Efficacy and Safety of Remdesivir in COVID-19 Positive Dialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort and Inclusion Criteria
2.2. Exclusion Criteria
2.3. Study Method
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 17 January 2022).
- Azghandi, M.; Kerachian, M.A. Detection of novel coronavirus (SARS-CoV-2) RNA in peripheral blood specimens. J. Transl. Med. 2020, 18, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E. COVID-19 in dialysis patients: Outlasting and outsmarting a pandemic. Kidney Int. 2020, 98, 1402–1404. [Google Scholar] [CrossRef]
- Aydin Bahat, K.; Parmaksiz, E.; Sert, S. The clinical characteristics and course of COVID-19 in hemodialysis patients. Hemodial. Int. 2020, 24, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Jamil, Z.; Almajhdi, F.N.; Khalid, S.; Asghar, M.; Ahmed, J.; Waheed, Y. Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients. Antibiotics 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 17 January 2022).
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death|Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results (accessed on 17 January 2022).
- Veiga, V.C.; Prats, J.A.G.G.; Farias, D.L.C.; Rosa, R.G.; Dourado, L.K.; Zampieri, F.G.; MacHado, F.R.; Lopes, R.D.; Berwanger, O.; Azevedo, L.C.; et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: Randomised controlled trial. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [CrossRef] [PubMed]
- Frost, M.T.; Jimenez-Solem, E.; Ankarfeldt, M.Z.; Nyeland, M.E.; Andreasen, A.H.; Petersen, T.S. The Adaptive COVID-19 Treatment Trial-1 (ACTT-1) in a real-world population: A comparative observational study. Crit. Care 2020, 24. [Google Scholar] [CrossRef] [PubMed]
