Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review
Abstract
:1. Introduction
2. Results
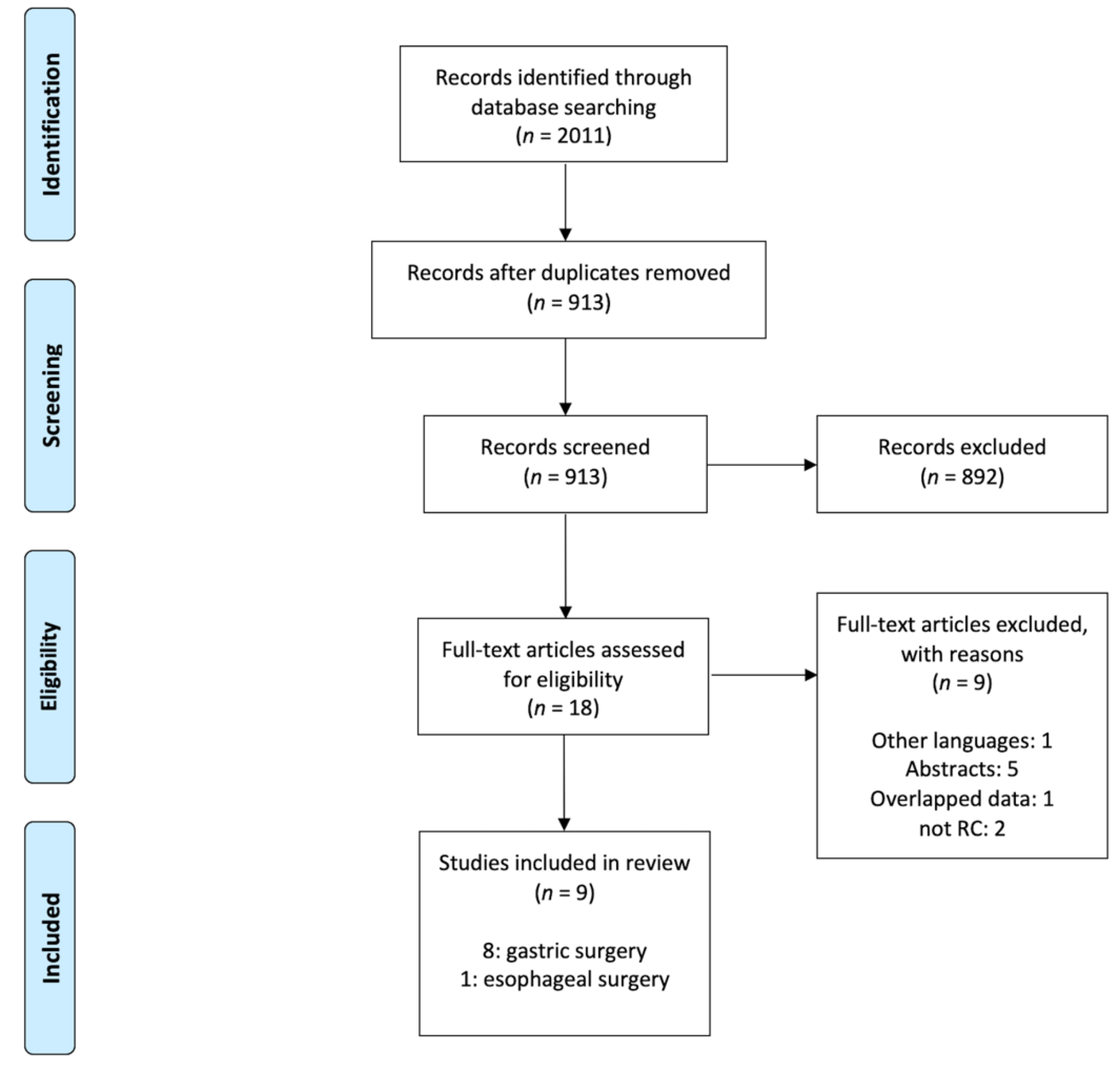
2.1. Study Selection
2.2. Reported Outcomes
2.2.1. Antimicrobial Prophylaxis: Do’s or Don’ts
2.2.2. Types of Antibiotics
2.2.3. Antimicrobial Regimen Selection in Gastric Surgery: Single- or Multiple-Dose
2.2.4. Antimicrobial Regimen Selection in Esophageal Surgery: Single- or Multiple-Dose
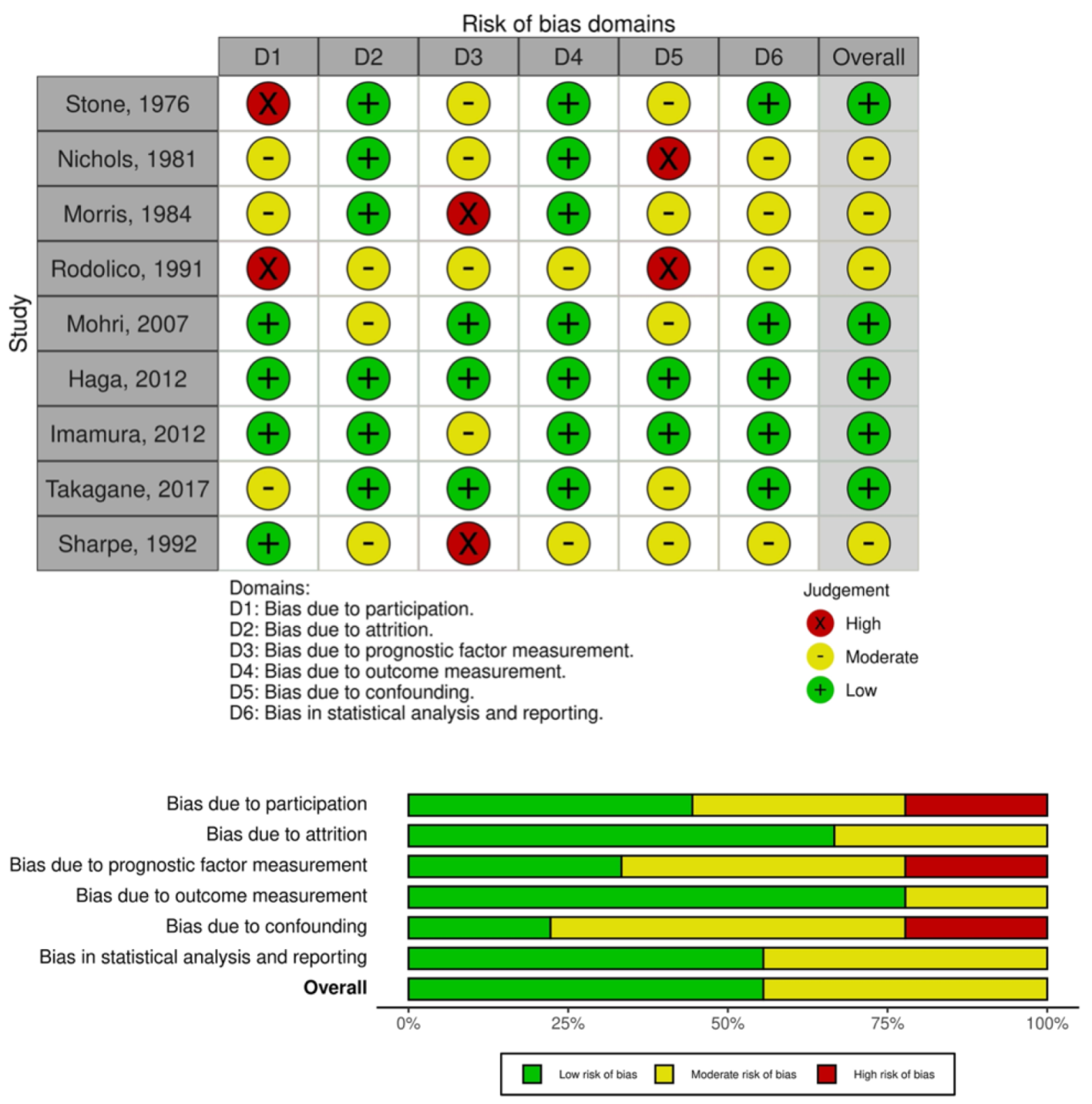
2.3. Quality Assessment
3. Discussion
- Antimicrobic prophylaxis reduces SSI in upper-GI surgical patients;
- Preoperative administration of antibiotics results in a more effective reduction in SSI rate;
- Single-dose is non-inferior to multiple-dose prophylaxis in terms of SSIs reduction rate;
- Short-term prophylaxis shows the same effect of long-term prophylaxis in terms of the risk of occurrence of SSI.
- An interesting meta-analysis [50] showed that the routine placement of drains was not necessary in elective surgery for gastric cancer, because of higher incidence of postoperative complication and longer hospital stay. Several authors, therefore, encouraged a drain-free strategy [51,52]. On the other hand, Haga et al. encountered no change in incidence of incisional SSI at the drain sites or organ/space SSI around the tips of the drains, advocating for placement of drains at the end of surgery [34].
