Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum
Abstract
1. Introduction
2. Materials and Methods
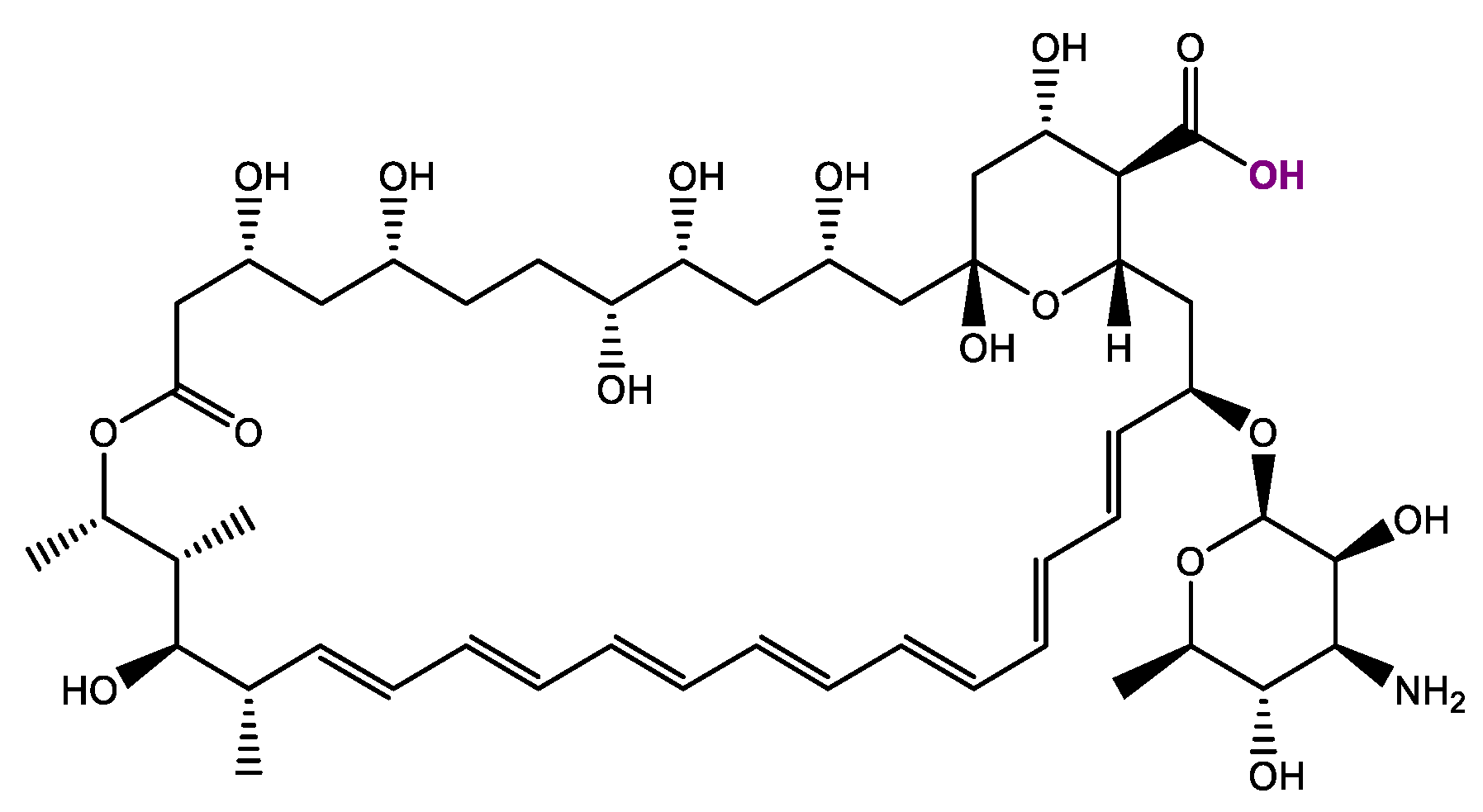
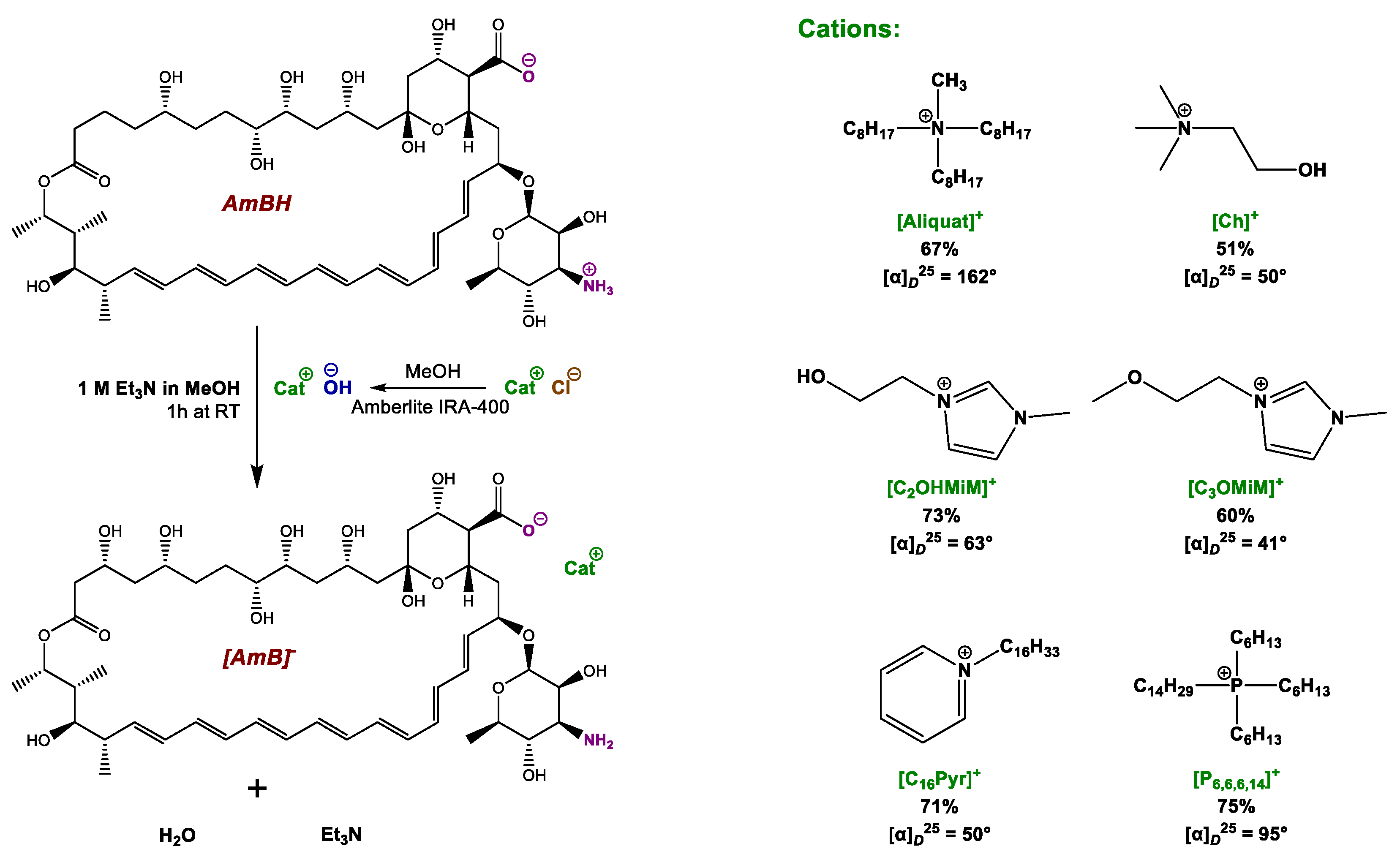
2.1. Synthesis and Chemical Characterization of OSILs Based on Amphotericin B
2.2. Biological Activity against Leishmania infantum
3. Results and Discussion
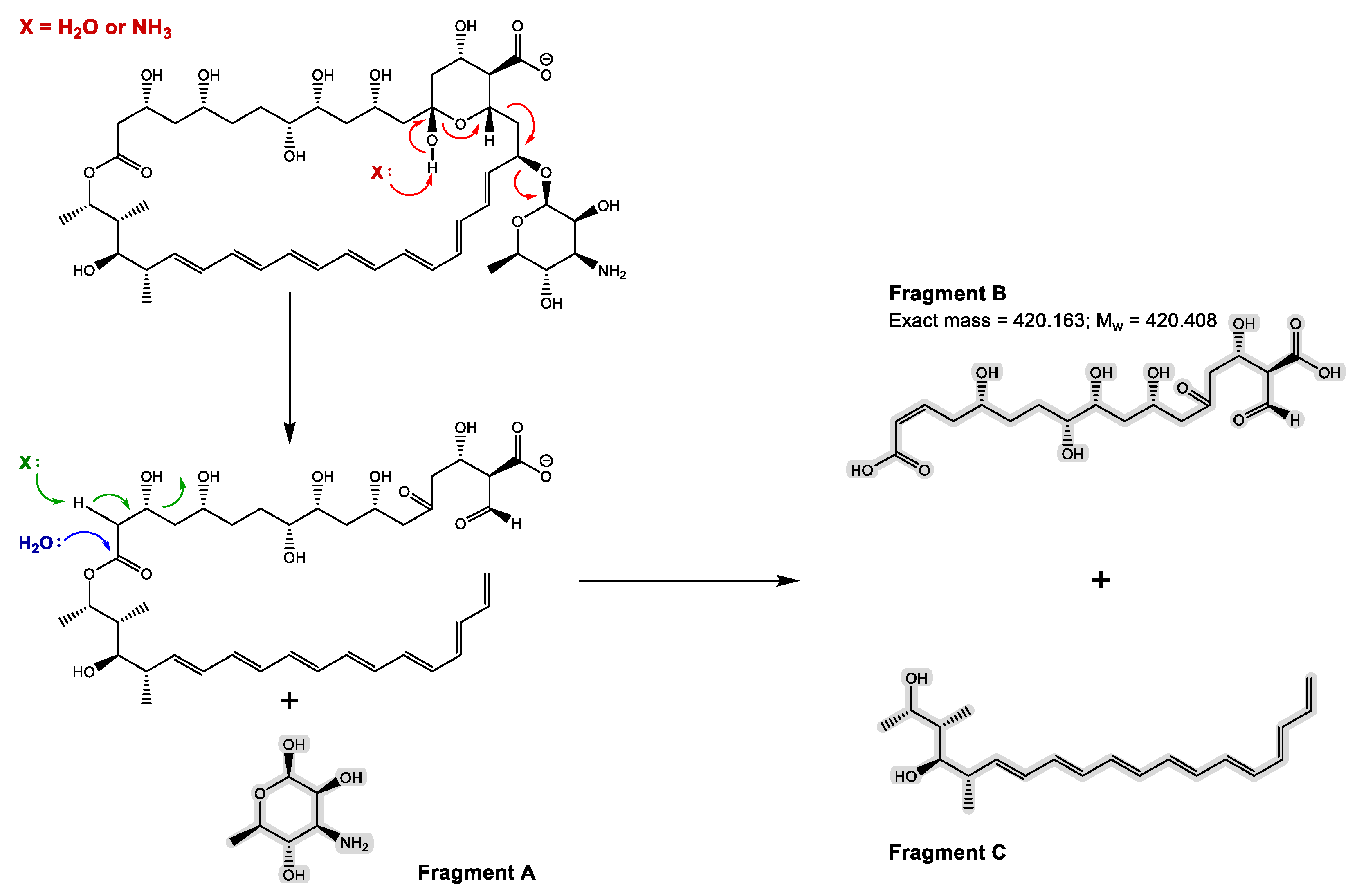
3.1. Synthesis and Chemical Characterization of OSILs Based on Amphotericin B
3.2. Biological Activity against Leishmania infantum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.W.; Billa, N.; Roberts, C.R.; Burley, J.C. Surfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriers. Colloids Surf. A—Physicochem. Eng. Asp. 2010, 372, 73–79. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Rueda, C.; Roman, E.; Quintin, J.; Terron, M.C.; Luque, D.; Netea, M.G.; Pla, J.; Zaragoza, O. Cell Wall Changes in Amphotericin B-Resistant Strains from Candida tropicalis and Relationship with the Immune Responses Elicited by the Host. Antimicrob. Agents Chemother. 2016, 60, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Szpilman, A.M.; Cereghetti, D.M.; Manthorpe, J.M.; Wurtz, N.R.; Carreira, E.M. Synthesis and Biophysical Studies on 35-Deoxy Amphotericin B Methyl Ester. Chem.-A Eur. J. 2009, 15, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Gariboldi, P.; Bruni, P.; Borowski, E. Conformational analysis of Amphotericin B. Biophys. Chem. 1997, 65, 91–100. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- Torrado, J.J.; Espada, R.; Ballesteros, M.P.; Torrado-Santiago, S. Amphotericin B formulations and drug targeting. J. Pharm. Sci. 2008, 97, 2405–2425. [Google Scholar] [CrossRef]
- Ching, M.S.; Raymond, K.; Bury, R.W.; Mashford, M.L.; Morgan, D.J. Absorption of Orally-Administered Amphotericin-B Lozenges. Br. J. Clin. Pharmacol. 1983, 16, 106–108. [Google Scholar] [CrossRef]
- Pappas, H.C.; Sylejmani, R.; Graus, M.S.; Donabedian, P.L.; Whitten, D.G.; Neumann, A.K. Antifungal Properties of Cationic Phenylene Ethynylenes and Their Impact on β-Glucan Exposure. Antimicrob. Agents Chemother. 2016, 60, 4519–4529. [Google Scholar] [CrossRef]
