Novel Phage Lysin Abp013 against Acinetobacter baumannii
Abstract
1. Introduction
2. Results
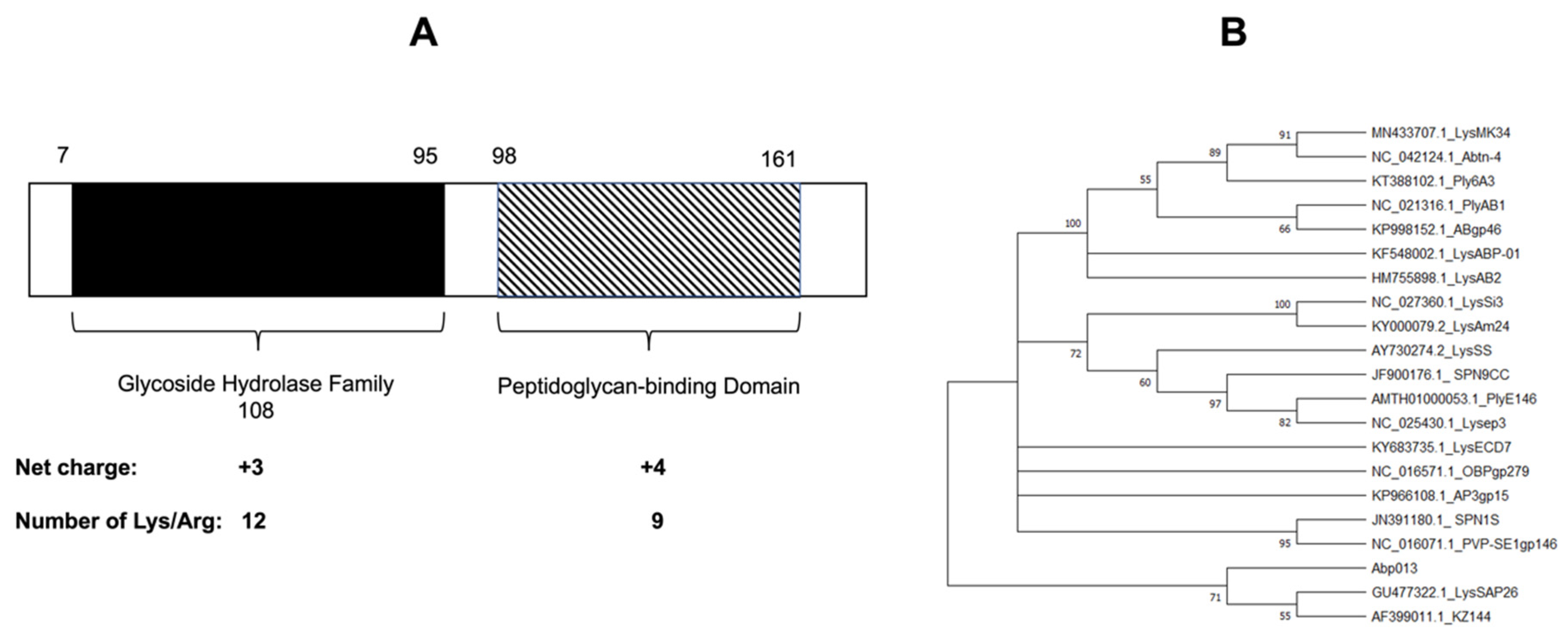
2.1. Identification and Bioinformatic Analysis of Abp013
2.2. Expression, Purification, and Functional Characterization of Abp013
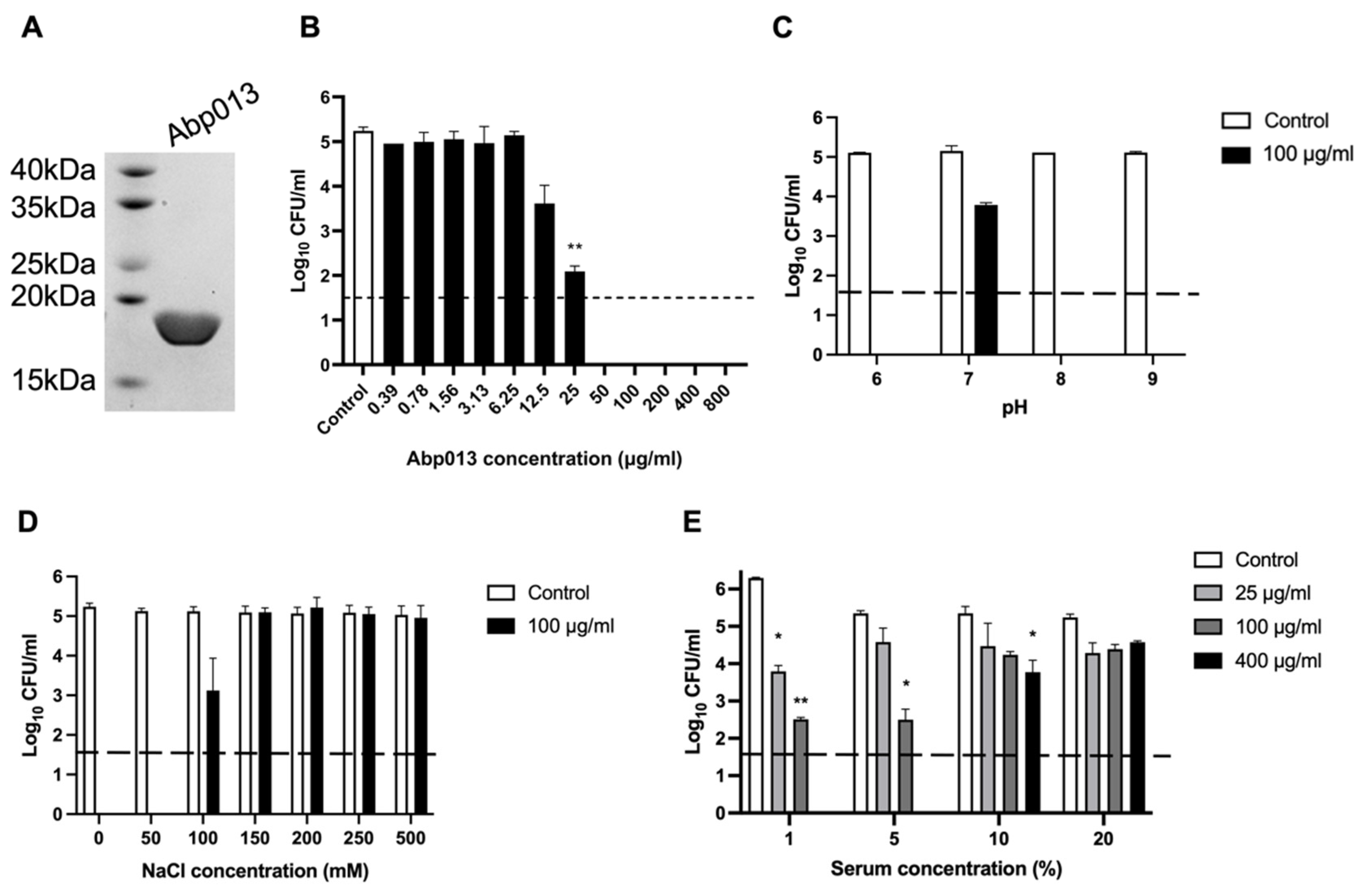
2.2.1. Expression and Purification of Abp013
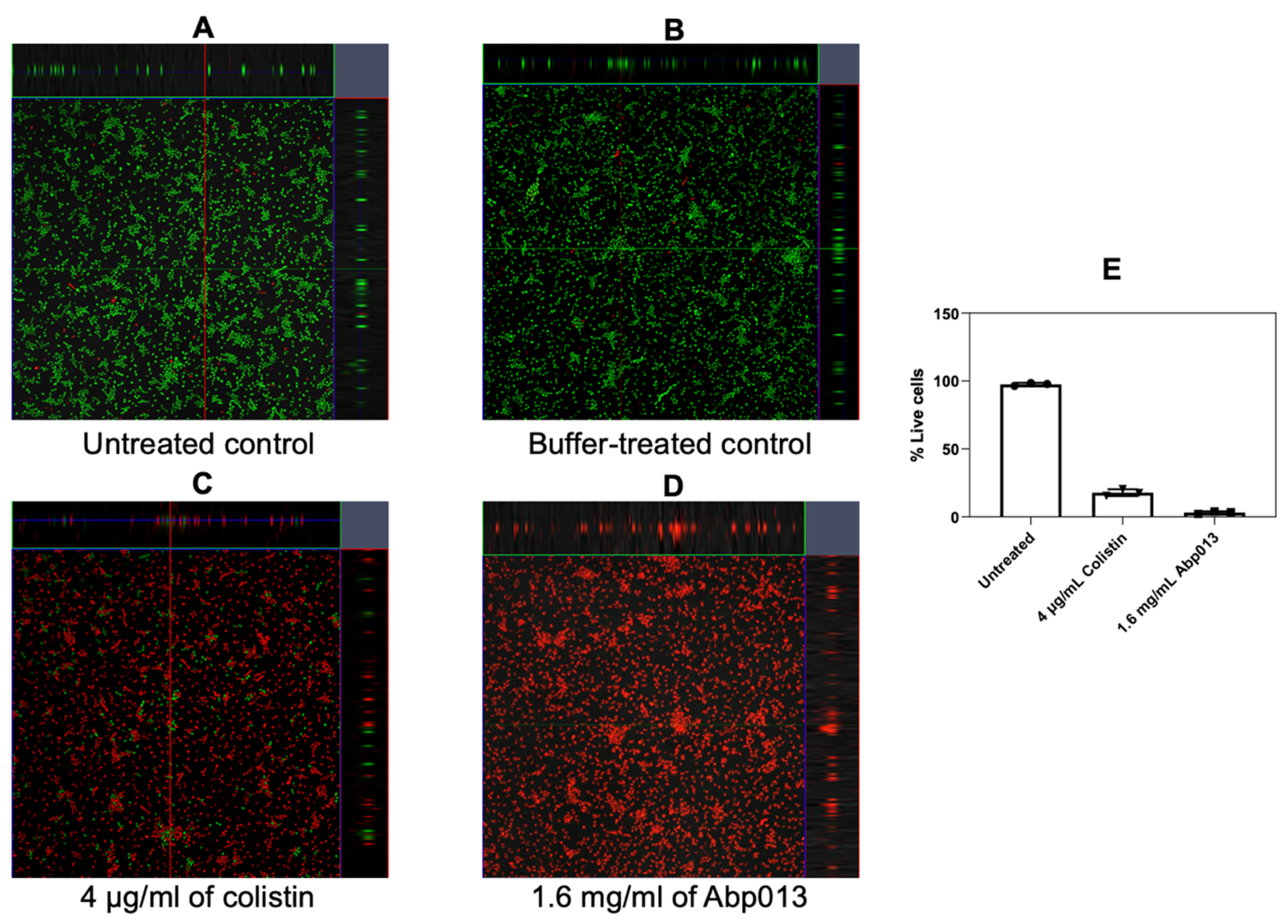
2.2.2. Dose Response of Abp013
2.2.3. Abp013 Characterization at Various pH and Salt Concentrations
2.2.4. Efficacy of Abp013 in Serum
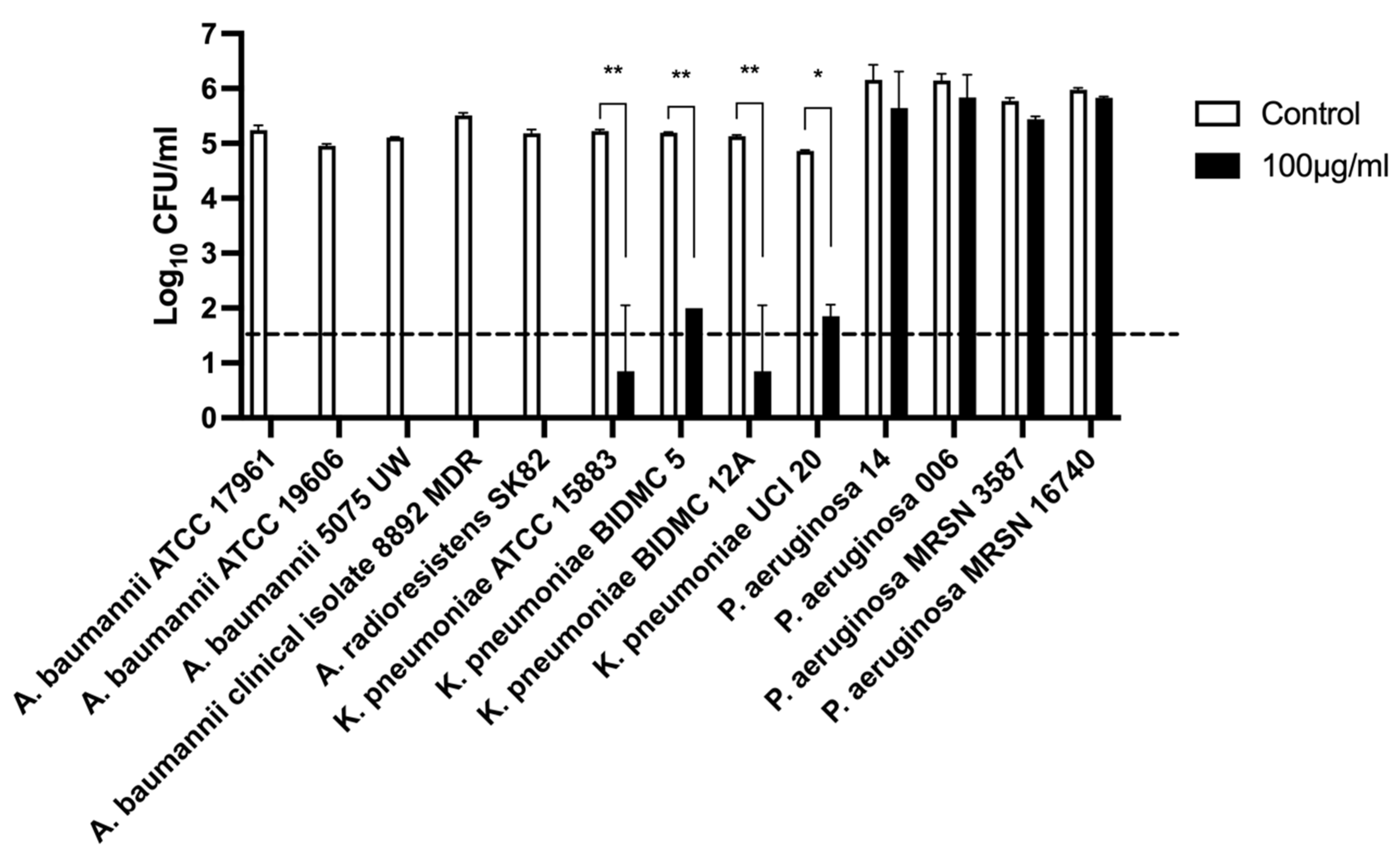
2.2.5. Host Lytic Spectra of Abp013
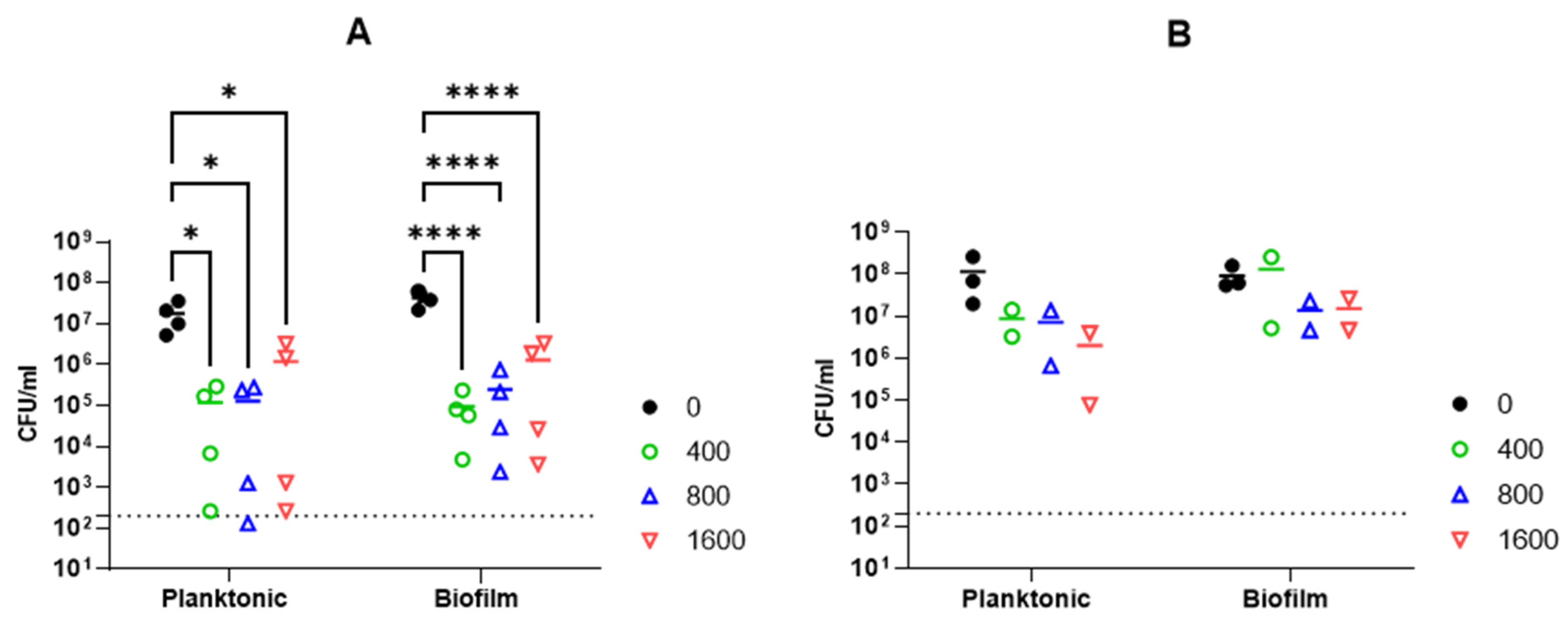
2.2.6. Activity against A. baumannii Biofilm
3. Discussion
4. Materials and Methods
4.1. The Bacterial Strains and Growth Conditions
4.2. Bioinformatics Analysis
4.3. Cloning, Expression, and Purification of Abp013
4.4. Characterization of the Lytic Activities of Abp013
4.5. Host Range Spectra Determination
4.6. Biofilm Assay
4.7. Confocal Microscopy
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial resistance in healthcare, agriculture and the environment: The biochemistry behind the headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Shrivastava, S.; Shrivastava, P.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76–77. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Heselpoth, R.D.; Swift, S.M.; Linden, S.B.; Mitchell, M.S.; Nelson, D.C. Enzybiotics: Endolysins and Bacteriocins. In Bacteriophages: Biology, Technology, Therapy; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 989–1030. [Google Scholar]
- Tafoya, D.A.; Hildenbrand, Z.L.; Herrera, N.; Molugu, S.K.; Mesyanzhinov, V.V.; Miroshnikov, K.A.; Bernal, R.A. Enzymatic characterization of a lysin encoded by bacteriophage EL. Bacteriophage 2013, 3, e25449. [Google Scholar] [CrossRef][Green Version]
- Grishin, A.V.; Karyagina, A.S.; Vasina, D.V.; Vasina, I.V.; Gushchin, V.A.; Lunin, V.G. Resistance to peptidoglycan-degrading enzymes. Crit. Rev. Microbiol. 2020, 46, 703–726. [Google Scholar] [CrossRef]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-Based Antibacterials as Therapeutics. Adv. Exp. Med. Biol. 2019, 1148, 233–253. [Google Scholar] [CrossRef]
- Binte Muhammad Jai, H.S.; Dam, L.C.; Tay, L.S.; Koh, J.J.W.; Loo, H.L.; Kline, K.A.; Goh, B.C. Engineered Lysins With Customized Lytic Activities against Enterococci and Staphylococci. Front. Microbiol. 2020, 11, 574739. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Sun, E.; Hua, L.; Peng, Z.; Wu, B. Characterization of a Lytic Bacteriophage vB_EfaS_PHB08 Harboring Endolysin Lys08 against Enterococcus faecalis Biofilms. Microorganisms 2020, 8, 1332. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Raz, A.; Molina, H.; Euler, C.W.; Fischetti, V.A. A highly active and negatively charged Streptococcus pyogenes lysin with a rare D-alanyl-L-alanine endopeptidase activity protects mice against streptococcal bacteremia. Antimicrob. Agents Chemother. 2014, 58, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.J.; Lin, N.T.; Hu, A.; Soo, P.C.; Chen, L.K.; Chen, L.H.; Chang, K.C. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Appl. Microbiol. Biotechnol. 2011, 90, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.B.; Chen, X.; Ho, M.K.Y.; Xia, J.; Leung, S.S.Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 2020, 589, 119833. [Google Scholar] [CrossRef]
- Huang, G.; Shen, X.; Gong, Y.; Dong, Z.; Zhao, X.; Shen, W.; Wang, J.; Hu, F.; Peng, Y. Antibacterial properties of Acinetobacter baumannii phage Abp1 endolysin (PlyAB1). BMC Infect. Dis. 2014, 14, 681. [Google Scholar] [CrossRef]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef]
- Kim, S.; Jin, J.S.; Choi, Y.J.; Kim, J. LysSAP26, a New Recombinant Phage Endolysin with a Broad Spectrum Antibacterial Activity. Viruses 2020, 12, 1340. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Rotem, S.; Radzishevsky, I.; Inouye, R.T.; Samore, M.; Mor, A. Identification of antimicrobial peptide regions derived from genomic sequences of phage lysins. Peptides 2006, 27, 18–26. [Google Scholar] [CrossRef] [PubMed]
