Factors Associated with Daptomycin-Induced Eosinophilic Pneumonia
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Inclusion and Exclusion Criteria
4.3. Data Collection
4.4. Statistical Analyses
4.5. Patient Consent Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Carugati, M.; Bayer, A.S.; Miró, J.M.; Park, L.P.; Guimarães, A.C.; Skoutelis, A.; Fortes, C.Q.; Durante-Mangoni, E.; Hannan, M.M.; Nacinovich, F.; et al. High-Dose Daptomycin Therapy for Left-Sided Infective Endocarditis: A Prospective Study From the International Collaboration on Endocarditis. Antimicrob. Agents Chemother. 2013, 57, 6213–6222. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information. These Highlights Do Not Include All the Information Needed to Use Cubicin Safely and Effectively. See Full Prescribing Information. for CUBICIN. Available online: https://www.merck.com/product/usa/pi_circulars/c/cubicin/cubicin_pi.pdf (accessed on 6 December 2021).
- Kim, p.W.; Sorbello, A.F.; Wassel, R.T.; Pham, T.M.; Tonning, J.M.; Nambiar, S. Eosinophilic Pneumonia in Patients Treated With Daptomycin: Review of the Literature and US FDA Adverse Event Reporting System Reports. Drug Saf. 2012, 35, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Boixader, L.; Villanueva, B.; Ulldemolins, M.; Benavent, E.; Padulles, A.; Ribera, A.; Borras, I.; Ariza, J.; Murillo, O. Risk Factors of Daptomycin-Induced Eosinophilic Pneumonia in a Population With Osteoarticular Infection. Antibiotics 2021, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Hites, M.; Taccone, F.S.; Wolff, F.; Cotton, F.; Beumier, M.; De Backer, D.; Roisin, S.; Lorent, S.; Surin, R.; Seyler, L.; et al. Case-Control Study of Drug Monitoring of β-Lactams in Obese Critically Ill Patients. Antimicrob. Agents Chemother. 2013, 57, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Lal, Y.; Assimacopoulos, A.P. Two Cases of Daptomycin-Induced Eosinophilic Pneumonia and Chronic Pneumonitis. Clin. Infect. Dis. 2010, 50, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Anstead, M.I.; Kuhn, R.J. Eosinophilic Pneumonia Induced by Daptomycin. J. Infect. 2007, 54, e211–e213. [Google Scholar] [CrossRef]
- Miller, B.A.; Gray, A.; Leblanc, T.W.; Sexton, D.J.; Martin, A.R.; Slama, T.G. Acute Eosinophilic Pneumonia Secondary to Daptomycin: A Report of Three Cases. Clin. Infect. Dis. 2010, 50, e63–e68. [Google Scholar] [CrossRef] [PubMed]
- Kakish, E.; Wiesner, A.M.; Winstead, P.S.; Bensadoun, E.S. Acute Respiratory Failure Due to Daptomycin Induced Eosinophilic Pneumonia. Respir. Med. CME 2008, 1, 235–237. [Google Scholar] [CrossRef][Green Version]
- FDA Drug Safety Communication: Eosinophilic Pneumonia Associated With the Use of Cubicin (Daptomycin). Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin (accessed on 6 December 2021).