- Dupont, B. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 2002, 49, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Risovic, V.; Sachs-Barrable, K.; Boyd, M.; Wasan, K.M. Potential mechanisms by which Peceol (R) increases the gastrointestinal absorption of Amphotericin B. Drug Dev. Ind. Pharm. 2004, 30, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, N.; Sawada, Y.; Murata, M. Mycosamine orientation of amphotericin B controlling interaction with ergosterol: Sterol-dependent activity of conformation-restricted derivatives with an amino-carbonyl bridge. J. Am. Chem. Soc. 2005, 127, 10667–10675. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Bau-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Daines, R.A.; Ogawa, Y.; Chakraborty, T.K. Total Synthesis Of Amphotericin-B.3. The Final Stages. J. Am. Chem. Soc. 1988, 110, 4696–4705. [Google Scholar] [CrossRef]
- Matsumori, N.; Umegawa, Y.; Oishi, T.; Murata, M. Bioactive fluorinated derivative of amphotericin B. Bioorg. Med. Chem. Lett. 2005, 15, 3565–3567. [Google Scholar] [CrossRef]
- Davis, S.A.; Della Ripa, L.A.; Hu, L.B.W.; Cioffi, A.G.; Pogorelov, T.V.; Rienstra, C.M.; Burke, M.D. C3-OH of Amphotericin B Plays an Important Role in Ion Conductance. J. Am. Chem. Soc. 2015, 137, 15102–15104. [Google Scholar] [CrossRef]
- Flores-Romero, J.D.; Rodriguez-Lozada, J.; Lopez-Ortiz, M.; Magana, R.; Ortega-Blake, I.; Regla, I.; Fernandez-Zertuche, M. Multigram Scale Synthesis of A21, A New Antibiotic Equally Effective and Less Toxic than Amphotericin B. Org. Process Res. Dev. 2016, 20, 1529–1532. [Google Scholar] [CrossRef]
- Sedlak, M.; Pravda, M.; Kubicova, L.; Mikulcikova, P.; Ventura, K. Synthesis and characterisation of a new pH-sensitive amphotericin B—poly(ethylene glycol)-b-poly(L-lysine) conjugate. Bioorg. Med. Chem. Lett. 2007, 17, 2554–2557. [Google Scholar] [CrossRef]
