Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans
Abstract
:1. Introduction
2. Results
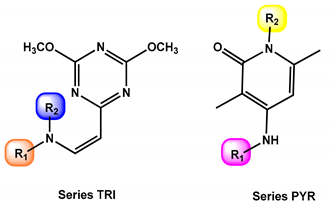
2.1. Screening of the Chemical Library
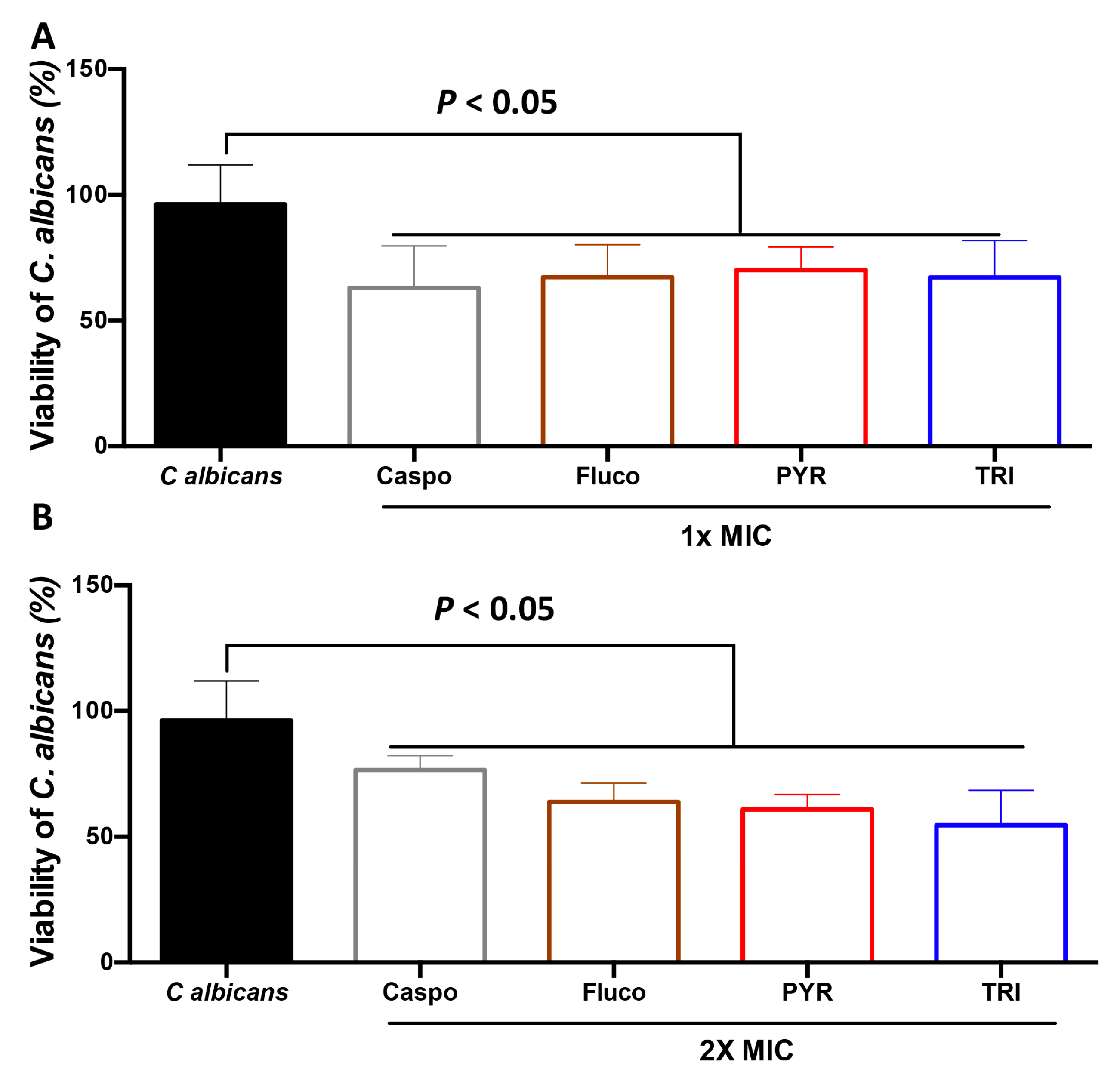
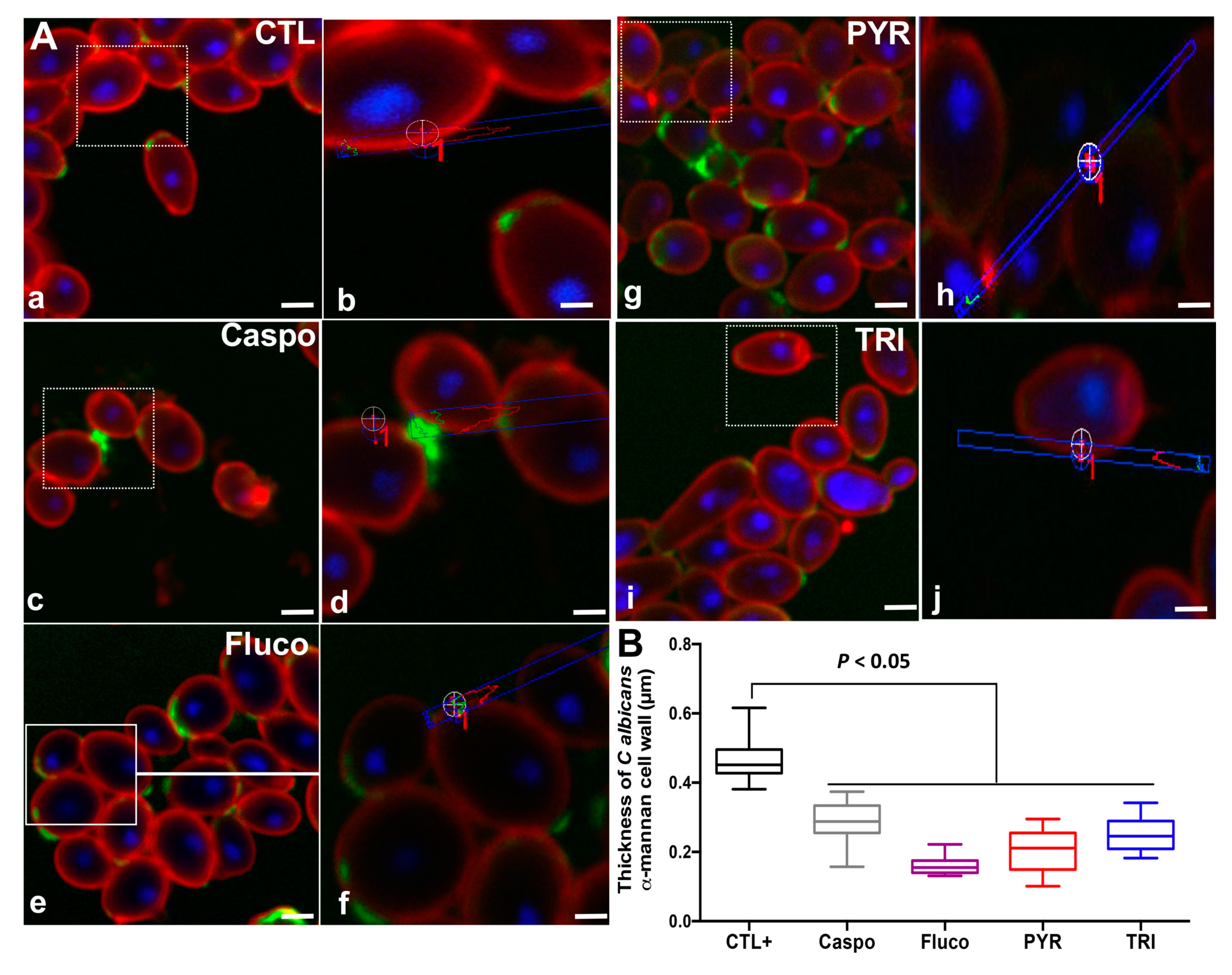
2.2. Effect of PYR and TRI on C. albicans Viability
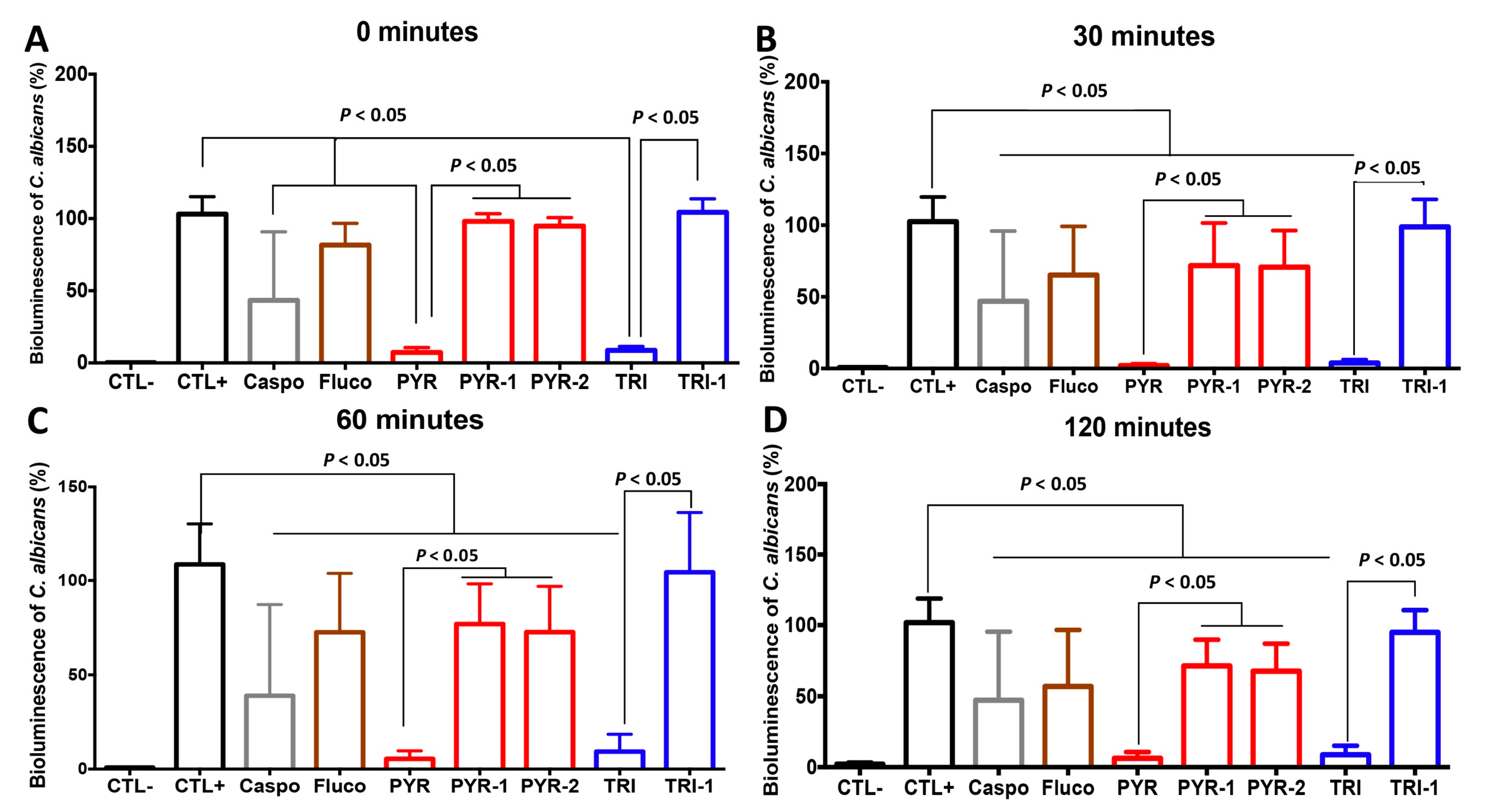
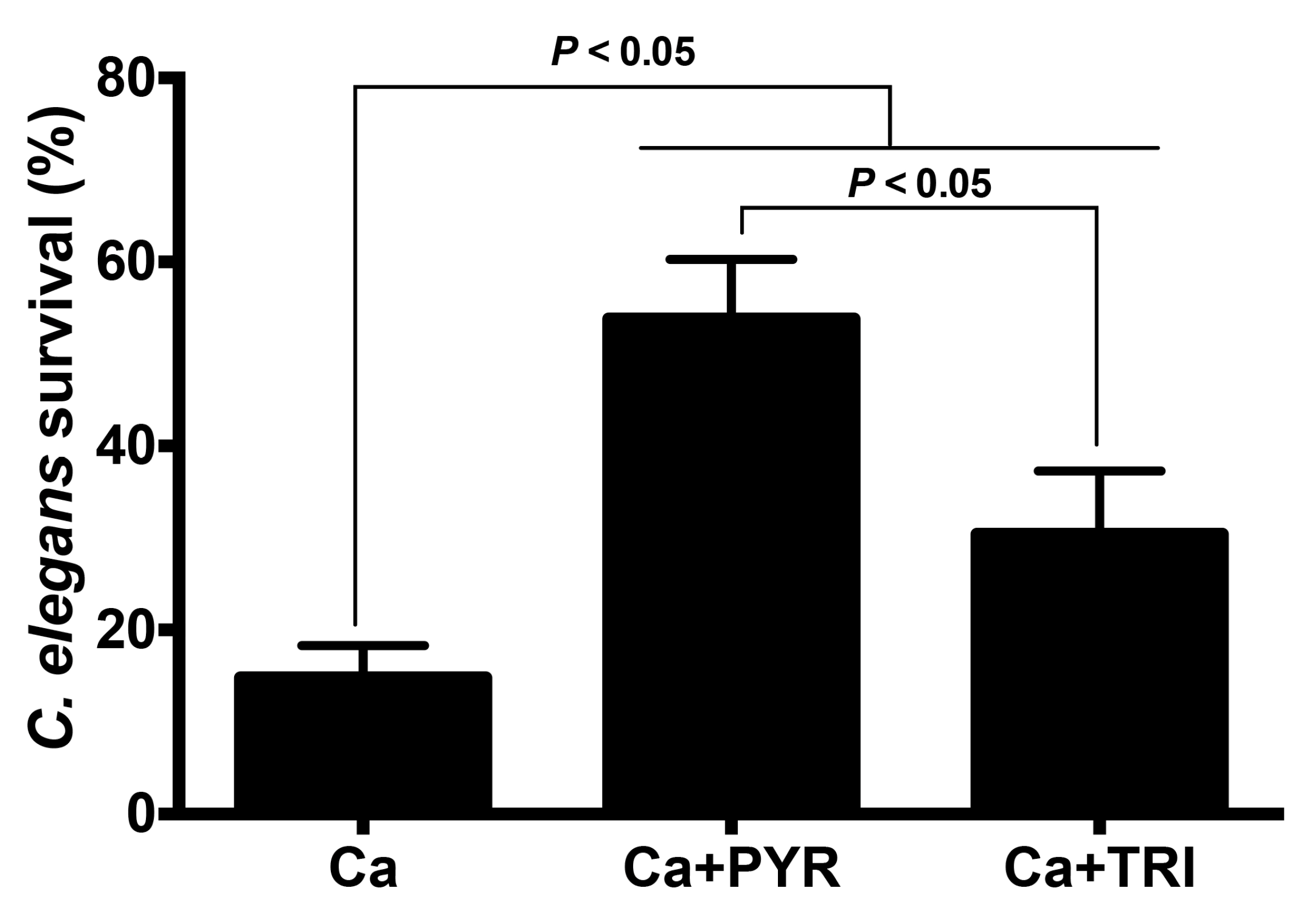
2.3. PYR and TRI Reduced the Virulence of C. albicans in the C. elegans Infection Model
3. Discussion
4. Materials and Methods
4.1. C. albicans Growth Conditions
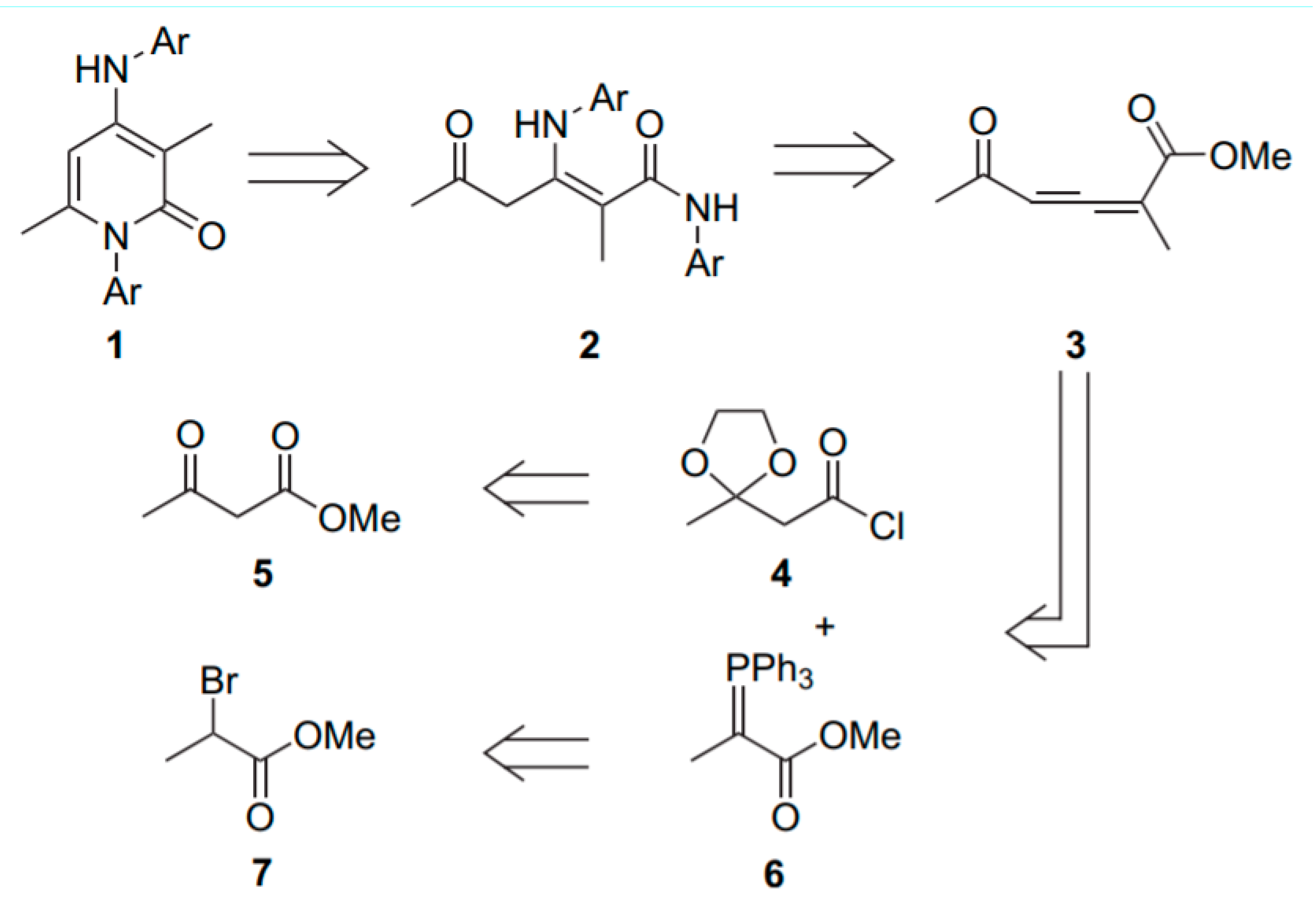
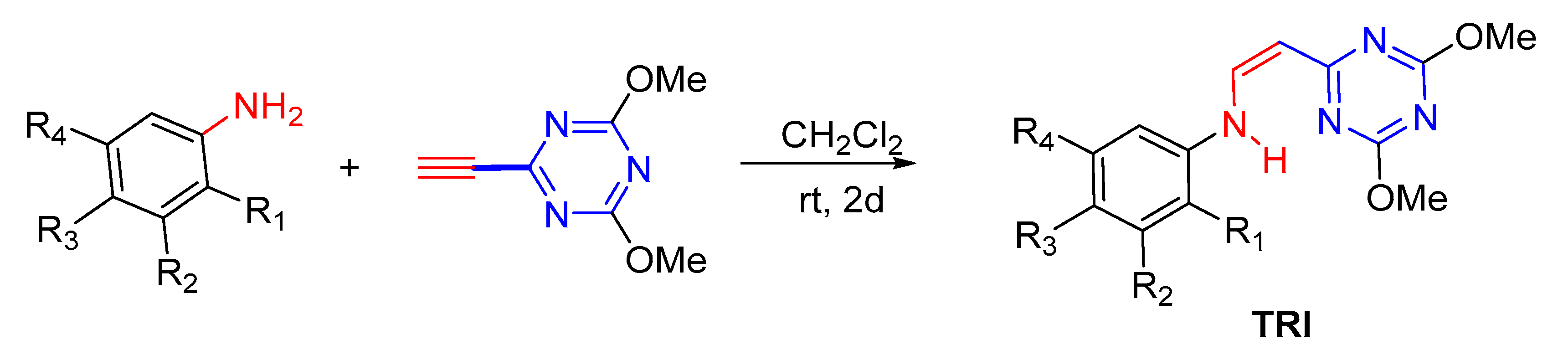
4.2. Chemical Synthesis of PYR and TRI
4.3. Antifungal Compounds
4.4. Fungal Viability Assays
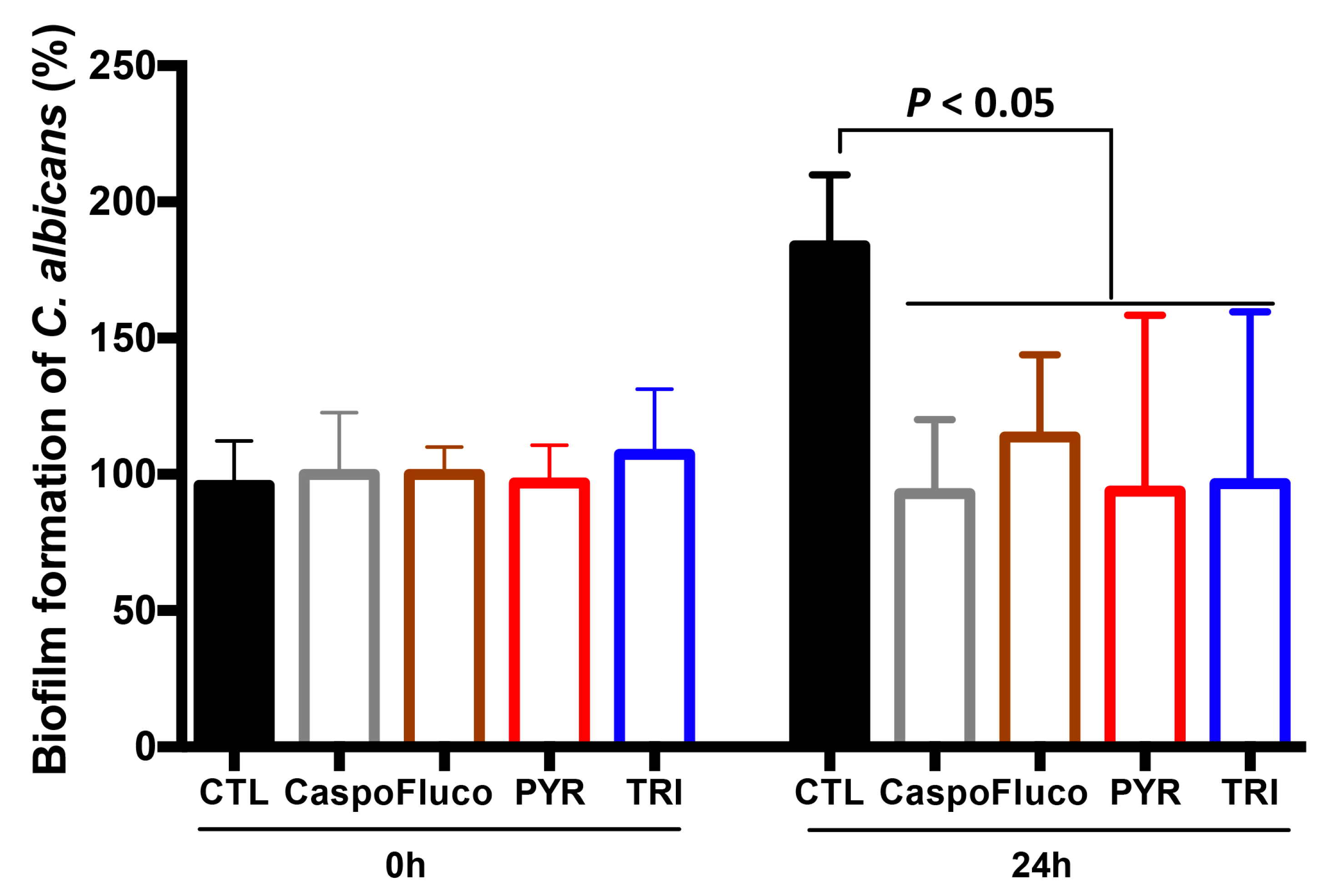
4.5. Effect of PYR and TRI on C. albicans Biofilm Formation
4.6. C. elegans Survival Assay and RT-PCR Quantification of Antimicrobial Genes of C. elegans
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef]
- Billamboz, M.; Fatima, Z.; Hameed, S.; Jawhara, S. Promising Drug Candidates and New Strategies for Fighting against the Emerging Superbug Candida auris. Microorganisms 2021, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Gabaldon, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Florl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Borghi, E.; Morace, G.; Borgo, F.; Rajendran, R.; Sherry, L.; Nile, C.; Ramage, G. New strategic insights into managing fungal biofilms. Front. Microbiol. 2015, 6, 1077. [Google Scholar] [CrossRef]