- Laparoscopy, in addition to accelerating recovery by decreasing pain and duration of hospital stay, has been shown to be associated with a lower risk of SSI after colorectal surgery [53,54,55]. To our knowledge, few studies compared laparoscopic and open approaches. Minimally invasive surgery was found to be a protective factor for SSI in gastric surgery [56]. In this review, the percentage of patients treated with a laparoscopic approach was too low to draw strong conclusions, and no robotic procedures have been reported yet.
- The accepted consensus is to perform adjuvant chemotherapy in gastric cancer patients with advanced stages of disease or with pN+ [57]. Nevertheless, a considerable number of patients do not start the therapy or drop out [58]. The minimally invasive approach, early hospital discharge, and no post-operative complications appears to have a great psychological impact and to improve therapeutic compliance [59,60]. On the contrary, SSI occurrence involves long hospitalization, consistent use of antibiotics, and wound drainage or rigorous wound debridement when appropriate [61], resulting in reduced adherence to chemotherapy.
4. Conclusions
5. Materials and Methods
5.1. Searches
5.2. Selection
5.3. Data Extraction
- -
- RCTs;
- -
- Comparison between different doses (no-drug, single-dose if performed during surgery, or multiple-dose if performed also postoperatively) of AMP for esophageal or gastric surgery. Multiple-dose AMP were also distinguished in short-term (within 24 h postoperatively) and long-term (beyond the first postoperative day) regimens;
- -
- -
- Studies lacking full-text available and not published in English language;
- -
5.4. Definition of Surgical Site Infection
- Purulent drainage, with or without laboratory confirmation, from the incision;
- Microorganism isolated from an aseptically obtained culture of fluid or tissue from the incision;
- At least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, heat, or fever (>38 °C);
- Spontaneous wound dehiscence (superficial incisional SSI);
- Abscess or other evidence of infection involving the fascia, muscle layer or the intra-abdominal cavity found on direct examination, during reoperation, or by histopathological or radiological findings.
5.5. Summary Measures
5.6. Quality Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chodak, G.W. Use of Systemic Antibiotics for Prophylaxis in Surgery. Arch. Surg. 1977, 112, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Aga, E.; Keinan-Boker, L.; Eithan, A.; Mais, T.; Rabinovich, A.; Nassar, F. Surgical site infections after abdominal surgery: Incidence and risk factors. A prospective cohort study. Infect. Dis. 2015, 47, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. The Hospital Infection Control Practices Advisory Committee Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef]
- Pollock, A. Surgical Prophylaxis—The Emerging Picture. Lancet 1988, 331, 225–230. [Google Scholar] [CrossRef]
- Hall, J.C.; Watts, J.M.; Press, L.; O’Brien, P.; Turnidge, J.; McDonald, P. Single-Dose Antibiotic Prophylaxis in Contaminated Abdominal Surgery. Arch. Surg. 1989, 124, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992, 326, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Polk, H.C.; Christmas, A.B. Prophylactic antibiotics in surgery and surgical wound infections. Am. Surg. 2000, 66, 105–111. [Google Scholar] [PubMed]
- Bratzler, D.W.; Houck, P.M.; Richards, C.; Steele, L.; Dellinger, E.P.; Fry, D.E.; Wright, C.; Ma, A.; Carr, K.; Red, L. Use of Antimicrobial Prophylaxis for Major Surgery. Arch. Surg. 2005, 140, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, T.L.; Sawyer, R.G. The end of postoperative antimicrobial prophylaxis? Lancet Infect. Dis. 2012, 12, 357–358. [Google Scholar] [CrossRef]