- Neuberger, A.; Van Deenen, L.L. Modern Physical Methods in Biochemistry, Part A; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Thanki, K.; Date, T.; Jain, S. Improved Oral Bioavailability and Gastrointestinal Stability of Amphotericin B through Fatty Acid Conjugation Approach. Mol. Pharm. 2019, 16, 4519–4529. [Google Scholar] [CrossRef]
- Belakhov, V.V.; Kolodyaznaya, V.A.; Garabadzhiu, A.V. Chemical modification of heptaene macrolide antibiotic Amphotericin B under conditions of the Atherton-Todd reaction. Russ. J. Gen. Chem. 2014, 84, 1953–1961. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.V.; Grammatikova, N.E.; Isakova, E.B.; Mirchink, E.P.; Treshchalin, I.D.; Pereverzeva, E.R.; Bykov, E.E.; et al. Discovery of Amphamide, a Drug Candidate for the Second Generation of Polyene Antibiotics. ACS Infect. Dis. 2020, 6, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Korolev, A.M.; Trenin, A.S.; Dezhenkova, L.G.; Shtil, A.A.; Polshakov, V.I.; Savelyev, O.Y.; Olsufyeva, E.N. New conjugates of polyene macrolide amphotericin B with benzoxaboroles: Synthesis and properties. J. Antibiot. 2016, 69, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Baibek, A.; Ucuncu, M.; Short, B.; Ramage, G.; Lilienkampf, A.; Bradley, M. Dyeing fungi: Amphotericin B based fluorescent probes for multiplexed imaging. Chem. Commun. 2021, 57, 1899–1902. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Prudencio, C.; Vieira, M.; van der Auweraer, S.; Ferraz, R. Recycling Old Antibiotics with Ionic Liquids. Antibiotics 2020, 9, 578. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [CrossRef]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- Suresh, C.; Zhao, H.; Gumbs, A.; Chetty, C.S.; Bose, H.S. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Hough, W.L.; Smiglak, M.; Rodriguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Galonde, N.; Nott, K.; Debuigne, A.; Deleu, M.; Jerome, C.; Paquot, M.; Wathelet, J.P. Use of ionic liquids for biocatalytic synthesis of sugar derivatives. J. Chem. Technol. Biotechnol. 2012, 87, 451–471. [Google Scholar] [CrossRef]