- Powell, J.R.; Ausubel, F.M. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; Volume 415, pp. 403–427. [Google Scholar]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallo, G.V.; Kurz, C.L.; Couillault, C.; Pujol, N.; Granjeaud, S.; Kohara, Y.; Ewbank, J.J. Inducible antibacterial defense system in C. elegans. Curr. Biol. 2002, 12, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Troemel, E.R.; Chu, S.W.; Reinke, V.; Lee, S.S.; Ausubel, F.M.; Kim, D.H. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006, 2, e183. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, J.J. Signaling in the immune response. In WormBook; 2006; pp. 1–12. Available online: http://www.wormbook.org/chapters/www_signalingimmuneresponse/signalingimmuneresponse.html (accessed on 23 January 2006).
- Bortolus, C.; Billamboz, M.; Charlet, R.; Lecointe, K.; Sendid, B.; Ghinet, A.; Jawhara, S. A Small Aromatic Compound Has Antifungal Properties and Potential Anti-Inflammatory Effects against Intestinal Inflammation. Int. J. Mol. Sci. 2019, 20, 321. [Google Scholar] [CrossRef] [Green Version]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Florl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Maubon, D.; Garnaud, C.; Calandra, T.; Sanglard, D.; Cornet, M. Resistance of Candida spp. to antifungal drugs in the ICU: Where are we now? Intensive Care Med. 2014, 40, 1241–1255. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, X.; Wang, J.; Xie, Z.; Li, L.; Chen, M.; Chen, S.; Peng, Y. Synthesis, molecular docking and alpha-glucosidase inhibition of 2-((5,6-diphenyl-1,2,4-triazin-3-yl)thio)-N-arylacetamides. Bioorg. Med. Chem. Lett. 2017, 27, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sirohi, T.S.; Singh, H.; Yadav, R.; Roy, R.K.; Chaudhary, A.; Pandeya, S.N. 1,2,4-triazine analogs as novel class of therapeutic agents. Mini-Rev. Rev. Med. Chem. 2014, 14, 168–207. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Shinde, D.B. One pot synthesis and SAR of some novel 3-substituted 5,6-diphenyl-1,2,4-triazines as antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Siddiqui, N. Anticonvulsant evaluation of clubbed indole-1,2,4-triazine derivatives: A synthetic approach. Eur. J. Med. Chem. 2014, 80, 509–522. [Google Scholar] [CrossRef]
- Zhan, P.; Li, X.; Li, Z.; Chen, X.; Tian, Y.; Chen, W.; Liu, X.; Pannecouque, C.; De Clercq, E. Structure-based bioisosterism design, synthesis and biological evaluation of novel 1,2,4-triazin-6-ylthioacetamides as potent HIV-1 NNRTIs. Bioorg. Med. Chem. Lett. 2012, 22, 7155–7162. [Google Scholar] [CrossRef]