- Kao, L.S.; Ghaferi, A.A.; Ko, C.Y.; Dimick, J.B. Reliability of Superficial Surgical Site Infections as a Hospital Quality Measure. J. Am. Coll. Surg. 2011, 213, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.S.; Leekha, S.; Magder, L.S.; Pineles, L.; Anderson, D.J.; Trick, W.; Woeltje, K.F.; Kaye, K.S.; Lowe, T.J.; Harris, A.D. Electronically Available Comorbidities Should Be Used in Surgical Site Infection Risk Adjustment. Clin. Infect. Dis. 2017, 65, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011, 14, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabtree, T.D.; Pelletier, S.J.; Gleason, T.G.; Pruett, T.L.; Sawyer, R. Clinical characteristics and antibiotic utilization in surgical patients with Clostridium difficile-associated diarrhea. Am. Surg. 1999, 65, 507–512. [Google Scholar]
- Childs, S.J.; Debessonet, D.A.; Merlin, A.S. Antibiotic prophylaxis in elective genitourinary tract surgery: A comparison of single-dose pre-operative cefotaxime and multiple-dose cefoxitin. J. Antimicrob. Chemother. 1984, 14, 271–275. [Google Scholar] [CrossRef]
- Kannan, A.; Ravichandran, M.; Sundaramurthi, S.; Win, M.; Tara, A.; Ruo, S.W.; Sultan, W.; Yanamala, V.L.; Mohammed, A.R.H.; Dominic, J.L. Is Single-Dose Antimicrobial Prophylaxis Sufficient to Control Infections in Gastrointestinal Oncological Surgeries? Cureus 2021, 13, e16939. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Samore, M.H.; Lichtenberg, D.; Carmeli, Y. Prolonged Antibiotic Prophylaxis after Cardiovascular Surgery and Its Effect on Surgical Site Infections and Antimicrobial Resistance. Circulation 2000, 101, 2916–2921. [Google Scholar] [CrossRef] [Green Version]
- Hogenauer, C.; Hammer, H.F.; Krejs, G.J.; Reisinger, E.C. Mechanisms and Management of Antibiotic-Associated Diarrhea. Clin. Infect. Dis. 1998, 27, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-D. Extended antimicrobial prophylaxis after gastric cancer surgery: A systematic review and meta-analysis. World J. Gastroenterol. 2013, 19, 2104–2109. [Google Scholar] [CrossRef]
- Wiström, J.; Norrby, S.R.; Myhre, E.B.; Eriksson, S.; Granström, G.; Lagergren, L.; Englund, G.; Nord, C.E.; Svenungsson, B. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J. Antimicrob. Chemother. 2001, 47, 43–50. [Google Scholar] [CrossRef]
- Kreisel, D.; Savel, T.G.; Silver, A.L.; Cunningham, J.D. Surgical Antibiotic Prophylaxis and Clostridium difficile Toxin Positivity. Arch. Surg. 1995, 130, 989–993. [Google Scholar] [CrossRef]
- Hall, J.C.; Mander, J.; Christiansen, K.; Reid, C.; Cooney, M.; Gibb, S.M. Cost-Efficiency Of A Long-Acting Cephalosporin Agent. ANZ J. Surg. 1988, 58, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tong, S.; Yu, B.; Tang, W.; Wu, Z.; Wang, S.; Wu, Y.; Lu, W.; Luo, M.; Wang, J. Single-dose ceftriaxone versus multiple-dose cefuroxime for prophylaxis of surgical site infection. Zhonghua Wai Ke Za Zhi 2003, 41, 372–374. [Google Scholar] [PubMed]
- Sumiyama, Y.; Kusunoki, M. Randomized clinical trial about the period of antimicrobial prophylaxis administration in total gastrectomy for gastric cancer. 2008. JPRN-UMIN000001062.
- Svaninger, G.; Forssell, H.; Leth, R.; Lind, T.; Lundell, L.; Olbe, L. Antibiotic prophylaxis in high-risk gastric surgery. A prospective, randomized clinical comparison of cefuroxime and doxycycline. Acta Chir. Scand. 1987, 153, 577–580. [Google Scholar] [PubMed]
- Fukushima, R.; Konishi, T.; Mohri, Y.; Noie, T.; Ono, S.; Omura, K.; Sueyoshi, S.; Takagane, A.; Kusunoki, M.; Shibata, T.; et al. A prospective randomized study to assess the optimal duration of antimicrobial prophylaxis in total gastrectomy. Surg. Infect. 2014, 15, S-11. [Google Scholar] [CrossRef]
- Han, J.H.; Jeong, O.; Ryu, S.Y.; Jung, M.R.; Park, Y.K. Efficacy of Single-Dose Antimicrobial Prophylaxis for Preventing Surgical Site Infection in Radical Gastrectomy for Gastric Carcinoma. J. Gastric Cancer 2014, 14, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Aberg, C.; Thore, M. Single versus triple dose antimicrobial prophylaxis in elective abdominal surgery and the impact on bacterial ecology. J. Hosp. Infect. 1991, 18, 149–154. [Google Scholar] [CrossRef]
