LC-HRMS-Based Identification of Transformation Products of the Drug Salinomycin Generated by Electrochemistry and Liver Microsome
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Investigation
2.2. Liver Microsomes Assay
3. Materials and Methods
3.1. Chemicals
3.2. ECR/ESI-HRMS
3.3. Microsomal Assay
3.4. LC-HRMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huczynski, A. Salinomycin—A New Cancer Drug Candidate. Chem. Biol. Drug Des. 2012, 79, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Kevin, I.D.A.; Meujo, D.A.F.; Hamann, M.T. Polyether ionophores: Broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin. Drug Discov. 2009, 4, 109–146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Huczyński, A. Salinomycin and its derivatives—A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef]
- Pico, Y.; Barcelo, D. Transformation products of emerging contaminants in the environment and high-resolution mass spectrometry: A new horizon. Anal. Bioanal. Chem. 2015, 407, 6257–6273. [Google Scholar] [CrossRef] [PubMed]
- Kotthoff, L.; Keller, J.; Lörchner, D.; Mekonnen, T.F.; Koch, M. Transformation products of organic contaminants and residues—Overview of current simulation methods. Molecules 2019, 24, 753. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Kolar, B.; et al. Safety and efficacy of Sacox® microGranulate (salinomycin sodium) for chickens for fattening and chickens reared for laying. EFSA J. 2017, 15, e04670. [Google Scholar] [CrossRef]
- Olejnik, M.; Radko, L.; Jedziniak, P. Identification of metabolites of anticancer candidate salinomycin using liquid chromatography coupled with quadrupole time-of-flight and hybrid triple quadrupole linear ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 629–634. [Google Scholar] [CrossRef]
- Radko, L.; Olejnik, M.; Posyniak, A. Primary Human Hepatocytes, But not HepG2 or Balb/c 3T3 Cells, Efficiently Metabolize Salinomycin and Are Resistant to Its Cytotoxicity. Molecules 2020, 25, 1174. [Google Scholar] [CrossRef]
- Radko, L.; Olejnik, M. Cytotoxicity of anticancer candidate salinomycin and identification of its metabolites in rat cell cultures. Toxicol Vitr. 2018, 52, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Vertesy, L.; Heil, K.; Fehlhaber, H.W.; Ziegler, W. Microbial decomposition of salinomycin. J. Antibiot. 1987, 40, 388–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munaretto, J.S.; Yonkos, L.; Aga, D.S. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting. Environ. Pollut. 2016, 212, 392–400. [Google Scholar] [CrossRef]