- Aiswarya, D.; Arumugam, V.; Dineshkumar, T.; Gopalakrishnan, N.; Lamech, T.M.; Nithya, G.; Sastry, B.V.R.H.; Vathsalyan, P.; Dhanapriya, J.; Sakthirajan, R. Use of Remdesivir in Patients with COVID-19 on Hemodialysis: A Study of Safety and Tolerance. Kidney Int. Rep. 2021, 6, 586–593. [Google Scholar] [CrossRef] [PubMed]
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed on 11 January 2022).
- Interleukin-6 Inhibitors|COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-6-inhibitors/ (accessed on 12 January 2022).
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; Sax, P.E.; Wohl, D.A.; Casciano, R.; et al. Remdesivir treatment in hospitalized patients with COVID-19: A comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Fintzi, J.; Bonnett, T.; Sweeney, D.A.; Huprikar, N.A.; Ganesan, A.; Frank, M.G.; McLellan, S.L.F.; Dodd, L.E.; Tebas, P.; Mehta, A.K. Deconstructing the Treatment Effect of Remdesivir in the Adaptive COVID-19 Treatment Trial-1: Implications for Critical Care Resource Utilization. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Adamsick, M.L.; Gandhi, R.G.; Bidell, M.R.; Elshaboury, R.H.; Bhattacharyya, R.P.; Kim, A.Y.; Nigwekar, S.; Rhee, E.P.; Sise, M.E. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1384–1386. [Google Scholar] [CrossRef]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Head Neck Surg. 2010, 143, 331. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Creput, C.; Fumeron, C.; Toledano, D.; Diaconita, M.; Izzedine, H. COVID-19 in Patients Undergoing Hemodialysis: Prevalence and Asymptomatic Screening During a Period of High Community Prevalence in a Large Paris Center. Kidney Med. 2020, 2, 716–723.e1. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, F.; Wu, S.K.; Xiang-Heng, L.; Li, W.; Li, G.S.; Wang, J. Clinical characteristics of 31 hemodialysis patients with 2019 novel coronavirus: A retrospective study. Ren. Fail. 2020, 42, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.; Alam, A.; Salman, B.; Saeed, F.; Memon, S.; Chughtai, J.; Ahmed, S.; Tariq, S.; Imtiaz, S. Clinical Course and Outcome of ESRD Patients on Maintenance Hemodialysis Infected with COVID-19: A Single-Center Study. Int. J. Nephrol. Renovasc. Dis. 2021, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Luke, D.R.; Tomaszewski, K.; Damle, B.; Schlamm, H.T. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J. Pharm. Sci. 2010, 99, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakare, S.; Gandhi, C.; Modi, T.; Bose, S.; Deb, S.; Saxena, N.; Katyal, A.; Patil, A.; Patil, S.; Pajai, A.; et al. Safety of Remdesivir in Patients with Acute Kidney Injury or CKD. Kidney Int. Rep. 2021, 6, 206–210. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Kopel, J.; Perisetti, A.; Roghani, A.; Aziz, M.; Gajendran, M.; Goyal, H. Racial and Gender-Based Differences in COVID-19. Front. Public Health 2020, 8, 418. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.; Chantrel, F.; Krummel, T.; Bazin-Kara, D.; Faller, A.L.; Muller, C.; Nussbaumer, T.; Ismer, M.; Benmoussa, A.; Brahim-Bouna, M.; et al. Impact of first-wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: The observational COVIDIAL study. Nephrol. Dial. Transpl. 2020, 35, 1338–1411. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.T.; Leung, S.T.; Chin, T.W.Y.; Lo, C.S.Y.; Lui, M.M.S.; Lee, J.C.Y.; Chiu, K.W.H.; Chung, T.W.H.; et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 2020, 296, E72–E78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Rathnasinghe, R.; Ferres, M.; Tan, G.S.; Barrera, A.; Pickett, B.E.; Methe, B.A.; Das, S.; Budnik, I.; Halpin, R.A.; et al. Microbiome disturbance and resilience dynamics of the upper respiratory tract during influenza A virus infection. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, Z. COVID-2019-associated overexpressed Prevotella proteins mediated host-pathogen interactions and their role in coronavirus outbreak. Bioinformatics 2020, 36, 4065–4069. [Google Scholar] [CrossRef]
- Tortonese, S.; Scriabine, I.; Anjou, L.; Loens, C.; Michon, A.; Benabdelhak, M.; Ouali, S.; Morin, G.; Laifi, M.; Dobosziewicz, H.; et al. COVID-19 in Patients on Maintenance Dialysis in the Paris Region. Kidney Int. Rep. 2020, 5, 1535–1544. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: A multicenter retrospective study from Wuhan, China. Crit. Care 2020, 24. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Valeri, A.M.; Robbins-Juarez, S.Y.; Stevens, J.S.; Ahn, W.; Rao, M.K.; Radhakrishnan, J.; Gharavi, A.G.; Mohan, S.; Ali Husain, S. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Zhu, G.; Zhang, Y.; Bi, Z.; Yu, Y.; Huang, B.; Fu, S.; Tan, Y.; Sun, J.; et al. Clinical Features of Maintenance Hemodialysis Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Clin. J. Am. Soc. Nephrol. 2020, 15, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Cámara, L.A.S.; Macías, N.; de Morales, A.M.; Rojas, Á.G.; Bascuñana, A.; Arroyo, D.; Vega, A.; Abad, S.; Verde, E.; et al. COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020, 98, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.J.; Deb, A.; Sarma, P.; Mallick, B.; Bhattacharjee, P. Comparative Safety and Efficacy of Remdesivir versus Remdesivir Plus Convalescent Plasma Therapy (CPT) and the Effect of Timing of Initiation of Remdesivir in COVID-19 Patients: An Observational Study from North East India. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Santenna, C.; Vidyasagar, K.; Amarneni, K.C.; Ghanta, S.N.; Sadasivam, B.; Pathan, S.; Padmavathi, R. The safety, tolerability and mortality reduction efficacy of remdesivir; based on randomized clinical trials, observational and case studies reported safety outcomes: An updated systematic review and meta-analysis. Ther. Adv. Drug Saf. 2021, 12. [Google Scholar] [CrossRef]
- Pettit, N.N.; Pisano, J.; Nguyen, C.T.; Lew, A.K.; Hazra, A.; Sherer, R.; Mullane, K.M. Remdesivir Use in the Setting of Severe Renal Impairment: A Theoretical Concern or Real Risk? Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
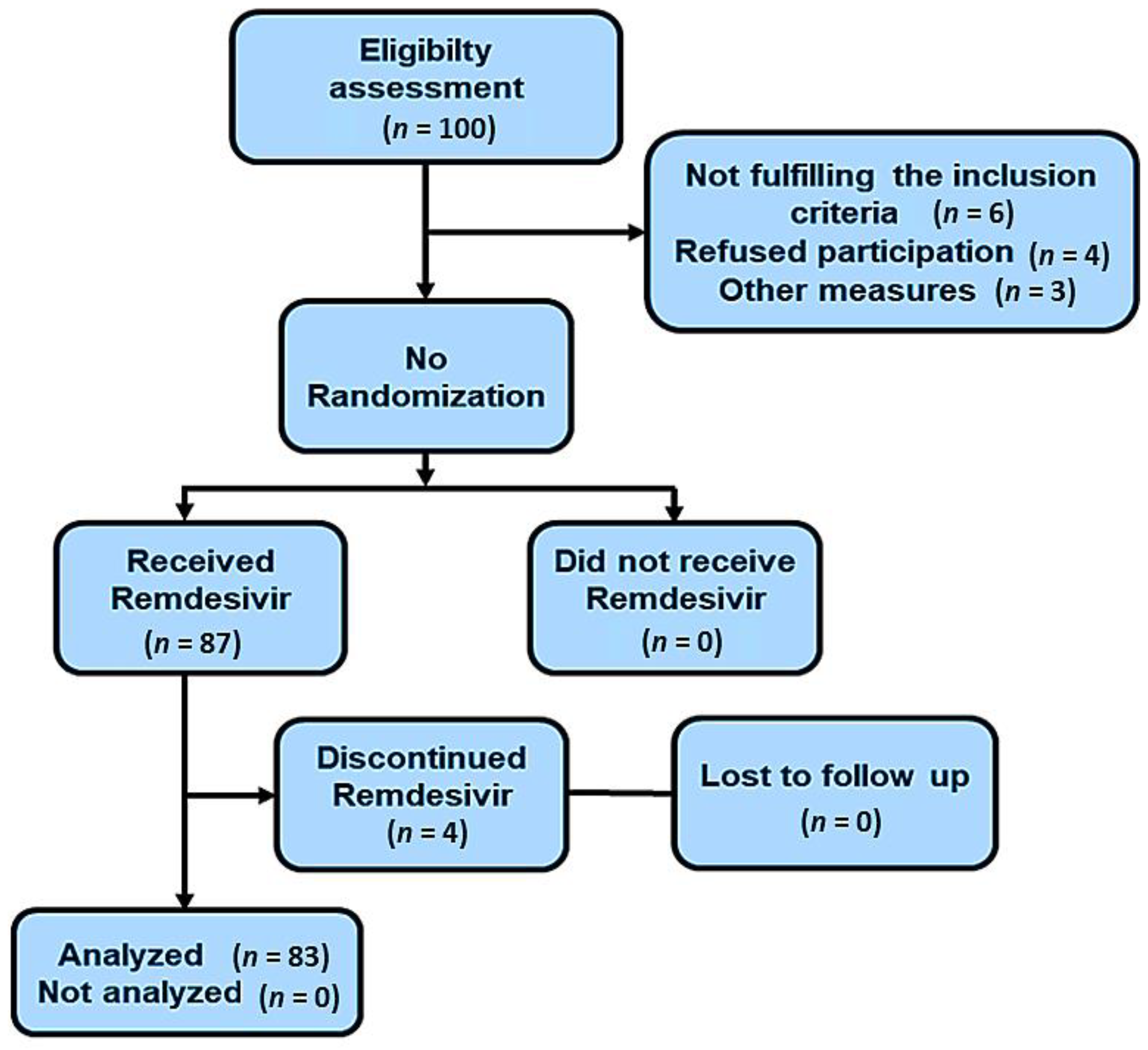
| Parameters | Baseline n (%) (n = 83) | Discharge n (%) (n = 76) | Death n (%) (n = 7) | p-Value |
|---|---|---|---|---|
| Age (years) | 59.43 ± 14.28 | 59.32 ± 62.50 | 60.57 ± 8.67 | 0.82 |
| Sex (M/F) | 31/50 (36.5/58.8) | 27/47 (35.5/61.8) | 4/3 (57.1%/42.8) | 0.5 |
| Cause of ESRD | ||||
| Diabetes | 29/54 (34.1) | 29 (38.1) | 4 (57.1) | 0.09 |
| Hypertension | 26/57 (30.6) | 26 (34.2) | 2 (28.57) | 0.09 |
| Others | 26 (30.6) | 21 (27.6) | 1 (14.2) | 0.08 |
| Symptoms | ||||
| Fever | 75/83 (90.3) | 63/76 (82.89) | 3/7 (42.8) (3) | 0.03 |
| Cough | 66/83 (79.5) | 68/76 (89) | 7/7 (100) | 1 |
| Dyspnea–mild | 76/83 (91.5) | 70/76 (92.1) | 6/7 (85.7) | 0.42 |
| Moderate | 4/83 (4.8) | 3/76 (3.9) | 1/7 (14) | - |
| Severe | 3/83 (3.6) | 0 | - | |
| Symptoms’ duration before admission, days, median (IQR) | 2 (1–5) | 2.0 (1–5) | 2 (1–4) | 0.15 |
| Hospitalization days, median (IQR) | 9 (6–14) | 9 (6–14) | 11 (7–14) | 0.06 |
| Day of initiation of remdesivir, median (IQR) | 2.0 (1–4) | 1 (0–1) | 1 (0–1) | 0.14 |