- Jeong, O.; Jung, M.R.; Ryu, S.Y.; Park, Y.-K.; Kim, M.C.; Kim, K.H.; Ryu, S.W.; Kwon, I.G.; Gil Son, Y. Multicenter Phase 2 Study about the Safety of No Antimicrobial Prophylaxis Use in Low-Risk Patients Undergoing Laparoscopic Distal Gastrectomy for Gastric Carcinoma (KSWEET-01 Study). Gastroenterol. Res. Pract. 2017, 2017, 8928353. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, M.; Saka, M.; Katayama, H.; Okinaka, K.; Morita, S.; Fukagawa, T.; Katai, H. A Prospective Cohort Study To Evaluate the Feasibility of Intraoperative Antimicrobial Prophylaxis in Open Gastrectomy for Gastric Cancer. Surg. Infect. 2015, 16, 833–839. [Google Scholar] [CrossRef]
- Stone, H.H.; Hooper, C.A.; Kolb, L.D.; Geheber, C.E.; Dawkins, E.J. Antibiotic Prophylaxis in Gastric, Biliary and Colonic Surgery. Ann. Surg. 1976, 184, 443–452. [Google Scholar] [CrossRef]
- Nichols, R.L.; Webb, W.R.; Jones, J.W.; Smith, J.W.; LoCicero, J. Efficacy of antibiotic prophylaxis in high risk gastroduodenal operations. Am. J. Surg. 1982, 143, 94–98. [Google Scholar] [CrossRef]
- Morris, D.; Young, D.; Burdon, D.; Keighley, M. Prospective randomized trial of single dose cefuroxime against mezlocillin in elective gastric surgery. J. Hosp. Infect. 1984, 5, 200–204. [Google Scholar] [CrossRef]
- Rodolico, G.; Puleo, S.; Blandino, G.; Scilletta, B.; Cavallaro, V.; Latteri, F.; Veroux, G.; Nicoletti, G. Aztreonam Versus Gentamicin for Short-Term Prophylaxis in Biliary and Gastric Surgery. Clin. Infect. Dis. 1991, 13, S616–S620. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Ishida, H.; Ishiguro, T.; Kumamoto, K.; Ishibashi, K.; Tsuji, Y.; Miyazaki, T. A Prospective Randomized Study to Assess the Optimal Duration of Intravenous Antimicrobial Prophylaxis in Elective Gastric Cancer Surgery. Int. Surg. 2012, 97, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, D.A.; Renwick, P.; Mathews, K.H.; Moghissi, K. Antibiotic prophylaxis in oesophageal surgery. Eur. J. Cardio-Thorac. Surg. 1992, 6, 561–564. [Google Scholar] [CrossRef]
- Mohri, Y.; Tonouchi, H.; Kobayashi, M.; Nakai, K.; Kusunoki, M. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br. J. Surg. 2007, 94, 683–688. [Google Scholar] [CrossRef]
- Takagane, A.; Mohri, Y.; Konishi, T.; Fukushima, R.; Noie, T.; Sueyoshi, S.; Omura, K.; Ono, S.; Kusunoki, M.; Mochizuki, H.; et al. Randomized clinical trial of 24 versus 72 h antimicrobial prophylaxis in patients undergoing open total gastrectomy for gastric cancer. Br. J. Surg. 2017, 104, e158–e164. [Google Scholar] [CrossRef] [Green Version]
- Imamura, H.; Kurokawa, Y.; Tsujinaka, T.; Inoue, K.; Kimura, Y.; Iijima, S.; Shimokawa, T.; Furukawa, H. Intraoperative versus extended antimicrobial prophylaxis after gastric cancer surgery: A phase 3, open-label, randomised controlled, non-inferiority trial. Lancet Infect. Dis. 2012, 12, 381–387. [Google Scholar] [CrossRef]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef]
- Laloto, T.L.; Gemeda, D.H.; Abdella, S.H. Incidence and predictors of surgical site infection in Ethiopia: Prospective cohort. BMC Infect. Dis. 2017, 17, 119. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) Report, Data Summary from October 1986–April 1996, Issued May 1996. A Report from the National Nosocomial Infections Surveillance (NNIS) System. Am. J. Infect. Control 1996, 24, 380–388. [Google Scholar] [CrossRef]
- De Lissovoy, G.; Fraeman, K.; Hutchins, V.; Murphy, D.; Song, D.; Vaughn, B.B. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control 2009, 37, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.; Mitchell, S.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohge, H.; Mayumi, T.; Haji, S.; Kitagawa, Y.; Kobayashi, M.; Kobayashi, M.; Mizuguchi, T.; Mohri, Y.; Sakamoto, F.; Shimizu, J.; et al. The Japan Society for Surgical Infection: Guidelines for the prevention, detection, and management of gastroenterological surgical site infection, 2018. Surg. Today 2020, 51, 1–31. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Grabsch, E.; Marshall, C.; Forbes, A. Single-Versus Multiple–Dose Antimicrobial Prophylaxis For Major Surgery: A Systematic Review. ANZ J. Surg. 1998, 68, 388–395. [Google Scholar] [CrossRef]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC Definitions of Nosocomial Surgical Site Infections, 1992: A Modification of CDC Definitions of Surgical Wound Infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. North Am. 2020, 30, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.; Phillips, E.J. Antibiotic allergy. Lancet 2018, 393, 183–198. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, H.; Xu, C.; Xu, G. Spotlights on Antibiotic-induced Acute Kidney Injury: The Evidence to Date. Iran. J. Kidney Dis. 2019, 13, 10–20. [Google Scholar]