- Durante-Mangoni, E.; Andini, R.; Parrella, A.; Mattucci, I.; Cavezza, G.; Senese, A.; Trojaniello, C.; Caprioli, R.; Diana, M.V.; Utili, R. Safety of Treatment With High-Dose Daptomycin in 102 Patients With Infective Endocarditis. Int. J. Antimicrob. Agents 2016, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Estes, K.S.; Derendorf, H. Comparison of the Pharmacokinetic Properties of Vancomycin, Linezolid, Tigecyclin, and Daptomycin. Eur. J. Med. Res. 2010, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.M.; Aronoff, G.R.; Morrison, G.; Golper, T.A.; Pulliam, J.; Wolfson, M.; Singer, I. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children, 5th ed.; American College of Physicians: Philadelphia, PA, USA, 1983. [Google Scholar]
- Kullar, R.; McClellan, I.; Geriak, M.; Sakoulas, G. Efficacy and Safety of Daptomycin in Patients with Renal Impairment: A Multicenter Retrospective Analysis. Pharmacotherapy 2014, 34, 582–589. [Google Scholar] [CrossRef] [PubMed]

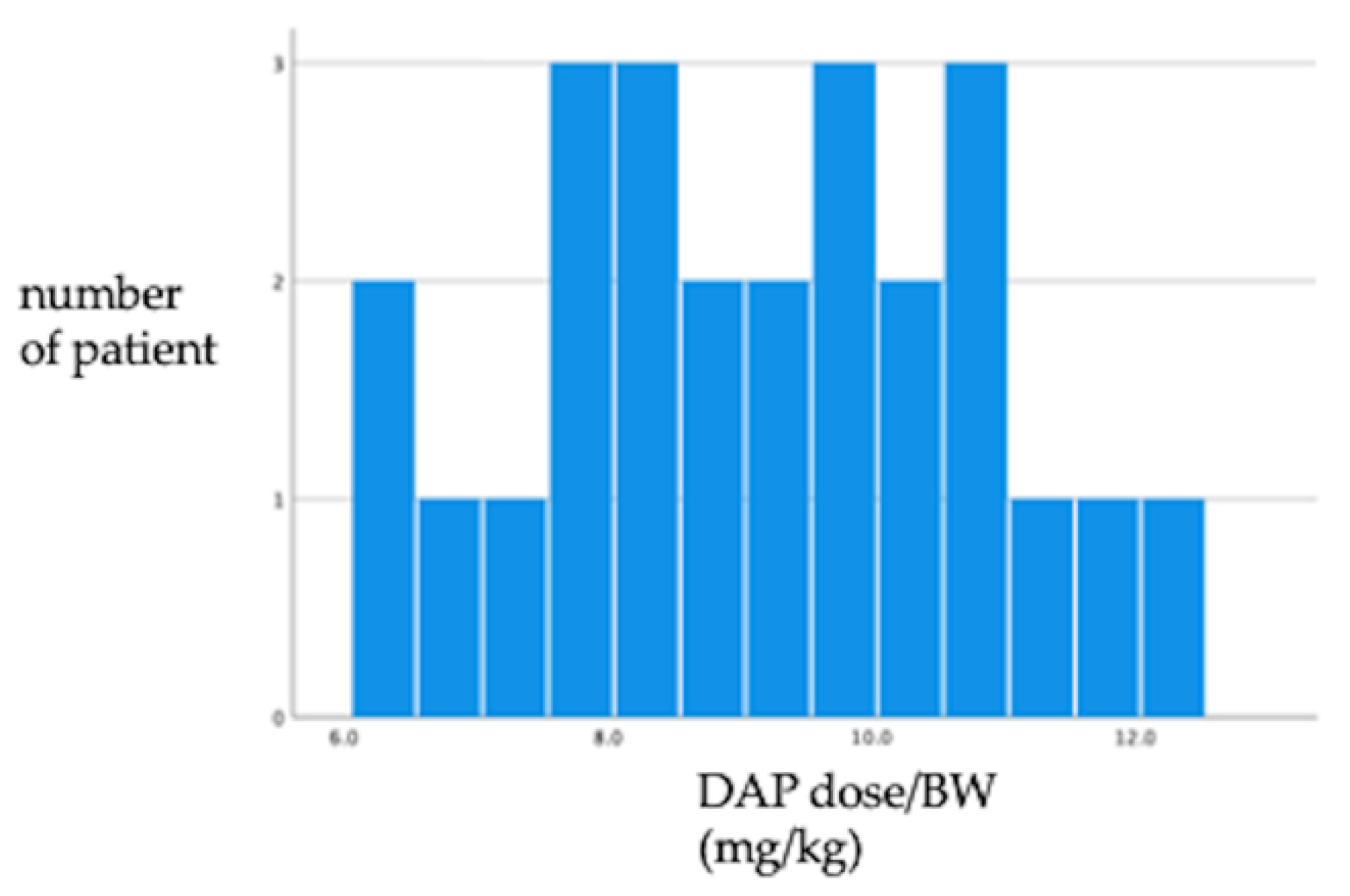
| Patient Characteristics | EP (n = 25) |
|---|---|
| Median duration from first DAP administration to EP onset (IQR, minimum–maximum), days | 18.00 (26.5, 3–49) |
| Definite, n (%) | 0 (0%) |
| Probable, n (%) | 9 (36%) |
| Possible, n (%) Clinical improvement following DAP withdrawal, n (%) | 16 (64%) 14 (56%) |
| DAP with EP (n = 25) | DAP without EP (n = 425) | p-Value | |
|---|---|---|---|
| Age in years, median (IQR) | 72.0 (26) | 64.0 (32) | 0.030 |
| Male, n (%) | 18 (72.0%) | 249 (58.6%) | 0.185 |
| BW (IQR), kg | 53.8 (17.0) | 58.9 (21.6) | 0.079 |
| BMI (IQR), kg/m2 | 20.0 (6.3) | 21.9 (6.0) | 0.040 |
| BW/IBW > 1, n (%) | 9 (36.0) | 192 (45.2) | 0.297 |
| BW/ABW > 1, n (%) | 11 (44.0) | 240 (56.5) | 0.203 |
| Heart failure, n (%) | 10 (40.0) | 134 (31.5) | 0.378 |
| Diabetes mellitus, n (%) | 6 (24.0) | 147 (34.6) | 0.277 |