- Sun, P.; Cabrera, M.L.; Huang, C.H.; Pavlostathis, S.G. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ. Sci. Technol. 2014, 48, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Schlusener, M.P.; von Arb, M.A.; Bester, K. Elimination of macrolides, tiamulin, and salinomycin during manure storage. Arch. Environ. Contam. Toxicol. 2006, 51, 21–28. [Google Scholar] [CrossRef]
- Sun, P.; Yao, H.; Minakata, D.; Crittenden, J.C.; Pavlostathis, S.G.; Huang, C.H. Acid-catalyzed transformation of ionophore veterinary antibiotics: Reaction mechanism and product implications. Environ. Sci. Technol. 2013, 47, 6781–6789. [Google Scholar] [CrossRef]
- Wells, J.L.; Bordner, J.; Bowles, P.; McFarland, J.W. Novel degradation products from the treatment of salinomycin and narasin with formic acid. J. Med. Chem. 1988, 31, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Pavlostathis, S.G.; Huang, C.H. Photodegradation of veterinary ionophore antibiotics under UV and solar irradiation. Environ. Sci. Technol. 2014, 48, 13188–13196. [Google Scholar] [CrossRef]
- Hansen, M.; Bjorklund, E.; Krogh, K.A.; Brandt, A.; Halling-Sorensen, B. Biotic transformation of anticoccidials in soil using a lab-scale bio-reactor as a precursor-tool. Chemosphere 2012, 86, 212–215. [Google Scholar] [CrossRef]
- Herl, T.; Matysik, F.-M. Recent Developments in Electrochemistry–Mass Spectrometry. ChemElectroChem 2020, 7, 2498–2512. [Google Scholar] [CrossRef]
- Hoffmann, T.; Hofmann, D.; Klumpp, E.; Küppers, S. Electrochemistry-mass spectrometry for mechanistic studies and simulation of oxidation processes in the environment. Anal. Bioanal. Chem. 2011, 399, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Portychova, L.; Schug, K.A. Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: A review. Anal. Chim. Acta 2017, 993, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Göldner, V.; Fangmeyer, J.; Karst, U. Development of an electrochemical flow-through cell for the fast and easy generation of isotopically labeled metabolite standards. Drug Test. Anal. 2021, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef]
- Bruins, A.P. An overview of electrochemistry combined with mass spectrometry. TrAC Trends Anal. Chem. 2015, 70, 14–19. [Google Scholar] [CrossRef]
- Miao, X.S.; March, R.E.; Metcalfe, C.D. Fragmentation study of salinomycin and monensin A antibiotics using electrospray quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 149–154. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]


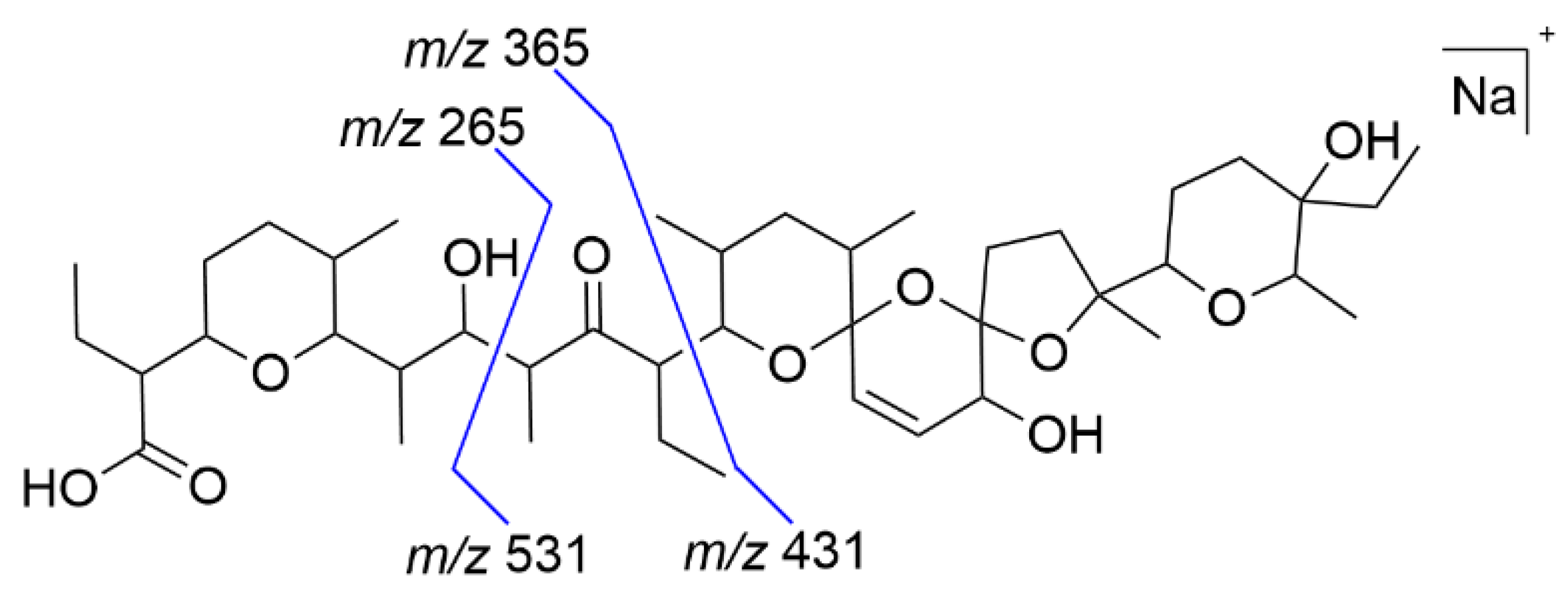


| Transformation Reaction | Experiment | References |
|---|---|---|