- Wang, Z.; Chen, J.; Su, K.; Dong, Z. Abdominal drainage versus no drainage post-gastrectomy for gastric cancer. Cochrane Database Syst. Rev. 2015, 2015, CD008788. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.P.; Zhang, Y.C.; Zhang, Y.L.; Yin, L.N.; Wang, J. Drain versus No-Drain after Gastrectomy for Patients with Advanced Gastric Cancer: Systematic Review and Meta-Analysis. Dig. Surg. 2011, 28, 178–189. [Google Scholar] [CrossRef]
- Shimoike, N.; Akagawa, S.; Yagi, D.; Sakaguchi, M.; Tokoro, Y.; Nakao, E.; Tamura, T.; Fujii, Y.; Mochida, Y.; Umemoto, Y.; et al. Laparoscopic gastrectomy with and without prophylactic drains in gastric cancer: A propensity score-matched analysis. World J. Surg. Oncol. 2019, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.J.; Datta, G.; Cunnick, G.; Gatzen, C.; Huang, A. Surgical site infection rate is lower in laparoscopic than open colorectal surgery. Color. Dis. 2010, 12, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Aimaq, R.; Akopian, G.; Kaufman, H.S. Surgical Site Infection Rates in Laparoscopic Versus Open Colorectal Surgery. Am. Surg. 2011, 77, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.P.; El-Gazzaz, G.H.; Vogel, J.D.; Remzi, F.H. Laparoscopic Approach Significantly Reduces Surgical Site Infections after Colorectal Surgery: Data from National Surgical Quality Improvement Program. J. Am. Coll. Surg. 2010, 211, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, M.; Sugita, H.; Otsuki, S.; Sato, Y.; Nakagawa, M.; Kojima, K. Laparoscopic distal gastrectomy reduced surgical site infection as compared with open distal gastrectomy for gastric cancer in a meta-analysis of both randomized controlled and case-controlled studies. Int. J. Surg. 2015, 15, 61–67. [Google Scholar] [CrossRef]
- De Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2016, 20, 20–30. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, W.; Lin, J. Delaying adjuvant chemotherapy in advanced gastric cancer patients: Risk factors and its impact on survival outcome. Curr. Probl. Cancer 2020, 44, 100577. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery after Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Guzman-Pruneda, F.A.; Husain, S.; Jones, C.D.; Beal, E.; Porter, E.; Grove, M.; Moffatt-Bruce, S.; Schmidt, C.R. Compliance with preoperative care measures reduces surgical site infection after colorectal operation. J. Surg. Oncol. 2018, 119, 497–502. [Google Scholar] [CrossRef]
- Mellinghoff, S.C.; Otto, C.; Cornely, O.A. Surgical site infections. Curr. Opin. Infect. Dis. 2019, 32, 517–522. [Google Scholar] [CrossRef]
- Hochreiter, M.; Uhling, M.; Sisic, L.; Bruckner, T.; Heininger, A.; Hohn, A.; Ott, K.; Schmidt, T.; Berger, M.M.; Richter, D.C.; et al. Prolonged antibiotic prophylaxis after thoracoabdominal esophagectomy does not reduce the risk of pneumonia in the first 30 days: A retrospective before-and-after analysis. Infection 2018, 46, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ruol, A.; Bertiato, G.; Boscarino, S.; Cusinato, R.; Pascarella, M.; Tonin, E.; Santi, S.; Ancona, E. Short-Term Prophylaxis with Ceftriaxone Plus Metronidazole in Esophageal Cancer Surgery. J. Chemother. 2000, 12, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.M.; Thom, K.A.; Preas, M.A. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection (2017): A summary, review, and strategies for implementation. Am. J. Infect. Control 2018, 46, 602–609. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, J.P.; Braun, B.I.; Hellinger, W.C.; Kusek, L.; Bozikis, M.R.; Bush, A.J.; Dellinger, E.P.; Burke, J.P.; Simmons, B.; Kritchevsky, S. Timing of Antimicrobial Prophylaxis and the Risk of Surgical Site Infections. Ann. Surg. 2009, 250, 10–16. [Google Scholar] [CrossRef]
