Effect of Antibiotic Prophylaxis on Surgical Site Infection in Thyroid and Parathyroid Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
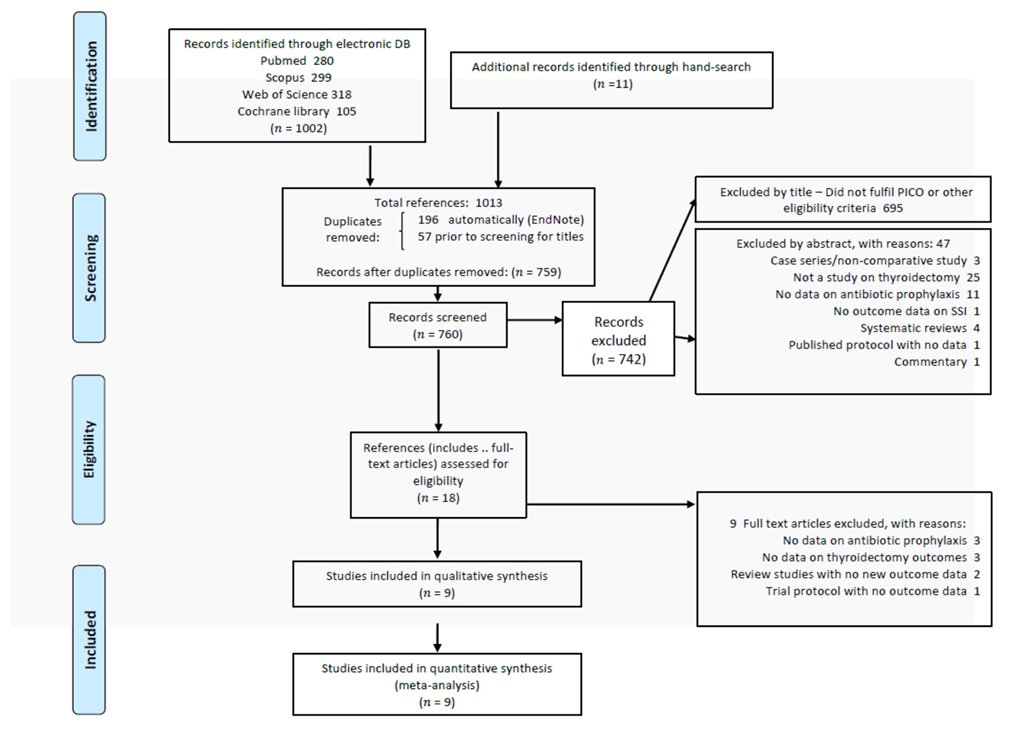
2.1. Literature Search and Selection of Studies
2.2. Characteristics of Interventions and Populations in the Included Studies
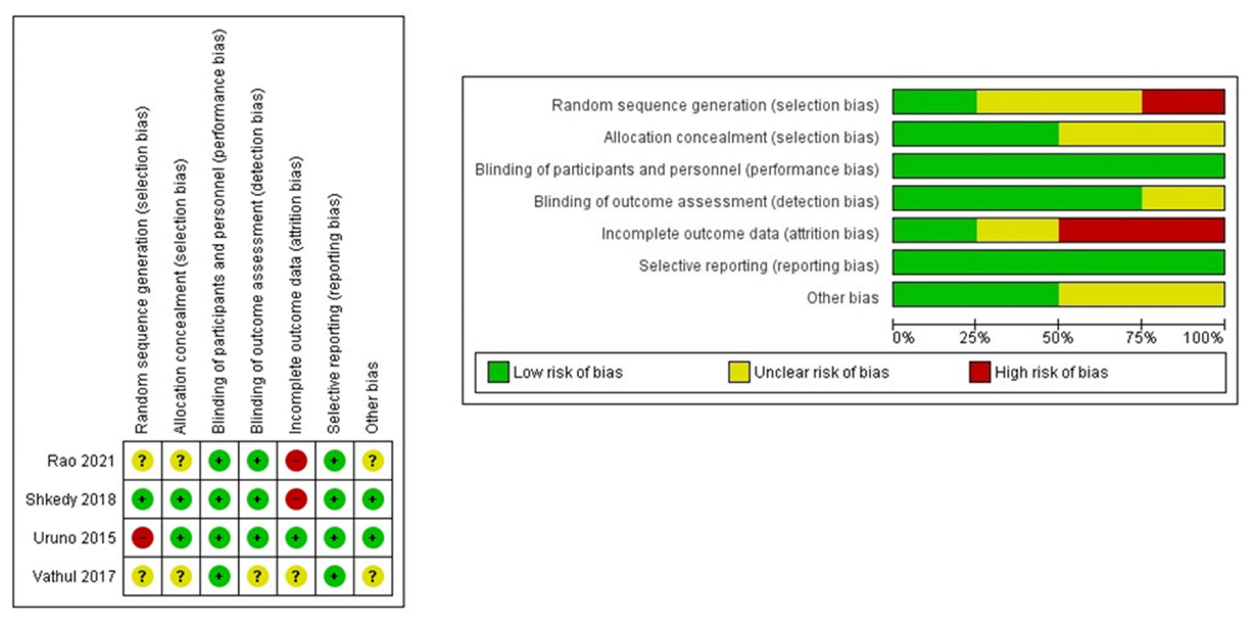
2.2.1. RCTs (Cochrane Tool)
2.2.2. nRCTs (MINORS Tool)
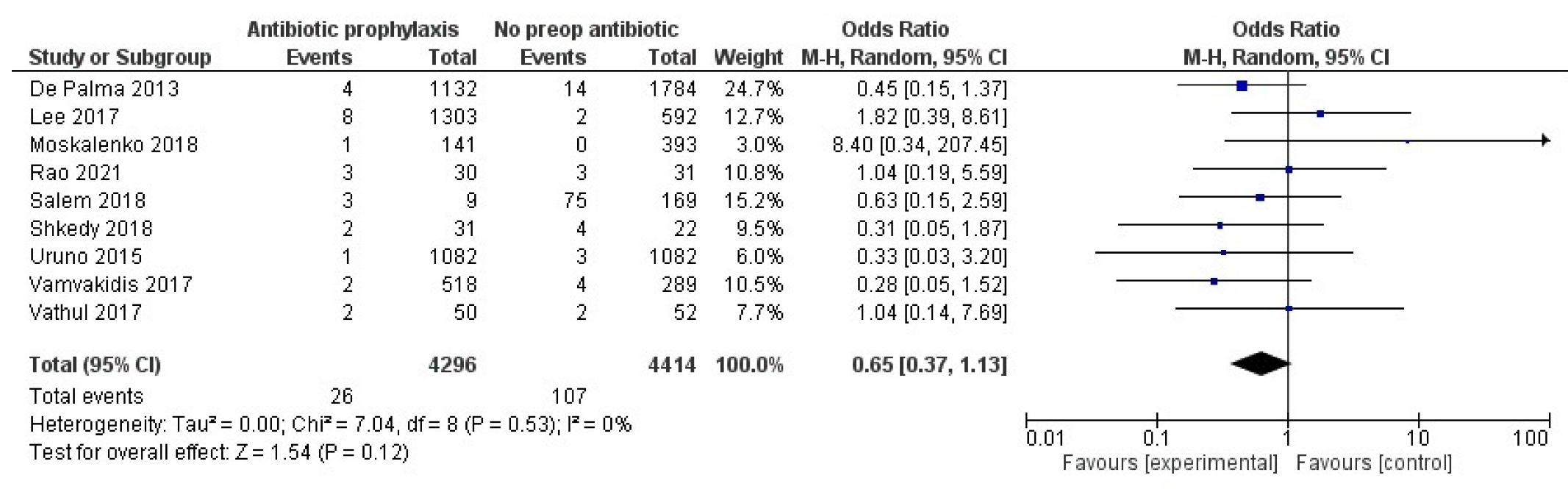
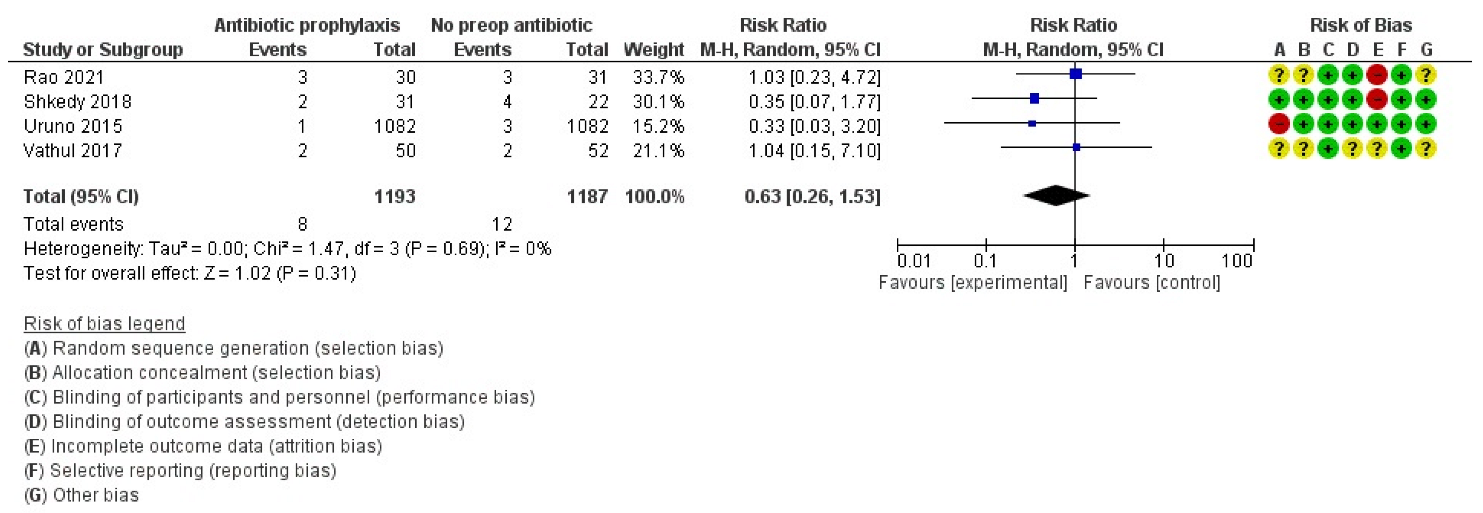
2.3. Primary Outcome
3. Discussion
4. Materials and Methods
4.1. Literature Search
- (Patients) adult patients who underwent thyroidectomy or thyroid lobectomy or parathyroidectomy;
- (Intervention) preoperative AP;
- (Comparator interventions) no preoperative AP or placebo;
- (Outcomes) SSI rate.
- (Methods-study design) Randomized controlled trials (RCTs) and observational studies. The choice to include both study designs was motivated by the aim to include as much evidence available as possible from existing comparative studies. Observational studies may more frequently enroll larger patient cohorts than RCTs, so given SSIs in thyroid and parathyroid surgery are a relatively rare event, including larger retrospective studies was expected to offer a higher chance to observe a meaningful treatment effect size if data could be pooled in a meta-analysis.
4.2. Selection of Studies
- -
- Studies were included if they specifically reported on SSIs by providing numerical data (generic report of postoperative complications or report of no complications without specific mention of SSIs was not considered sufficient for inclusion)
- -
- Adult participants (>16 years of age) diagnosed with thyroid and parathyroid diseases undergoing surgery. Study groups including clean neck interventions on organs other than thyroid or parathyroids or lymph nodes were acceptable only if any odd cases were making up for less than 5% of a study population and their prevalence was less than the SSI rate in a study group.