| m/z 531 (C-C cleavage, β-hydroxy-ketone position) | degradation by poultry litter | [13] |
| degradation by broiler litter | [14] | |
| manure storage | [15] | |
| hydrolysis (acid-catalyzed) | [16] | |
| treatment with formic acid | [17] | |
| photodegradation | [18] | |
| transformation in soil | [19] | |
| microbial decomposition | [12,14] | |
| m/z 265 (C-C cleavage) | hydrolysis (acid-catalyzed) | [16] |
| treatment with formic acid | [17] | |
| photodegradation | [18] | |
| Hydroxylation (+O) | human hepatoma cells (HepG2) | [9] |
| primary human hepatocytes (PHH) | [10] | |
| rat primary hepatocytes (PRH) | [11] | |
| rat hepatoma cells (FaO) | [11] | |
| Hydroxylation + Demethylation | photodegradation | [18] |
| human hepatoma cells (HepG2) | [9] | |
| primary human hepatocytes (PHH) | [10] | |
| Di-/Tri-hydroxylation | photodegradation | [18] |
| primary human hepatocytes (PHH) | [10] | |
| rat primary hepatocytes (PRH) | [11] | |
| Dehydrogenation | rat primary hepatocytes (PRH) | [11] |
| Isomeric changes | hydrolysis (acid-catalyzed) | [16] |
| EC-TP | Mass Meas. | Mass Calc. | Sum Formula | Suggested Modification | Intensity |
|---|---|---|---|---|---|
| 1 * | 791.4801 | 791.4795 | C41H70O12NNa | −CO, +2O, −4H, +NH4 | vw |
| 2 | 786.5116 | 786.5108 | C41H74O10NNa2 | −CO, +NH4, + Na | m |
| 3 | 777.4804 | 777.4764 | C41H70O12Na | −CO, +2O | vw |
| 4 | 759.4956 | 759.5000 | C42H72O10Na | −O, +2H | vs |
| 5 § | 745.4859 | 745.4866 | C41H70O10Na | −CO | vs |
| 6 * | 743.4710 | 743.4710 | C41H68O10Na | −CO, −2H | m |
| 7 § | 727.4764 | 727.4761 | C41H68O9Na | −CO, −H2O | s |
| 8 | 717.4552 | 717.4553 | C39H66O10Na | −CO, −C2H4 | vw |
| 9 | 687.4817 | 687.4811 | C39H68O8Na | −CO, −CO2, −CH2 | w |
| 10 * | 669.4732 | 669.4706 | C39H66O7Na | −CO, −CO2, −CH2, −H2O | w |
| 11 * | 651.4621 | 651.4600 | C39H64O6Na | −CO, −CO2, −CH2, −2H2O | vw |
| SAL | 773.4816 | 773.4815 | C42H70O11Na |
| rt [s] | Mass Meas. | Mass Calc. | Sum Formula | Suggested Modification | Intensity | |
|---|---|---|---|---|---|---|
| HLM | ||||||
| TP-R1 | 65.20 | 821.4456 | 821.4453 | C42H70O13K | −Na, +2O, +K | vw |
| TP-R2 | 81.35 | 805.4510 | 805.4504 | C42H70O12K | −Na, +O, +K | m |
| TP-R3 | 91.57 | 787.4582 | 787.4608 | C42H68O12Na | −2H, +O | vw |
| TP-R4 | 108.00 | 789.4754 | 789.4764 | C42H70O12Na | +O | w |
| TP-R5 | 118.56 | 771.4633 | 771.4659 | C42H68O11Na | −2H | vw |
| SAL | 142.61 | 773.4815 | 773.4815 | C42H70O11Na | vs | |
| RLM | ||||||
| TP-H1 | 44.33 | 821.4445 | 821.4453 | C42H70O13K | −Na, +2O, +K | vs |
| TP-H2 | 53.60 | 819.4307 | 819.4296 | C42H68O13K | −Na, −2H, +2O, +K | m |