- Morita, S.; Nishisho, I.; Nomura, T.; Fukushima, Y.; Morimoto, T.; Hiraoka, N.; Shibata, N. The Significance of the Intraoperative Repeated Dosing of Antimicrobials for Preventing Surgical Wound Infection in Colorectal Surgery. Surg. Today 2005, 35, 732–738. [Google Scholar] [CrossRef]
- Swoboda, S.M.; Merz, C.; Kostuik, J.; Trentler, B.; Lipsett, P.A. Does Intraoperative Blood Loss Affect Antibiotic Serum and Tissue Concentrations? Arch. Surg. 1996, 131, 1165–1172. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2, 2021; Cochrane, Canada, 2021; Available online: https://training.cochrane.org/handbook/current (accessed on 29 September 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Abis, G.S.A.; Stockmann, H.B.A.C.; Van Egmond, M.; Bonjer, H.J.; Vandenbroucke-Grauls, C.M.J.E.; Oosterling, S.J. Selective Decontamination of the Digestive Tract in Gastrointestinal Surgery: Useful in Infection Prevention? A Systematic Review. J. Gastrointest. Surg. 2013, 17, 2172–2178. [Google Scholar] [CrossRef]
- Schardey, H.M.; Joosten, U.; Finke, U.; Staubach, K.H.; Schauer, R.; Heiss, A.; Kooistra, A.; Rau, H.G.; Nibler, R.; Lüdeling, S.; et al. The Prevention of Anastomotic Leakage after Total Gastrectomy with Local Decontamination. Ann. Surg. 1997, 225, 172–180. [Google Scholar] [CrossRef]
- Farran, L.; Llop, J.; Sans, M.; Kreisler, E.; Miró, M.; Galan, M.; Rafecas, A. Efficacy of enteral decontamination in the prevention of anastomotic dehiscence and pulmonary infection in esophagogastric surgery. Dis. Esophagus 2008, 21, 159–164. [Google Scholar] [CrossRef]
- Gaynes, R.P.; Culver, D.H.; Horan, T.C.; Edwards, J.R.; Richards, C.; Tolson, J.S.; System, T.N.N.I.S. Surgical Site Infection (SSI) Rates in the United States, 1992–1998: The National Nosocomial Infections Surveillance System Basic SSI Risk Index. Clin. Infect. Dis. 2001, 33, S69–S77. [Google Scholar] [CrossRef]
| Study ID, Year | Country | Study Period | Number of Participants (I Coh/II Coh) | Age (Years) | Surgical Procedure | Duration Follow-Up (Days) | I Cohort | II Cohort | Reported Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Stone, 1976 | USA (single center) | 1974–1976 | 96 (A = 22; B = 27; C = 24; D = 23) | 47.6 (2–86) | Open approach Elective admission Gastric surgery | Uncertain | Patients were divided into four treatment categories: A = cefazolin being administered 8–12 h pre-operatively; B = cefazolin being administered just prior to operation; C = cefazolin being administered after operation; | D = not antibiotic. | SSI: Group A = 1 (5%) Group B = 1 (4%) Group C = 4 (17%) Group D = 5 (22%) |
| Nichols, 1981 | USA (single center) | 1978–1980 | 39 (19/20) | I coh: (39–76) II coh: (21–78) | Open approach Gastroduodenal surgery | Uncertain | Patients received a total of 4 g of cefamandole:
| Patients received equal volumes of inert placebo at the same intervals. | SSI (p < 0.01): I Cohort = 1 (5%) II Cohort = 7 (35%) |
| Morris, 1984 | USA (single center) | Undefined | 78 (40/38) | Undefined | Open approach
| Uncertain | Patients received cefuroxime 1.5 g after induction of anesthesia. | Patients received mezlocillin 2 g after induction of anesthesia. | Incisional SSI: I Cohort = 1 (2.5%) II Cohort = 5 (13%) Organ/space SSI: I Cohort = 1 (2.5%) II Cohort = 3 (8%) Overall SSI (p = 0.03): I Cohort = 2 (5%) II Cohort = 8 (21%) |
| Rodolico, 1991 | Italy (single center) | Undefined | 30 (15/15) | I coh: 59 ± 13 (34–84) II coh: 58 ± 12 (32–72) | Open approach
| Uncertain | Patients received a total of 3 g of aztreonam:
| Patients received a total of 240 mg of gentamicin:
| SSI (p = 0.03): I Cohort = 0 II Cohort = 4 (27%) |
| Mohri, 2007 | Japan (multicenter) | 2001–2004 | 486 (243/243) | I coh: 68 (22–91) II coh: 68 (23–90) | Open approach Elective admission