| Mean laboratory values | ||||
| NLR | 13.22 ± 10.56 | 13.7 ± 11.41 | 11.2857 ± 5.96 | 0.93 |
| ALT (U/L) | 39.67 ± 46.71 | 37.96 ± 41.05 | 58.28 ± 91.17 | 0.32 |
| Ferritin (ng/mL) | 1395.57 ± 963.89 | 1449.47 ± 978.03 | 807.43 ± 547.52 | 0.06 |
| LDH (U/L) | 735.88 ± 522.96 | 735.93 ± 523.73 | 735.29 ± 555.831 | 0.27 |
| CRP (mg/dL) | 2.17 ± 2.57 | 1.99 ± 2.38 | 4.19 ± 4.07 | 0.44 |
| ALC | 897.08 ± 556.05 | 847.89 ± 542.06 | 1431.14 ± 435.211 | 0.05 |
| CT scan of chest | ||||
| None | 7 (8.2) | 5 (6.5) | 2 (28.57) | 0.38 |
| STAGE 1 | 21 (24.87) | 19 (25) | 2 (28.57) | |
| STAGE 2 | 32 (37.7) | 31 (40.7) | 1 (14.28) | |
| STAGE 3 | 16 (18.9) | 14 (18.4) | 2 (28.57) | |
| STAGE 4 | 7 (8.2) | 7 (9.21) | 0 | |
| Oxygen requirement | ||||
| Oxygen mask | 35 (42.1) | 33 (43.4) | 2 (28.5) | 0.53 |
| Non-rebreathing Mask | 18 (21.6) | 16 (21.0) | 2 (28.5) | |
| CPAP | 18 (21.6) | 16 (21.0) | 2 (28.5) | |
| Mechanical ventilation | 12 (14.4) | 11 (14.4) | 1 (14.2) | |
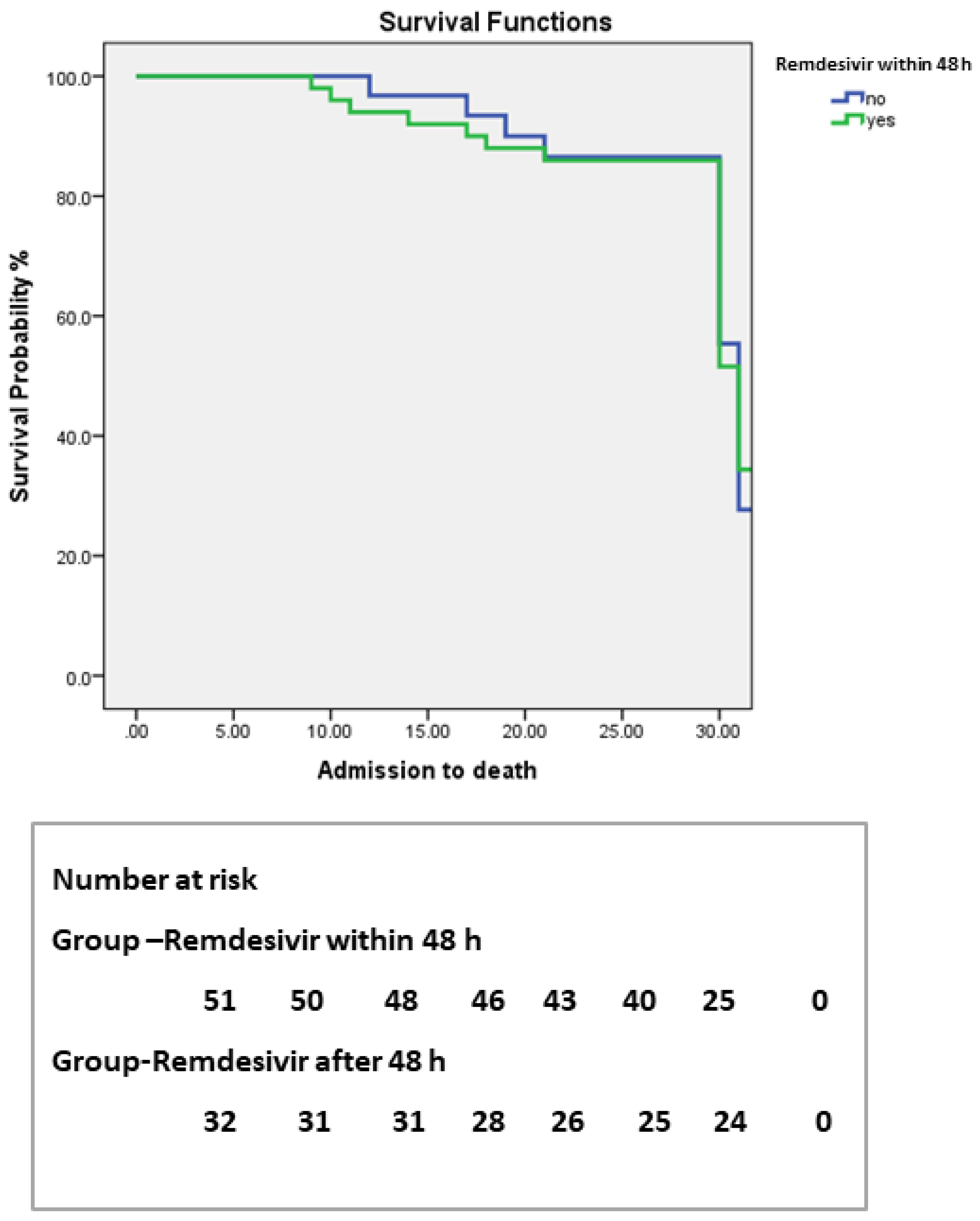
| Parameters | Initiation before 48 h (n–51) | Initiation after 48 h (n–32) | p-Value |
|---|---|---|---|
| Hospitalization days, median (IQR) | 9 (7–11) | 11 (9–12) | 0.03 |
| Days to swab negative, median (IQR) | 7 (6–9) | 9 (8–11) | 0.02 |
| Need of mechanical ventilation, median (IQR) | 9 (7–11) | 14 (11–14) | 0.01 |
| Death n (%) | 4 (7.8) | 4 (12.5) | 0.8 |
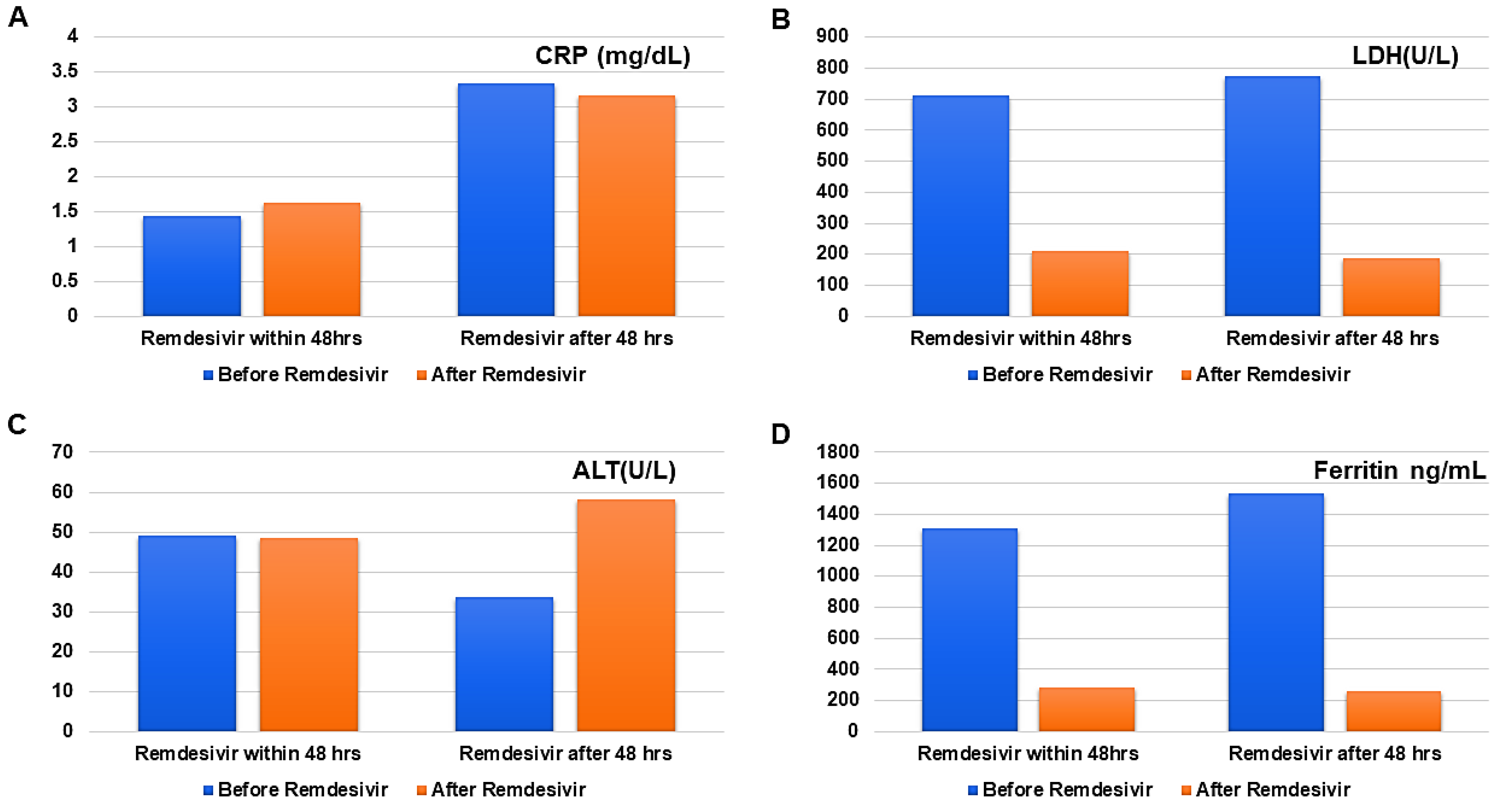
| Parameters | Before Remdesivir | After Remdesivir | p-Value |
|---|---|---|---|
| Remdesivir initiated within 48 h | |||
| ALT (U/L) | 49.25 ± 59.84 | 48.411 ± 48.36 | 0.00 |
| CRP (mg/dL) | 1.43 ± 1.51 | 1.63± 3.45 | 0.90 |
| Ferritin (ng/mL) | 1310.33 ± 1067.05 | 281.91 ± 180.54 | 0.00 |
| LDH (U/L) | 711.31 ± 329.91 | 211.01 ± 76.49 | 0.00 |