| Respiratory disease, n (%) | 18 (72.0) | 255 (60.0) | 0.233 |
| Myocardial infarction, n (%) | 3 (12.0) | 48 (11.3) | 0.914 |
| Collagen disease, n (%) | 2 (8.00) | 41 (9.6) | 0.785 |
| Hepatic disease, n (%) | 5 (20.0) | 68 (16.0) | 0.598 |
| Solid tumor, n (%) | 11 (44.0) | 157 (36.9) | 0.478 |
| Hematological malignancy, n (%) | 1 (4.00) | 58 (13.6) | 0.165 |
| Chronic kidney disease, n (%) | 9 (36.0) | 89 (20.9) | 0.076 |
| Hemodialysis, n (%) | 10 (40.0) | 57 (13.4) | <0.001 |
| Cerebrovascular disease, n (%) | 2 (8.00) | 66 (15.5) | 0.307 |
| Hypertension, n (%) | 16 (64.0) | 210 (49.4) | 0.156 |
| HIV, n (%) | 0 (0) | 1 (0.2) | 0.808 |
| Immunosuppressor, n (%) | 1 (4.0) | 35 (8.2) | 0.448 |
| Biological agent, n (%) | 0 (0) | 1 (0) | 0.808 |
| Steroid, n (%) | 5 (20.0) | 98 (23.1) | 0.723 |
| Statin, n (%) | 6 (24.0) | 86 (20.2) | 0.650 |
| Shock (SBP < 90), n (%) | 7 (28.0) | 158 (37.2) | 0.355 |
| qSOFA > 2, n (%) | 11 (44.0) | 193 (45.4) | 0.441 |
| DAP dosage (IQR) (mg/day) | 525 (235) | 350 (230) | 0.046 |
| DAP dosage /BW (IQR) (mg/kg) | 9.00 (2.7) | 7.50 (2.60) | <0.001 |
| DAP dosage/IBW (IQR) (mg/kg) | 8.90 (2.70) | 7.40 (3.84) | 0.025 |
| DAP dosage/ABW (IQR) (mg/kg) | 8.93 (2.33) | 7.44 (3.25) | 0.020 |
| Total dosage of DAP (IQR) (mg) | 7875 (9300) | 4800 (8100) | 0.032 |
| WBC (IQR) (/μL) | 8400 (2950) | 7600 (5600) | 0.625 |
| Blood eosinophilia (IQR) (/μL) | 419 (910) | 96 (255) | <0.001 |
| HGB (IQR) (g/dL) | 10.0 (3.0) | 9.50 (2.8) | 0.744 |
| PLT (IQR) (/μL) | 21.2 (15.8) | 21.1 (20.0) | 0.643 |
| BUN (IQR) (mg/dL) | 20.0 (30.0) | 18.4 (24.7) | 0.968 |
| sCr (IQR) (mg/dL) | 1.01 (1.92) | 0.92 (1.18) | 0.929 |
| eGFR (IQR) (mL/min/1.73 m2) | 62.1 (78.0) | 58.8 (61.9) | 0.671 |
| CCr (IQR) (mL/min/1.73 m2) | 68.2 (66.1) | 58.9 (82.4) | 0.361 |
| LDH (IQR) (IU/l) | 231.5 (155) | 227 (139) | 0.450 |