| 45 | Patients received 1 g of cefazolin or 1.5 g of ampicillin-sulbactam before surgical incision and every 3 h as intraoperative supplements. | Patients received intraoperative schedule and additional doses every 12 h postoperatively, until a total of 7 doses. | Incisional SSI: I Cohort = 14 (5.8%) II Cohort = 11 (4.5%) Organ/Space SSI: I Cohort = 12 (4.9%) II Cohort = 10 (4.1%) Overall SSI: I Cohort = 23 (9.5%) 1 II Cohort = 21 (8.6%) |
| Haga, 2012 | Japan (single center) | 2007–2010 | 325 (164/161) | I coh: 68 (33–90) II coh: 68 (39–91) | Open (88.3%) or laparoscopic (11.7%) approach Elective admission Total or distal gastrectomy | 30 | Patients received 1 g of cefazolin. | Patients received intraoperative schedule and an additional 5 doses every 12 h postoperatively. | Incisional SSI: I Cohort = 14 (8.5%) II Cohort = 7 (4.3%) Organ/space SSI: I Cohort = 11 (6.7%) II Cohort = 6 (3.7%) Overall surgical incision 2: I Cohort = 15 (9.1%) II Cohort = 10 (6.2%) |
| Imamura, 2012 | Japan (multicenter) | 2005–2007 | 355 (176/179) | I coh: 66 (36–84) II coh: 65 (35–84) | Open (96%) or laparoscopic (4%) approach Distal gastrectomy | 30 | Patients received 1 g of cefazolin before surgical incision and every 3 h as intraoperative supplements. | Patients received intraoperative schedule and cefazolin 1 g once after closure and twice daily for two postoperative days. | Superficial incisional SSI: I Cohort = 1 (0.6%) I Cohort = 5 (2.8%) Deep incisional SSI: I Cohort = 0 II Cohort = 0 Organ or space SSI: I Cohort = 7 (4%) II Cohort = 11 (6.1%) Overall SSI: I Cohort = 8 (4.6%) II Cohort = 16 (8.9%) |
| Takagane, 2017 | Japan (multicenter) | 2008–2012 | 464 (228/236) | I coh: 65.5 ± 9.2 II coh: 64.7 ± 10 | Open approach Total gastrectomy | 30 | Patients received 1.5 g ampicillin-sulbactam for 24 h postoperatively. | Patients received 1.5 g ampicillin-sulbactam for 72 h postoperatively. | Superficial SSI: I Cohort = 3 (1.3%) II Cohort = 2 (0.8%) Deep SSI: I Cohort = 0 II Cohort = 2 (0.8%) Organ/space SSI: I Cohort = 17 (7.5%) II Cohort = 23 (9.7%) Overall SSI: I Cohort = 20 (8.8%) II Cohort = 26 (11%) 3 |
| Sharpe, 1992 | UK (single center) | Undefined | 226 (A = 41; B = 42; C = 46; D = 47; E = 50) | 63.5 (24–86) | Open approach Esophageal carcinoma (alimentary tract opened):
| Uncertain | When alimentary tract was opened, patients were divided into three treatment categories: A = cefoxitina 1.5 g and metronidazole 1 g at induction of anesthesia. When alimentary tract was not opened, patients were divided into two treatment categories: D = treated with cefoxitina 1.5 g on induction of anesthesia. | When alimentary tract was opened: >B = cefoxitina 1.5 g at induction of anesthesia and then cefoxitina 750 mg twice-daily for four days.C = cefoxitina 1.5 g and metronidazole 1 g at induction of anesthesia, then cefoxitina 750 mg twice-daily and metronidazole 500 mg four times per day for four days. When alimentary tract was not opened: E = treated with cefoxitina 1.5 g on induction of anesthesia, then cefoxitina 750 mg twice-daily for two days. | SSI: Group A = 4 (9.7%) Group B = 3 (7.2%) Group C = 1 (2.2%) Group D = 3 (6.4%) Group E = 2 (4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230. https://doi.org/10.3390/antibiotics11020230
Marano L, Carbone L, Poto GE, Calomino N, Neri A, Piagnerelli R, Fontani A, Verre L, Savelli V, Roviello F, et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics. 2022; 11(2):230. https://doi.org/10.3390/antibiotics11020230
Chicago/Turabian StyleMarano, Luigi, Ludovico Carbone, Gianmario Edoardo Poto, Natale Calomino, Alessandro Neri, Riccardo Piagnerelli, Andrea Fontani, Luigi Verre, Vinno Savelli, Franco Roviello, and et al. 2022. "Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review" Antibiotics 11, no. 2: 230. https://doi.org/10.3390/antibiotics11020230
APA StyleMarano, L., Carbone, L., Poto, G. E., Calomino, N., Neri, A., Piagnerelli, R., Fontani, A., Verre, L., Savelli, V., Roviello, F., & Marrelli, D. (2022). Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics, 11(2), 230. https://doi.org/10.3390/antibiotics11020230