- -
- Studies focused on SSI outcomes were included only if data on the proportion of patients receiving antibiotic prophylaxis among SSI cases could be obtained.
- -
- Exclusion criteria were:
- -
- case reports, technical notes, expert opinions, tutorials, commentaries, protocols with no data, narrative reviews with no original data
- -
- comparative studies on clean-contaminated neck surgery
- -
- therapeutic, postoperative administration of antibiotic therapy
- -
- laboratory studies
4.3. Data Extraction
- -
- study characteristics (authors, publication year, country of origin, study design, sample size, and time interval for each study),
- -
- participants’ characteristics (sex, age, inclusion/exclusion criteria and diagnosis),
- -
- surgery characteristics (procedure type, operating time in minutes, rate of drain positioning, rate of radical neck dissection),
- -
- intervention characteristics (antibiotic, dose, frequency and primary end points), and outcome results (number of events in each group, total infections, length of hospital stay).
4.4. Risk of Bias Assessment
4.5. Measures of Treatment Effect
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy and Strings for Literature Search
Appendix A.1. Pubmed: ISI Web of Science Search String
Appendix A.2. Scopus Search String
Appendix A.3. Cochrane Library Search String
References
- Mangram, A.J.; Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Li, H.; Wang, L.-B.; Li, A.-H.; Chen, L.-J.; Lu, Q.J. Thyroid surgery without antibiotic prophylaxis: Experiences with 1030 patients from a teaching hospital in China. World J. Surg. 2014, 38, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, A.; Bergenfelz, A.; Jansson, S.; Kristoffersson, A.; Mårtensson, H.; Reihnér, E.; Wallin, G.; Lausen, I.J. Complications to thyroid surgery: Results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch. Surg. 2008, 393, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, G.; Rovera, F.; Boni, L.; Castano, P.; Dionigi, R.J. Surgical site infections after thyroidectomy. Surg. Infect. 2006, 7 (Suppl. 2), s-117–s-120. [Google Scholar] [CrossRef]
- Dionigi, G.; Rovera, F.; Boni, L.; Dionigi, R.J. Surveillance of surgical site infections after thyroidectomy in a one-day surgery setting. Int. J. Surg. 2008, 6, S13–S15. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef]
- Patel, K.N.; Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; et al. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef]
- Haugen, B.R.; Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Gentile, I.; Rosato, L.; Avenia, N.; Testini, M.; D’Ajello, M.; Antonino, A.; De Palma, M. Do Italian surgeons use antibiotic prophylaxis in thyroid surgery. Ann. Ital. Chir. 2014, 85, 33–37. [Google Scholar]
- Moalem, J.; Ruan, D.T.; Farkas, R.L.; Shen, W.T.; Kebebew, E.; Duh, Q.Y.; Clark, O.H. Patterns of antibiotic prophylaxis use for thyroidectomy and parathyroidectomy: Results of an international survey of endocrine surgeons. J. Am. Coll. Surg. 2010, 210, 949–956. [Google Scholar] [CrossRef]