- During, K.; Porsch, P.; Mahn, A.; Brinkmann, O.; Gieffers, W. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999, 449, 93–100. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Shi, Y.; Yin, S.; Shen, W.; Chen, J.; Chen, Y.; Chen, Y.; You, B.; Gong, Y.; et al. Characterization and genome annotation of a newly detected bacteriophage infecting multidrug-resistant Acinetobacter baumannii. Arch. Virol. 2019, 164, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, N.; Jawdat, F.; Al-Bakri, A.G.; Shehabi, A.A. Major biologic characteristics of Acinetobacter baumannii isolates from hospital environmental and patients’ respiratory tract sources. Am. J. Infect. Control. 2014, 42, 401–404. [Google Scholar] [CrossRef]
- Dekic, S.; Hrenovic, J.; Ivankovic, T.; van Wilpe, E. Survival of ESKAPE pathogen Acinetobacter baumannii in water of different temperatures and pH. Water Sci. Technol. 2018, 78, 1370–1376. [Google Scholar] [CrossRef]
- Wu, M.; Hu, K.; Xie, Y.; Liu, Y.; Mu, D.; Guo, H.; Zhang, Z.; Zhang, Y.; Chang, D.; Shi, Y. A Novel Phage PD-6A3, and Its Endolysin Ply6A3, With Extended Lytic Activity against Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3302. [Google Scholar] [CrossRef]