- Bica, K.; Rijksen, C.; Nieuwenhuyzen, M.; Rogers, R.D. In search of pure liquid salt forms of aspirin: Ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.; Lobo, L.; Oliveira, I.S.; Gomes, J.; Teixeira, C.; Nogueira, F.; Marques, E.F.; Ferraz, R.; Gomes, P. Building on Surface-Active Ionic Liquids for the Rescuing of the Antimalarial Drug Chloroquine. Int. J. Mol. Sci. 2020, 21, 5334. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Santos, M.M.; Ferraz, R.; Prudencio, C.; Fernandes, M.H.; Costa-Rodrigues, J.; Branco, L.C. A Novel Approach for Bisphosphonates: Ionic Liquids and Organic Salts from Zoledronic Acid. Chemmedchem 2019, 14, 1767–1770. [Google Scholar] [CrossRef]
- Silva, A.T.; Cerqueira, M.J.; Prudencio, C.; Fernandes, M.H.; Costa-Rodrigues, J.; Teixeira, C.; Gomes, P.; Ferraz, R. Antiproliferative Organic Salts Derived from Betulinic Acid: Disclosure of an Ionic Liquid Selective against Lung and Liver Cancer Cells. ACS Omega 2019, 4, 5682–5689. [Google Scholar] [CrossRef]
- Stoimenovski, J.; Dean, P.M.; Izgorodina, E.I.; MacFarlane, D.R. Protic pharmaceutical ionic liquids and solids: Aspects of protonics. Faraday Discuss. 2012, 154, 335–352. [Google Scholar] [CrossRef]
- Azevedo, A.M.O.; Costa, S.P.F.; Dias, A.F.V.; Marques, A.H.O.; Pinto, P.; Bica, K.; Ressmann, A.K.; Passos, M.L.C.; Araujo, A.; Reis, S.; et al. Anti-inflammatory choline based ionic liquids: Insights into their lipophilicity, solubility and toxicity parameters. J. Mol. Liq. 2017, 232, 20–26. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Beck, T.S.; Rodrigues, L.V.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Soares, F.; Martins, M.A.P. Novel ibuprofenate- and docusate-based ionic liquids: Emergence of antimicrobial activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Mukherjee, A.; Muller, P.; Rogers, R.D.; Myerson, A.S. Exploring the role of ionic liquids to tune the polymorphic outcome of organic compounds. Chem. Sci. 2018, 9, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Duman, A.N.; Ozturk, I.; Tuncel, A.; Ocakoglu, K.; Colak, S.G.; Hosgor-Limoncu, M.; Yurt, F. Synthesis of new water-soluble ionic liquids and their antibacterial profile against gram-positive and gram-negative bacteria. Heliyon 2019, 5, e02607. [Google Scholar] [CrossRef] [PubMed]
