, O.; Pischke, S.E.; Fure, H.; Landsem, A.; Bergseth, G.; Schjalm, C.; et al. A conformational change of complement c5 is required for thrombin-mediated cleavage, revealed by a novel ex vivo human whole blood model preserving full thrombin activity. J. Immunol. 2021, 207, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- DiScipio, R.G.D.; Daffern, P.J.D.; Schraufstätter, I.U.; Sriramarao, P. Human polymorphonuclear leukocytes adhere to complement factor h through an interaction that involves amb2 (cd11b/cd18). J. Immunol. 1998, 160, 4057–4066. [Google Scholar]
- Losse, J.; Zipfel, P.F.; Jozsi, M. Factor h and factor h-related protein 1 bind to human neutrophils via complement receptor 3, mediate attachment to candida albicans, and enhance neutrophil antimicrobial activity. J. Immunol. 2010, 184, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Soloviev, D.A.; Jawhara, S.; Fonzi, W.A. Regulation of innate immune response to candida albicans infections by alphambeta2-pra1p interaction. Infect. Immun. 2011, 79, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, N.; Kruppa, M.D. Standard growth media and common techniques for use with candida albicans. Methods Mol. Biol. 2009, 499, 197–201. [Google Scholar]
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Eucast definitive document edef 7.1: Method for the determination of broth dilution mics of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Claudino, A.L.R.; Peixoto, R.F.; Melhem, M.S.C.; Szeszs, M.W.; Lyon, J.P.; Chavasco, J.K.; Franco, M.C. Correlation between clsi, eucast and etest methodologies for amphotericin b and fluconazole antifungal susceptibility testing of candida spp. Clinical isolates. Pharmazie 2008, 63, 286–289. [Google Scholar]
- Clinical and Laboratory Standards Institute. CLSI document M27: Reference method for broth dilution antifungal susceptibility testing of yeasts. In Clinical and Laboratory Standards Institute (CLSI), 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Noble, S.M.; Johnson, A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen candida albicans. Eukaryot Cell 2005, 4, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Rambach, G.; Fleischer, V.; Harpf, V.; Lackner, M.; Meinitzer, A.; Maier, H.; Engesser, J.; Lass-Florl, C.; Speth, C. Comparative immunopathogenesis in a murine model of inhalative infection with the mucormycetes lichtheimia corymbifera and rhizopus arrhizus. PLoS ONE 2020, 15, e0234063. [Google Scholar] [CrossRef]
- Janssen, B.J.; De Celle, T.; Debets, J.J.; Brouns, A.E.; Callahan, M.F.; Smith, T.L. Effects of anesthetics on systemic hemodynamics in mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1618–H1624. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpf, V.; Kenno, S.; Rambach, G.; Fleischer, V.; Parth, N.; Weichenberger, C.X.; Garred, P.; Huber, S.; Lass-Flörl, C.; Speth, C.; et al. Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis. Antibiotics 2022, 11, 257. https://doi.org/10.3390/antibiotics11020257
Harpf V, Kenno S, Rambach G, Fleischer V, Parth N, Weichenberger CX, Garred P, Huber S, Lass-Flörl C, Speth C, et al. Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis. Antibiotics. 2022; 11(2):257. https://doi.org/10.3390/antibiotics11020257
Chicago/Turabian StyleHarpf, Verena, Samyr Kenno, Günter Rambach, Verena Fleischer, Nadia Parth, Christian X. Weichenberger, Peter Garred, Silke Huber, Cornelia Lass-Flörl, Cornelia Speth, and et al. 2022. "Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis" Antibiotics 11, no. 2: 257. https://doi.org/10.3390/antibiotics11020257
APA StyleHarpf, V., Kenno, S., Rambach, G., Fleischer, V., Parth, N., Weichenberger, C. X., Garred, P., Huber, S., Lass-Flörl, C., Speth, C., & Würzner, R. (2022). Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis. Antibiotics, 11(2), 257. https://doi.org/10.3390/antibiotics11020257