- De Palma, M.; Grillo, M.; Borgia, G.; Pezzullo, L.; Lombardi, C.P.; Gentile, I.J. Antibiotic prophylaxis and risk of infections in thyroid surgery: Results from a national study (UEC—Italian Endocrine Surgery Units Association). Updat. Surg. 2013, 65, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Medas, F.; Canu, G.L.; Cappellacci, F.; Romano, G.; Amato, G.; Erdas, E.; Calo, P.G. Antibiotic Prophylaxis for Thyroid and Parathyroid Surgery: A Systematic Review and Meta-analysis. Otolaryngol.-Head Neck Surg. 2021, 164, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, M.; Asai, M.; Beem, K.; Pezzi, T.A.; Brophy, C.L.; Noonan, K.; Pezzi, C.M. Incidence of surgical site infections after thyroid and parathyroid surgery: No role for antimicrobial prophylaxis. Am. Surg. 2018, 84, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.V.; D’Souza, C. Is Prophylactic Antibiotic Therapy Needed in Thyroidectomy? Indian J. Otolaryngol. Head Neck Surg. 2021, 4. [Google Scholar] [CrossRef]
- Salem, F.; Almquist, M.; Nordenström, E.; Dahlberg, J.; Hessman, O.; Lundgren, C.; Bergenfelz, A.J. A Nested Case–Control Study on the Risk of Surgical Site Infection after Thyroid Surgery. World J. Surg. 2018, 42, 2454–2461. [Google Scholar] [CrossRef]
- Alonso-Garcia, M.; Toledano-Munoz, A.; Aparicio-Fernandez, J.M.; De-la-Rosa-Astacio, F.M.; Rodriguez-Villar, D.; Gil-de-Miguel, A.; Duran-Poveda, M.; Rodriguez-Caravaca, G. Adequacy of antibiotic prophylaxis and incidence of surgical site infections in neck surgery. Sci. Rep. 2021, 11, 7. [Google Scholar]
- Bures, C.; Klatte, T.; Gilhofer, M.; Behnke, M.; Breier, A.-C.; Neuhold, N.; Hermann, M.J.S. A prospective study on surgical-site infections in thyroid operation. Surgery 2014, 155, 675–681. [Google Scholar] [CrossRef]
- Elbur, A.I.; Yousif, M.A.E.R.; Elsayed, A.S.A.; Abdel-Rahman, M.E. An audit of prophylactic surgical antibiotic use in a Sudanese Teaching Hospital. Int. J. Clin. Pharm. 2013, 35, 149–153. [Google Scholar] [CrossRef]
- Elfenbein, D.M.; Schneider, D.F.; Chen, H.; Sippel, R.S. Surgical site infection after thyroidectomy: A rare but significant complication. J. Surg. Res. 2014, 190, 170–176. [Google Scholar] [CrossRef]
- Fachinetti, A.; Chiappa, C.; Arlant, V.; Kim, H.Y.; Liu, X.L.; Sun, H.; Dionigi, G.; Rovera, F. Antibiotic prophylaxis in thyroid surgery. Gland Surg. 2017, 6, 525–529. [Google Scholar] [CrossRef]
- Lu, Q.; Xie, S.-Q.; Chen, S.-Y.; Chen, L.-J.; Qin, Q.J. Experience of 1166 thyroidectomy without use of prophylactic antibiotic. Biomed Res. Int. 2014, 2014, 758432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Man, L.X.; Beswick, D.M.; Johnson, J.T. Antibiotic prophylaxis in uncontaminated neck dissection. Laryngoscope 2011, 121, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Nct. Antimicrobial Prophylaxis in Thyroid and Parathyroid Surgery. 2013. Available online: https://clinicaltrials.gov/show/NCT01805856 (accessed on 23 March 2013).
- Uruno, T.; Masaki, C.; Suzuki, A.; Ohkuwa, K.; Shibuya, H.; Kitagawa, W.; Nagahama, M.; Sugino, K.; Ito, K.J. Antimicrobial prophylaxis for the prevention of surgical site infection after thyroid and parathyroid surgery: A prospective randomized trial. World J. Surg. 2015, 39, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Kim, S.Y.; Kim, S.-M.; Chang, H.J.; Kim, B.-W.; Lee, Y.; Chang, H.-S.; Park, C.S.; Lim, C.Y. Perioperative Antibiotic Prophylaxis May Not Be Required Routinely in Thyroid Surgery. J. Endocr. Surg. 2017, 17, 160–167. [Google Scholar] [CrossRef][Green Version]