- Krauth, F.; Dahse, H.M.; Ruttinger, H.H.; Frohberg, P. Synthesis and characterization of novel 1,2,4-triazine derivatives with antiproliferative activity. Bioorg. Med. Chem. 2010, 18, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.F.; Fabio, P.F.; Lee, V.J.; Kuck, N.A.; Testa, R.T. Pyrido[3,4-e]-1,2,4-triazines and related heterocycles as potential antifungal agents. J. Med. Chem. 1989, 32, 2474–2485. [Google Scholar] [CrossRef]
- Breinhold, J.; Ludvigsen, S.; Rassing, B.R.; Rosendahl, C.N.; Nielsen, S.E.; Olsen, C.E. Oxysporidinone: A novel, antifungal N-methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J. Nat. Prod. 1997, 60, 33–35. [Google Scholar] [CrossRef]
- Ando, K.; Matsuura, I.; Nawata, Y.; Endo, H.; Sasaki, H.; Okytomi, T.; Saehi, T.; Tamura, G. Funiculosin, a new antibiotic. II. Structure elucidation and antifungal activity. J. Antibiot. 1978, 31, 533–538. [Google Scholar] [CrossRef]
- Gupta, A.K.; Skinner, A.R. Ciclopirox for the treatment of superficial fungal infections: A review. Int. J. Dermatol. 2003, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vancraeyneste, H.; Charlet, R.; Guerardel, Y.; Choteau, L.; Bauters, A.; Tardivel, M.; Francois, N.; Dubuquoy, L.; Soloviev, D.; Poulain, D.; et al. Short fungal fractions of beta-1,3 glucans affect platelet activation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H725–H734. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Mogensen, E.; Maggiotto, F.; Fradin, C.; Sarazin, A.; Dubuquoy, L.; Maes, E.; Guerardel, Y.; Janbon, G.; Poulain, D. Murine model of dextran sulfate sodium-induced colitis reveals Candida glabrata virulence and contribution of beta-mannosyltransferases. J. Biol. Chem. 2012, 287, 11313–11324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampakakis, E.; Okoli, I.; Mylonakis, E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008, 3, 1925–1931. [Google Scholar] [CrossRef]
- Kamaladevi, A.; Balamurugan, K. Role of PMK-1/p38 MAPK defense in Caenorhabditis elegans against Klebsiella pneumoniae infection during host-pathogen interaction. Pathog. Dis. 2015, 73, ftv021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.C.R.; Fuchs, B.B.; Alves, V.S.; Jayamani, E.; Colombo, A.L.; Mylonakis, E. Pathogenesis of the Candida parapsilosis Complex in the Model Host Caenorhabditis elegans. Genes 2018, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, I.; Griffon, A.; Tichit, L.; Montanana-Sanchis, F.; Wang, G.; Reinke, V.; Waterston, R.H.; Hillier, L.W.; Ewbank, J.J. A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS ONE 2011, 6, e19055. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Boisse, T.; Rigo, B.; Millet, R.; Hénichart, J.P. From dicarbonylallene to 1-aryl-3,6-dimethyl-4-aminoaryl-2-pyridones: A one-pot versatile and uncatalyzed synthesis. Tetrahedron 2007, 63, 10511–10520. [Google Scholar] [CrossRef]