- Ruokonen, S.K.; Sanwald, C.; Robciuc, A.; Hietala, S.; Rantamaki, A.H.; Witos, J.; King, A.W.T.; Lammerhofer, M.; Wiedmer, S.K. Correlation between Ionic Liquid Cytotoxicity and Liposome-Ionic Liquid Interactions. Chem.-A Eur. J. 2018, 24, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Gurau, G.; Narita, A.; Barber, P.S.; Cojocaru, O.A.; Rogers, R.D. Drug specific, tuning of an ionic liquid’s hydrophilic-lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. [Google Scholar] [CrossRef]
- Jameson, L.P.; Dzyuba, S.V. Effect of imidazolium room-temperature ionic liquids on aggregation of amphotericin B: A circular dichroism study. RSC Adv. 2015, 5, 80325–80329. [Google Scholar] [CrossRef]
- Cabezas, Y.; Legentil, L.; Robert-Gangneux, F.; Daligault, F.; Belaz, S.; Nugier-Chauvin, C.; Tranchimand, S.; Tellier, C.; Gangneux, J.P.; Ferrieres, V. Leishmania cell wall as a potent target for antiparasitic drugs. A focus on the glycoconjugates. Org. Biomol. Chem. 2015, 13, 8393–8404. [Google Scholar] [CrossRef]
- Ouellette, M.; Drummelsmith, J.; Papadopoulou, B. Leishmaniasis: Drugs in the clinic, resistance and new developments. Drug Resist. Updates 2004, 7, 257–266. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; de Moraes, J.; Andricopulo, A.D. Approaches to advance drug discovery for neglected tropical diseases. Drug Discov. Today 2022, 27, 2278–2287. [Google Scholar] [CrossRef]
- Sundar, S.; Agarwal, D. Visceral Leishmaniasis-Optimum Treatment Options in Children. Pediatr. Infect. Dis. J. 2018, 37, 492–494. [Google Scholar] [CrossRef]
- Choi, H.L.; Jain, S.; Postigo, J.A.R.; Borisch, B.; Dagne, D.A. The global procurement landscape of leishmaniasis medicines. PLoS Negl. Trop. Dis. 2021, 15, e0009181. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Rajput, B. Comparative analysis of the omics technologies used to study antimonial, amphotericin B, and pentamidine resistance in leishmania. J. Parasitol. Res. 2014, 2014, 726328. [Google Scholar] [CrossRef]