| Remdesivir initiated after 48 h | |||
| ALT (U/L) | 33.66 ± 35.53 | 58.25 ± 74.38 | 0.00 |
| CRP (mg/dL) | 3.34 ± 3.39 | 3.17 ± 3.46 | 0.89 |
| LDH (U/L) | 775.03 ± 735.44 | 187.37 ± 63.62 | 0.00 |
| Ferritin (ng/mL) | 1531.41 ± 768.20 | 256.87 ± 175.92 | 0.00 |
| Variable | HR | 95%CI | p-Value |
|---|---|---|---|
| Age | 1.00 | 0.041–25.0 | 0.99 |
| Remdesivir within 48 h | 1.25 | 0.196–8.00 | 0.81 |
| Number of days requiring oxygen | 0.42 | 0.018–10.13 | 0.59 |
| Comorbid conditions (diabetes, hypertension) | 1.00 | 0.046–21.91 | 0.99 |
| Number of days on ventilation | 0.90 | 0.033–24.49 | 0.95 |
| ALC | 1.00 | 1.000–1.00 | 0.10 |
| TLC | 1.17 | 1.035–1.33 | 0.01 |
| CRP | 1.00 | 1.000–1.00 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, B.; Hussain, T.; Jarrar, M.; Khalid, K.; Albaker, W.; Ambreen, A.; Waheed, Y. Efficacy and Safety of Remdesivir in COVID-19 Positive Dialysis Patients. Antibiotics 2022, 11, 156. https://doi.org/10.3390/antibiotics11020156
Butt B, Hussain T, Jarrar M, Khalid K, Albaker W, Ambreen A, Waheed Y. Efficacy and Safety of Remdesivir in COVID-19 Positive Dialysis Patients. Antibiotics. 2022; 11(2):156. https://doi.org/10.3390/antibiotics11020156
Chicago/Turabian StyleButt, Batool, Tajamul Hussain, Mu’taman Jarrar, Kashaf Khalid, Waleed Albaker, Asma Ambreen, and Yasir Waheed. 2022. "Efficacy and Safety of Remdesivir in COVID-19 Positive Dialysis Patients" Antibiotics 11, no. 2: 156. https://doi.org/10.3390/antibiotics11020156
APA StyleButt, B., Hussain, T., Jarrar, M., Khalid, K., Albaker, W., Ambreen, A., & Waheed, Y. (2022). Efficacy and Safety of Remdesivir in COVID-19 Positive Dialysis Patients. Antibiotics, 11(2), 156. https://doi.org/10.3390/antibiotics11020156