- Bustamante, B.; Martins, M.A.; Bonfietti, L.X.; Szeszs, M.W.; Jacobs, J.; Garcia, C.; Melhem, M.S.C. Species distribution and antifungal susceptibility profile of Candida isolates from bloodstream infections in Lima, Peru. J. Med. Microbiol. 2014, 63, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Doyle, T.C.; Nawotka, K.A.; Kawahara, C.B.; Francis, K.P.; Contag, P.R. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb. Pathog. 2006, 40, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, C.; Charlet, R.; Bettaieb, A.; Jawhara, S. H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice. Microorganisms 2020, 8, 2039. [Google Scholar] [CrossRef] [PubMed]


| Entry | CPD | R1 | R2 | %Inhibition at 32 µg/mLa | %Inhibition at 0.8 mmol/L (µg/mL) b |
|---|---|---|---|---|---|
| 1 | TRI | 4-CH3O-C6H4- | H | 32.1 | 98 (230) |
| 2 | TRI1 | C6H5- | H | 3.9 | 72 (206) |
| 3 | TRI2 | 2,5-(CH3O)2-C6H4- | H | 1.7 | 77 (255) |
| 4 | TRI3 | 2,4-(CH3O)2-C6H4- | H | 0.5 | ND |
| 5 | TRI4 | 3,4,5-(CH3O)3-C6H4- | H | 1.9 | ND |
| 6 | TRI5 | 3-(OH),4-(CH3O)-C6H4- | H | 0.8 | ND |
| 7 | TRI6 | 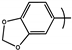 | H | 1.4 | ND |
| 8 | TRI7 | 3,5-(CH3O)2-C6H4- | H | 3.9 | ND |
| 9 | TRI8 | 3,5-(Cl)2-C6H4- | H | 0.5 | ND |
| 10 | TRI9 | CH3-CH2- | CH3-CH2- | 3.8 | 84 (190) |
| 11 | PYR | 4-Cl-C6H4- | 4-Cl-C6H4- | 14.0 | 98 (287) |
| 12 | PYR1 | 4-CH3-C6H4- | 4-CH3-C6H4- | 8.1 | 88 (255) |
| 13 | PYR2 | C6H5- | C6H5- | 3.3 | ND |