- Vamvakidis, K.; Rellos, K.; Tsourma, M.; Christoforides, C.; Anastasiou, E.; Zorbas, K.; Arambatzi, A.; Falagas, M.J.T. Antibiotic prophylaxis for clean neck surgery. Ann. R. Coll. Surg. Engl. 2017, 99, 410–412. [Google Scholar] [CrossRef]
- Shkedy, Y.; Stern, S.; Nachalon, Y.; Levi, D.; Menasherov, I.; Reifen, E.; Shpitzer, T.J. Antibiotic prophylaxis in clean head and neck surgery: A prospective randomised controlled trial. Clin. Otolaryngol. 2018, 43, 1508–1512. [Google Scholar] [CrossRef]
- Vathul, B.; Pari, M. Is antibiotic prophylaxis for open thyroidectomies necessary? A randomized trial in south indian population. Clujul Med. 2017, 90, S88. [Google Scholar]
- Myssiorek, D.; Ahmed, Y.; Parsikia, A.; Castaldi, M.; McNelis, J.J. Factors predictive of the development of surgical site infection in thyroidectomy–An analysis of NSQIP database. Int. J. Surg. 2018, 60, 273–278. [Google Scholar] [CrossRef]
- Mickenautsch, S. SYSTEM Research note on: Assessing publication bias. J. Minim. Interv. Dent. 2012, 5, 4–6. [Google Scholar]
- Edwards, J.R.; Peterson, K.D.; Mu, Y.; Banerjee, S.; Allen-Bridson, K.; Morrell, G.; Dudeck, M.A.; Pollock, D.A.; Horan, T.C. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am. J. Infect. Control. 2009, 37, 783–805. [Google Scholar] [CrossRef]
- Hardy, R.; Forsythe, J. Uncovering a rare but critical complication following thyroid surgery: An audit across the UK and Ireland. Thyroid 2007, 17, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Karlik, J.B.; Johnson-Obaseki, S.; Luo, L.; Javidnia, H.J.M. Severe group a streptococcus surgical site infection after thyroid lobectomy. Surg. Infect. 2013, 14, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Caulley, L.; Green, S. Risk factors for postoperative complications in total thyroidectomy: A retrospective, risk-adjusted analysis from the National Surgical Quality Improvement Program. Medicine 2017, 96, e5752. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.; Colley, L.; Lynd, L.D. Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials. J. Clin. Epidemiol. 2009, 62, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Shea, B.J.; Hamel, C.; Wells, G.A.; Bouter, L.M.; Kristjansson, E.; Grimshaw, J.; Henry, D.A.; Boers, M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009, 62, 1013–1020. [Google Scholar] [CrossRef]
- Hupe, M. EndNote X9. J. Electron. Resour. Med. Libr. 2019, 16, 117–119. [Google Scholar] [CrossRef]
- Tarsilla, M. Cochrane handbook for systematic reviews of interventions. J. Multidiscip. Eval. 2010, 6, 142–148. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 1–10. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
| Author, Publication Year, Country | Study Design | Inclusion Criteria | Time Interval | No. Pt | Mean Age, y | Male, % | Procedure | Thyroid Pathology | Radical Neck Dissection, % | Drain, % | Operative Time, min | Lenght of Stay, days | Antibiotic Prophylaxis | SSI, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Palma, 2013, Italy | Case control, Multicenter | Thyroid surgery within the study interval | 1 January 2009–31 December 2011 | 2926 | 52+/−14.6 | 22.3 | TT, n-TT, TL | Benign and malignant (15%-DTC, MTC, anaplastic ca) | NA | 91.4 | NA | NA | 1132 | 4/1132, 0.35 |
| 1784 | 14/1784, 0.78 | |||||||||||||