- Lopez, C.A.; Zgurskaya, H.; Gnanakaran, S. Molecular characterization of the outer membrane of Pseudomonas aeruginosa. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183151. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Barros, M.; Vennemann, T.; Gallagher, D.T.; Yin, Y.; Linden, S.B.; Heselpoth, R.D.; Spencer, D.J.; Donovan, D.M.; Moult, J.; et al. A bacteriophage endolysin that eliminates intracellular streptococci. eLife 2016, 5, e13152. [Google Scholar] [CrossRef] [PubMed]
- Larpin, Y.; Oechslin, F.; Moreillon, P.; Resch, G.; Entenza, J.M.; Mancini, S. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria. PLoS ONE 2018, 13, e0192507. [Google Scholar] [CrossRef]
- Raz, A.; Serrano, A.; Hernandez, A.; Euler, C.W.; Fischetti, V.A. Isolation of Phage Lysins That Effectively Kill Pseudomonas aeruginosa in Mouse Models of Lung and Skin Infection. Antimicrob. Agents Chemother. 2019, 63, e00024-19. [Google Scholar] [CrossRef]
- Gerstmans, H.; Grimon, D.; Gutierrez, D.; Lood, C.; Rodriguez, A.; van Noort, V.; Lammertyn, J.; Lavigne, R.; Briers, Y. A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci. Adv. 2020, 6, eaaz1136. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar] [PubMed]
- Sharma, U.; Vipra, A.; Channabasappa, S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today 2018, 23, 848–856. [Google Scholar] [CrossRef]