| 14 | PYR3 | 4-CH3O-C6H4- | 4-CH3O-C6H4- | 4.2 | ND |
| 15 | PYR4 | 3,4-(CH3O)2-C6H4- | 3,4-(CH3O)2-C6H4- | 2.5 | ND |
| 16 | PYR5 | 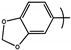 | 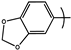 | 3.4 | ND |
| 17 | PYR6 | 3,4-(CH3O)2-C6H4- | 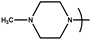 | 3.0 | ND |
| 18 | PYR7 | 3,4-(CH3O)2-C6H4- | 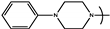 | 3.6 | ND |
| Strain | Description | MIC Caspofungin µg/mL | MIC Fluconazole µg/mL | MIC PYR µg/mL | MIC TRI µg/mL | Strain Ref. |
|---|---|---|---|---|---|---|
| C. albicans SC5314 | Wild-type | 0.03 | 0.5 | 12.5 | 50 | [13] |
| Bioluminescent C. albicans | C. albicans strain CAI4 (ura3::imm434/ura3::imm434) | 0.03 | 0.5 | 12.5 | 50 | [12] |
| C. albicans 17292c3367 | Venous catheter, caspofungin-resistant | 8 | 0.5 | 12.5 | 50 | This study |
| C. albicans 15343c3523 | Blood, caspofungin-resistant | 2 | 0.5 | 12.5 | 50 | This study |
| C. albicans 15351c6859 | Venous catheter, caspofungin-resistant | 4 | 1 | 12.5 | 50 | This study |
| C. albicans 14402c5521 | Abdominal lesions, fluconazole-resistant | 0.125 | 256 | 12.5 | 50 | This study |
| Strain | Viability of C. albicans at 2× MIC (µg/mL) | Viability of C. albicans at 4x MIC (µg/mL) | ||
|---|---|---|---|---|
| TRI vs. Caspo (%) | TRI vs. Fluco (%) | PYR vs. Caspo (%) | PYR vs. Fluco (%) | |