| Uruno, 2015, Japan | RCT, Single Center | Clean neck surgery for thyroid or parathyroid disease (Excl.: no consent, sternotomy, resection of trachea, larynx, pharynx, oesophagus, penicillin allergy) | November 2010–April 2012 | 2164 | 52+/−15.1 | 15.6 | TT, n-TT, TL, PTX (4%) | Benign and malignant | 9 | NA | 74.7+/−38.1 | 4 | 1082 (541 Piperacillin, 541 Cefazolin) | 1/1082, 0.09 |
| 52+/−14.7 | 14.9 | 8.7 | 76.1+/−34.0 | 1082 | 3/1082, 0.28 | |||||||||
| Lee, 2017, Korea | Retrospective cohort, Single Center | Thyroid surgery, single Institution, single surgeon (Excl.: endoscopic, robotic surgery) | January 2013–June 2013 | 1895 | 44+/−11.4 | 21.7 | TT, lt-TT | Malignant (89% CCND, 11% LND) | 12.2 | 100 | 108.6+/−56.6 | NA | 1303 | 8/1303 |
| July 2013–December 2013 | 43.6+/−10.5 | 26.4 | 9.6 | 100 | 99.0+/−44.0 | 592 | 2/592 | |||||||
| Vamvakidis, 2017, Greece | Retrospective cohort, Single Center | Clean neck surgery | 2010–2014 | 807 | 49 | 20.3 | TT, PTX | Benign and malignant (45%-PTC, MTC, other) | 8.8 | NA | 168.5 | NA | 518 | 2/518, 0.4 |
| 289 | 4/289, 1.4 | |||||||||||||
| Moskalenko, 2018, USA | Retrospective cohort, Single Center | Thyroid or parathyroid surgery, data from NSQIP database, single center | November 2007–June 2015 | 534 | 59.6 | 23.2 | TT, TL, PTX (32.9%) | NA | NA | 7/151, 5 | 79 | NA | 141 | 1/141, 0.7 |
| 60.6 | 21.7 | 4/393, 1 | 105 | 393 | 0/393, 0 | |||||||||
| Salem, 2018, Sweden | Nested Case-Control, Multicenter | Thyroid surgery, data from SQRTPA database | 2004–2010 | 218 ** | 53 | 26.6 | TT, TL | Benign and Malignant | 2.5 | NA | NA | NA | 9 | 3/9,33.9 |
| 19.2 | 169 | 75/16944.3 | ||||||||||||
| Shkedy, 2018, Israel | RCT, Single Center | Clean revision H&N surgery, >18 years, no preop indication to AP (Excl.: irradiation, other factors requiring abx, tracheostomy, concurrent infection, penicillin allergy, immunosuppression,) | January 2014–January 2017 | 53 | 54.5+/−15.7 | 19.4 | ND, TT, TL, PTX (3.7%), PTD (3.7%) | NA | 32.2 | NA | NA | 3.6+/−1.1 | 31 | 2/31, 6.5 |
| 55.5+/−14.2 | 36.4 | 13.6 | 3.5+/−1.1 | 22 | 4/22, 18.2 | |||||||||
| Vathul, 2018, India | RCT, Single Center | Benign (FNAC), TT or TL, >18 <70 years, not Immunocompromised | NA | 102 | NA | NA | TT, TL | Benign | - | 50 | NA | 4 | 50 | 2/50, 4 |
| 52 | 3 | 52 | 2/52, 3.8 | |||||||||||
| Rao, 2021, India | RCT, Single Center | Benign thyroid disease, >16 <80 years, consent (Excl.: diabetes, infective or hematologic disease, other infection, BMI > 25, steroids or immunosuppression, malignancy, drain > 70 mL) | 2021 | 67 | 44.33+/−7.9 | NA | TT, TL | Benign | - | 33 | NA | NA | 33 | 3/33, 9 |
| 43.11+/−6.9 | 34 | 34 | 3/34, 8.8 |
| Author, Publication Year, Country | Preoperative Patient Skin Prepping | Antibiotic Prophylaxis | Timing | Route | Follow Up | SSI Definition |
|---|---|---|---|---|---|---|
| De Palma, 2013, Italy | NA | cephalosporins or aminopenicillins ± beta lactamase inhibitors | NA | IV | NA | NA |
| Uruno, 2015, Japan | Chlorhexidine gluconate solution | Piperacillin, 2 g or Cefazolin, 1 g | Immediately after intubation—if operating time > 3 h, further dose | IV | 30 days | CDC guidelines for incisional SSI |
| Lee, 2017, Korea | NA | NA | NA | NA | 30 days | CDC guidelines for incisional SSI |
| Vamvakidis, 2017, Greece | NA | Cefuroxime | NA | IV | NA | NA |
| Moskalenko, 2018, USA | At the discretion of the operating surgeon (Povidone-iodine used in 96% of patients, or Chloraprep 3.2%) | Cefazolin, Vancomycin, or Clindamycin | NA | IV | 30 days | NSQIP criteria (CDC’s definitions for superficial incisional infection, deep incisional infection) |