- Sedlacek, M.J.; Walker, C. Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol. Immunol. 2007, 22, 333–339. [Google Scholar] [CrossRef]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef]
- Borriello, G.; Richards, L.; Ehrlich, G.D.; Stewart, P.S. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2006, 50, 382–384. [Google Scholar] [CrossRef]
- Maciejewska, B.; Źrubek, K.; Espaillat, A.; Wiśniewska, M.; Rembacz, K.P.; Cava, F.; Dubin, G.; Drulis-Kawa, Z. Modular endolysin of Burkholderia AP3 phage has the largest lysozyme-like catalytic subunit discovered to date and no catalytic aspartate residue. Sci. Rep. 2017, 7, 14501. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Phylogenetics and Evolution; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Zdobnov, E.M.; Apweiler, R. InterProScan—An integration platform for the signature-recognition methods in InterPro. Bioinformatics 2001, 17, 847–848. [Google Scholar] [CrossRef]
- Farrow, J.M.; Pesci, E.C.; Slade, D.J. Genome Sequences for Two Acinetobacter baumannii Strains Obtained Using the Unicycler Hybrid Assembly Pipeline. Microbiol. Resour. Announc. 2021, 10, e00017-21. [Google Scholar] [CrossRef]
| Strain | Reference/Source |
|---|---|
| A. baumannii ATCC 17961 | BEI |
| A. baumannii ATCC 19606 | BEI |
| A. baumannii 5075-UW | BEI |
| A. baumannii clinical isolate 8879 MDR | Singapore General Hospital, Department of Microbiology |
| A. radioresistens SK82 | BEI |