- Mbongo, N.; Loiseau, P.M.; Billion, M.A.; Robert-Gero, M. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 1998, 42, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pourshafie, M.; Morand, S.; Virion, A.; Rakotomanga, M.; Dupuy, C.; Loiseau, P.M. Cloning of S-adenosyl-L-Methionine: C-24-Delta-sterol-methyltransferase (ERG6) from Leishmania donovani and characterization of mRNAs in wild-type and amphotericin B-resistant promastigotes. Antimicrob. Agents Chemother. 2004, 48, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Borowski, E.; Salewska, N.; Boros-Majewska, J.; Serocki, M.; Chabowska, I.; Milewska, M.J.; Zietkowski, D.; Milewski, S. The Substantial Improvement of Amphotcricin B Selective Toxicity Upon Modification of Mycosamine with Bulky Substituents. Med. Chem. 2020, 16, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Paquet, V.; Volmer, A.A.; Carreira, E.M. Synthesis and in vitro biological properties of novel cationic derivatives of amphotericin B. Chem.-A Eur. J. 2008, 14, 2465–2481. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators Against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251. [Google Scholar] [CrossRef]
- Hartmann, D.O.; Shimizu, K.; Rothkegel, M.; Petkovic, M.; Ferraz, R.; Petrovski, Z.; Branco, L.C.; Lopes, J.N.C.; Pereira, C.S. Tailoring amphotericin B as an ionic liquid: An upfront strategy to potentiate the biological activity of antifungal drugs. RSC Adv. 2021, 11, 14441–14452. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araujo, J.M.M.; Rebelo, L.P.N.; da Ponte, M.N.; Prudencio, C.; Noronha, J.P.; Petrovski, Z. Development of novel ionic liquids based on ampicillin. Medchemcomm 2012, 3, 494–497. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Souter, A.J.; Hung, C.T.; Perrier, D.G.; Lam, F.C. A Stability Study of Amphotericin-B in Aqueous-Media Using Factorial Design. Proc. Univ. Otago Med. Sch. 1985, 63, 77–78. [Google Scholar]