| Salem, 2018, Sweden | NA | NA | NA | NA | six weeks | Local wound complication, SQRTPA criteria |
| Shkedy, 2018, Israel | NA | IV Cefazolin, 1 g (2 g if BMI > 40) | 30–60 min before surgery | IV | 30 days | CDC guidelines for incisional SSI |
| Vathul, 2018, India | NA | 3rd Gen Cephalosporins | 3–4 doses, NA | IV | three months | NA |
| Rao, 2021, India | Povidone iodine-Betadine for 2–5 min then 0.25 g benzalkonium chloride and 70 g 96% alcohol in concentric circles from the incision site (3 times) | Cefuroxime 1 g | Three doses eight hourly, from induction of anaesthesia | IV | six weeks | Southampton grading scale |
| Author, Publication Year, Country | Study Design | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Collection of Data | Endpoints Appropriate to the Aim of the Study | Unbiased Assessment of the Study Endpoint | Follow-Up Period Appropriate to the Aim of the Study | Loss to Follow-Up Less Than 5% | Prospective Calculation of the Study Size | An Adequate Control Group | Contemporary Groups | Baseline Equivalence of Groups | Adequate Statistical Analyses | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Palma, 2013, Italy | Case control, Multicenter | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 1 (DRAIN) | 2 | 15 |
| Lee, 2017, Korea | Retrospective cohort, Single Center | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 2 | 14 |
| Vamvakidis, 2017, Greece | Retrospective cohort, Single Center | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 18 |
| Moskalenko, 2018, USA | Retrospective cohort, Single Center | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Salem, 2018, Sweden | Matched Case-Control, Multicenter | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polistena, A.; Prete, F.P.; Avenia, S.; Cavallaro, G.; Di Meo, G.; Pasculli, A.; Rondelli, F.; Sanguinetti, A.; Sgaramella, L.I.; Avenia, N.; et al. Effect of Antibiotic Prophylaxis on Surgical Site Infection in Thyroid and Parathyroid Surgery: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 290. https://doi.org/10.3390/antibiotics11030290
Polistena A, Prete FP, Avenia S, Cavallaro G, Di Meo G, Pasculli A, Rondelli F, Sanguinetti A, Sgaramella LI, Avenia N, et al. Effect of Antibiotic Prophylaxis on Surgical Site Infection in Thyroid and Parathyroid Surgery: A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(3):290. https://doi.org/10.3390/antibiotics11030290
Chicago/Turabian StylePolistena, Andrea, Francesco Paolo Prete, Stefano Avenia, Giuseppe Cavallaro, Giovanna Di Meo, Alessandro Pasculli, Fabio Rondelli, Alessandro Sanguinetti, Lucia Ilaria Sgaramella, Nicola Avenia, and et al. 2022. "Effect of Antibiotic Prophylaxis on Surgical Site Infection in Thyroid and Parathyroid Surgery: A Systematic Review and Meta-Analysis" Antibiotics 11, no. 3: 290. https://doi.org/10.3390/antibiotics11030290
APA StylePolistena, A., Prete, F. P., Avenia, S., Cavallaro, G., Di Meo, G., Pasculli, A., Rondelli, F., Sanguinetti, A., Sgaramella, L. I., Avenia, N., Testini, M., & Gurrado, A. (2022). Effect of Antibiotic Prophylaxis on Surgical Site Infection in Thyroid and Parathyroid Surgery: A Systematic Review and Meta-Analysis. Antibiotics, 11(3), 290. https://doi.org/10.3390/antibiotics11030290