| P. aeruginosa MRSN 16740 | BEI |
| P. aeruginosa MRSN 3587 | BEI |
| P. aeruginosa 006 | Singapore General Hospital, Department of Microbiology |
| P. aeruginosa 14 | BEI |
| K. pneumoniae ATCC 13883 | ATCC |
| K. pneumoniae BIDMC 5 | BEI |
| K. pneumoniae BIDMC 12A | BEI |
| K. pneumoniae UCI 20 | BEI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, J.J.K.; Poh, W.H.; Hasnuddin, N.T.B.; Hew, E.Y.; Dam, L.C.; Sahili, A.E.; Rice, S.A.; Goh, B.C. Novel Phage Lysin Abp013 against Acinetobacter baumannii. Antibiotics 2022, 11, 169. https://doi.org/10.3390/antibiotics11020169
Chu JJK, Poh WH, Hasnuddin NTB, Hew EY, Dam LC, Sahili AE, Rice SA, Goh BC. Novel Phage Lysin Abp013 against Acinetobacter baumannii. Antibiotics. 2022; 11(2):169. https://doi.org/10.3390/antibiotics11020169
Chicago/Turabian StyleChu, Joash Jun Keat, Wee Han Poh, Nabilah Taqiah Binte Hasnuddin, En Yi Hew, Linh Chi Dam, Abbas El Sahili, Scott A. Rice, and Boon Chong Goh. 2022. "Novel Phage Lysin Abp013 against Acinetobacter baumannii" Antibiotics 11, no. 2: 169. https://doi.org/10.3390/antibiotics11020169
APA StyleChu, J. J. K., Poh, W. H., Hasnuddin, N. T. B., Hew, E. Y., Dam, L. C., Sahili, A. E., Rice, S. A., & Goh, B. C. (2022). Novel Phage Lysin Abp013 against Acinetobacter baumannii. Antibiotics, 11(2), 169. https://doi.org/10.3390/antibiotics11020169