| CK (IQR) (IU.L) | 26 (90) | 47 (84) | 0.102 |
| CRP (IQR) mg/dL | 10.6 (15.8) | 5.39 (9.2) | 0.010 |
| Positive blood culture within one month, n (%) | 8 (32.0) | 145 (34.1) | 0.828 |
| MRSA infection, n (%) | 4 (16.0) | 29 (6.8) | 0.087 |
| CNS infection, n (%) | 2 (8.0) | 58 (13.6) | 0.420 |
| Mortality at discharge, n (%) | 5 (20.0) | 87 (20.6) | 0.946 |
| Mortality within 30 days from DAP administration, n (%) | 4 (16.0) | 59 (13.9) | |
| Mortality within 90 days from DAP administration, n (%) | 4 (16.0) | 76 (17.9) | |
| ICU admission within 30 days from admission, n (%) | 5 (20.0) | 75 (17.6) | |
| Mechanical intubation, n (%) | 5 (20.0) | 65 (15.3) |
| OR (95%CI) | p-Value | |
|---|---|---|
| Age | 1.03 (1.00–1.05) | 0.042 |
| DAP dosage/BW (mg/kg) | 1.61 (1.25–2.07) | <0.001 |
| Hemodialysis | 4.42 (1.86–10.5) | 0.010 |
| MRSA infection | 2.04 (0.63–6.62) | 0.235 |
Definite
|
Probable
|
Possible
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, K.; Matsuo, T.; Tsuda, Y.; Rahman, M.; Uehara, Y.; Mori, N. Factors Associated with Daptomycin-Induced Eosinophilic Pneumonia. Antibiotics 2022, 11, 254. https://doi.org/10.3390/antibiotics11020254
Ishikawa K, Matsuo T, Tsuda Y, Rahman M, Uehara Y, Mori N. Factors Associated with Daptomycin-Induced Eosinophilic Pneumonia. Antibiotics. 2022; 11(2):254. https://doi.org/10.3390/antibiotics11020254
Chicago/Turabian StyleIshikawa, Kazuhiro, Takahiro Matsuo, Yasumasa Tsuda, Mahbubur Rahman, Yuki Uehara, and Nobuyoshi Mori. 2022. "Factors Associated with Daptomycin-Induced Eosinophilic Pneumonia" Antibiotics 11, no. 2: 254. https://doi.org/10.3390/antibiotics11020254
APA StyleIshikawa, K., Matsuo, T., Tsuda, Y., Rahman, M., Uehara, Y., & Mori, N. (2022). Factors Associated with Daptomycin-Induced Eosinophilic Pneumonia. Antibiotics, 11(2), 254. https://doi.org/10.3390/antibiotics11020254