| TP-H3 | 61.44 | 821.4439 | 821.4453 | C42H70O13K | −Na, +2O, +K | vs |
| TP-H4 | 65.20 | 821.4408 | 821.4453 | C42H70O13K | −Na, +2O, +K | s |
| TP-H5 | 69.91 | 819.4313 | 819.4296 | C42H68O13K | −Na, −2H, +2O, +K | w |
| TP-H6 | 81.35 | 805.4508 | 805.4504 | C42H70O12K | −Na, +O, +K | vs |
| TP-H7 | 86.37 | 803.4409 | 803.4347 | C42H68O12K | −Na, −2H, +O, +K | m |
| TP-H8 | 90.00 | 819.4582 | 819.4507 | C42H68O14Na | −2H, +3O | vw |
| TP-H9 | 91.57 | 787.4595 | 787.4608 | C42H68O12Na | −2H, +O | vw |
| TP-H10 | 102.10 | 787.4577 | 787.4608 | C42H68O12Na | −H2O, +2O | w |
| TP-H11 | 108.00 | 789.4707 | 789.4764 | C42H70O12Na | +O | vw |
| SAL | 142.61 | 773.4815 | 773.4815 | C42H70O11Na | w | |
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 400 °C | collision energy | 40 V |
| ion source gas 1 (nitrogen) | 55 L/min | declustering potential | 80 V |
| ion source gas 2 (nitrogen | 55 L/min | mass range | 100–800 Da |
| curtain gas (nitrogen) | 45 L/min | ||
| ion spray voltage floating | +5500 V | ||
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 400 °C | MS 1 | |
| ion source gas 1 (nitrogen) | 50 L/min | collision energy | 10 V |
| ion source gas 2 (nitrogen) | 55 L/min | declustering potential | 80 V |
| curtain gas (nitrogen) | 45 L/min | mass range | 100–900 Da |
| ion spray voltage floating | +5500 V | MS 2 | |
| gas temperature | 400 °C | collision energy | 85 V (LM) 70 V (EC) |
| collision energy spread | 20 V | ||
| declustering potential | 80 V | ||
| mass range | 100–900 Da | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoche, L.; Lisec, J.; Schwerdtle, T.; Koch, M. LC-HRMS-Based Identification of Transformation Products of the Drug Salinomycin Generated by Electrochemistry and Liver Microsome. Antibiotics 2022, 11, 155. https://doi.org/10.3390/antibiotics11020155
Knoche L, Lisec J, Schwerdtle T, Koch M. LC-HRMS-Based Identification of Transformation Products of the Drug Salinomycin Generated by Electrochemistry and Liver Microsome. Antibiotics. 2022; 11(2):155. https://doi.org/10.3390/antibiotics11020155
Chicago/Turabian StyleKnoche, Lisa, Jan Lisec, Tanja Schwerdtle, and Matthias Koch. 2022. "LC-HRMS-Based Identification of Transformation Products of the Drug Salinomycin Generated by Electrochemistry and Liver Microsome" Antibiotics 11, no. 2: 155. https://doi.org/10.3390/antibiotics11020155
APA StyleKnoche, L., Lisec, J., Schwerdtle, T., & Koch, M. (2022). LC-HRMS-Based Identification of Transformation Products of the Drug Salinomycin Generated by Electrochemistry and Liver Microsome. Antibiotics, 11(2), 155. https://doi.org/10.3390/antibiotics11020155