| Caspofungin-resistant C. albicans 15343c3523 | 14.81 ± 7.8 vs. 83.56 ± 32.05 * | 24.96 ± 2.23 vs. 33.47 ± 18.6 * | ||
| Caspofungin-resistant C. albicans 15351c6859 | 13.49 ± 17.04 vs. 194.03 ± 8.014 * | 22.08 ± 13.95 vs. 231.89 ± 50.58 * | ||
| Caspofungin-resistant C. albicans 17292c3367 | 29.54 ± 17.8 vs. 130.76 ± 6.101 * | 10.79 ± 8.04 vs. 125.11 ± 62.04 * | ||
| Fluconazole-resistant C. albicans 14402c5521 | 11.30 ± 13.2 vs. 33.68 ± 26.2 * | 10.95 ± 9.49 vs. 29.12 ± 20.03 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena, L.; Billamboz, M.; Charlet, R.; Desprès, B.; Sendid, B.; Ghinet, A.; Jawhara, S. Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans. Antibiotics 2022, 11, 72. https://doi.org/10.3390/antibiotics11010072
Mena L, Billamboz M, Charlet R, Desprès B, Sendid B, Ghinet A, Jawhara S. Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans. Antibiotics. 2022; 11(1):72. https://doi.org/10.3390/antibiotics11010072
Chicago/Turabian StyleMena, Laura, Muriel Billamboz, Rogatien Charlet, Bérangère Desprès, Boualem Sendid, Alina Ghinet, and Samir Jawhara. 2022. "Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans" Antibiotics 11, no. 1: 72. https://doi.org/10.3390/antibiotics11010072
APA StyleMena, L., Billamboz, M., Charlet, R., Desprès, B., Sendid, B., Ghinet, A., & Jawhara, S. (2022). Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans. Antibiotics, 11(1), 72. https://doi.org/10.3390/antibiotics11010072