- Carreira, A.R.F.; Rocha, S.N.; Silva, F.A.E.; Sintra, T.E.; Passos, H.; Ventura, S.P.M.; Coutinho, J.A.P. Amino-acid-based chiral ionic liquids characterization and application in aqueous biphasic systems. Fluid Phase Equilibria 2021, 542, 113091. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280965, Amphotericin B. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amphotericin-b (accessed on 1 November 2022).
| Compound | IC50 1 (nM) | RDIC 2 |
|---|---|---|
| AmBH | 86.6 [80.67 to 92.96] | - |
| [Aliquat][Cl] | 299.7 [190.1 to 472.3] | - |
| [Ch][Cl] | N.A. | - |
| [C2OHMIM][Cl] | N.A. | - |
| [C3OMIM][Cl] | N.A. | - |
| [C16Pyr][Cl] | 482.8 [356.1 to 654.5] | - |
| [P6,6,6,14][Cl] | 204.4 [183.2 to 228.2] | - |
| [Aliquat][AmB] | 80.38 [73.39 to 88.03] | 1.08 |
| [Ch][AmB] | 109.6 [95.64 to 125.6] | 0.79 |
| [C2OHMIM][AmB] | 119.5 [99.10 to 144.0] | 0.72 |
| [C3OMIM][AmB] | 88.26 [78.60 to 99.11] | 0.98 |
| [C16Pyr][AmB] | 103.7 [90.53 to 118.7] | 0.84 |
| [P6,6,6,14][AmB] | 61.4 [53.68 to 70.21] | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, R.; Santarém, N.; Santos, A.F.M.; Jacinto, M.L.; Cordeiro-da-Silva, A.; Prudêncio, C.; Noronha, J.P.; Branco, L.C.; Petrovski, Ž. Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum. Antibiotics 2022, 11, 1841. https://doi.org/10.3390/antibiotics11121841
Ferraz R, Santarém N, Santos AFM, Jacinto ML, Cordeiro-da-Silva A, Prudêncio C, Noronha JP, Branco LC, Petrovski Ž. Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum. Antibiotics. 2022; 11(12):1841. https://doi.org/10.3390/antibiotics11121841
Chicago/Turabian StyleFerraz, Ricardo, Nuno Santarém, Andreia F. M. Santos, Manuel L. Jacinto, Anabela Cordeiro-da-Silva, Cristina Prudêncio, João Paulo Noronha, Luis C. Branco, and Željko Petrovski. 2022. "Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum" Antibiotics 11, no. 12: 1841. https://doi.org/10.3390/antibiotics11121841
APA StyleFerraz, R., Santarém, N., Santos, A. F. M., Jacinto, M. L., Cordeiro-da-Silva, A., Prudêncio, C., Noronha, J. P., Branco, L. C., & Petrovski, Ž. (2022). Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum. Antibiotics, 11(12), 1841. https://doi.org/10.3390/antibiotics11121841












