Prescription of Choreito, a Japanese Kampo Medicine, with Antimicrobials for Treatment of Acute Cystitis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Ethical Issue
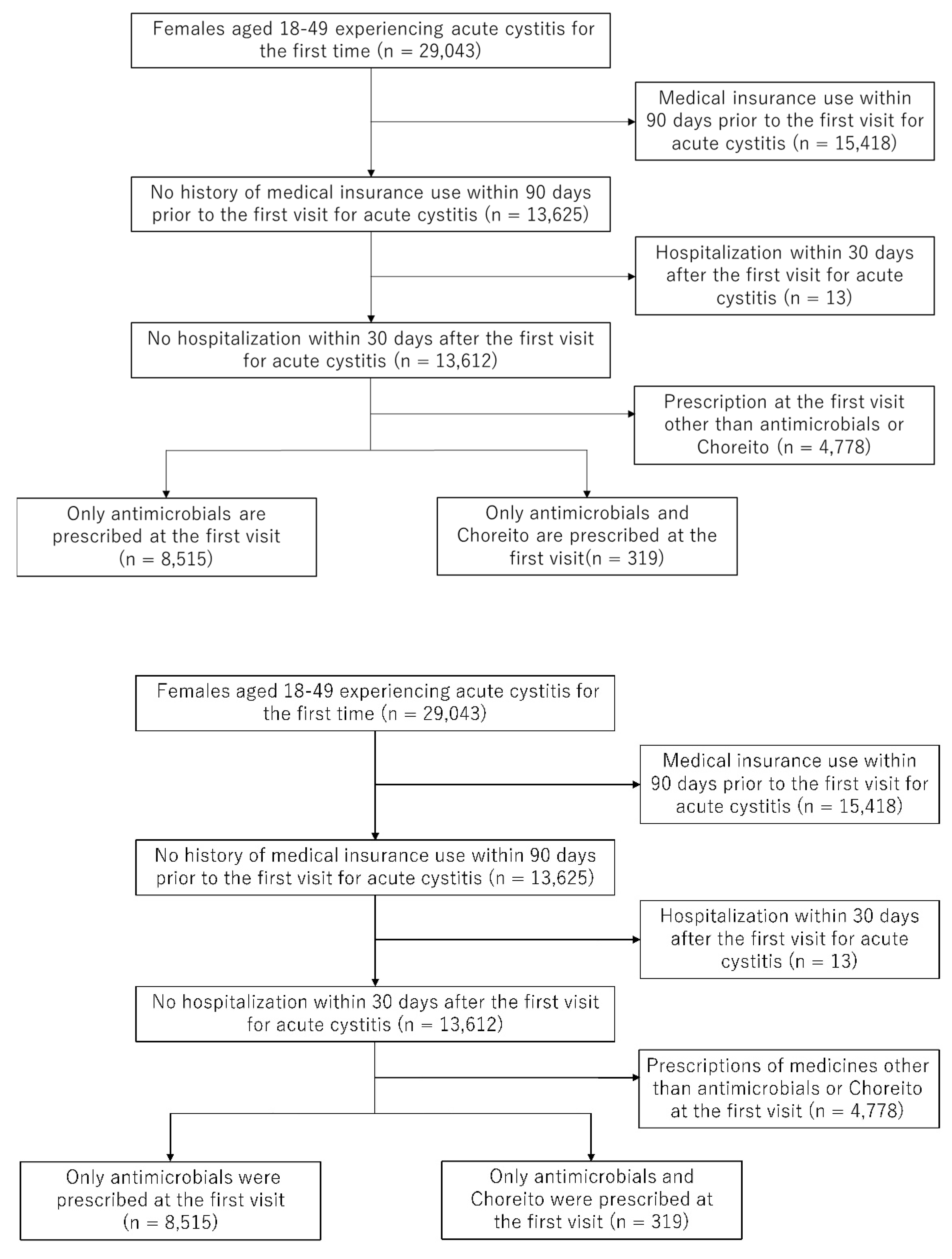
4.3. Study Population
4.4. Endpoints
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Hadj Messaoud, S.; Demonchy, E.; Mondain, V. Recurring cystitis: How can we do our best to help patients help themselves? Antibiotics 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Stamatiou, K.; Samara, E.; Alidjanov, J.F.; Pilatz, A.M.E.; Naber, K.G.; Wagenlehner, F.M.E. Clinical validation of the Greek version of the Acute Cystitis Symptom Score (ACSS)—Part II. Antibiotics 2021, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Waldorff, M.S.; Bjerrum, L.; Holm, A.; Siersma, V.; Bang, C.; Llor, C.; Cordoba, G. Influence of antimicrobial resistance on the course of symptoms in female patients treated for uncomplicated cystitis caused by Escherichia coli. Antibiotics 2022, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Aljeldah, M.M. Antimicrobial resistance and its spread is a global threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- George, P.B.L.; Rossi, F.; St-Germain, M.-W.; Amato, P.; Badard, T.; Bergeron, M.G.; Boissinot, M.; Charette, S.J.; Coleman, B.L.; Corbeil, J.; et al. Antimicrobial resistance in the environment: Towards elucidating the roles of bioaerosols in transmission and detection of antibacterial resistance genes. Antibiotics 2022, 11, 974. [Google Scholar] [CrossRef]
- Khoo, S.C.; Goh, M.S.; Alias, A.; Luang-In, V.; Chin, K.W.; Ling Michelle, T.H.; Sonne, C.; Ma, N.L. Application of antimicrobial, potential hazard and mitigation plans. Environ. Res. 2022, 215, 114218. [Google Scholar] [CrossRef]
- Sevilla-Navarro, S.; Catalá-Gregori, P.; Torres-Boncompte, J.; Orenga, M.T.; Garcia-Llorens, J.; Cortés, V. Antimicrobial resistance trends of Escherichia coli isolates: A three-year prospective study of poultry production in Spain. Antibiotics 2022, 11, 1064. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 September 2022).
- Fuyuno, I. Japan: Will the sun set on Kampo? Nature 2011, 480, S96. [Google Scholar] [CrossRef]
- Research Center for Medicinal Plant Resources; National Institute of Biomedical Innovation. Japan. Table of the Links of Kampo Product Information in Japanese Pharmacopoeia (JP) and/or Package Insert (Ver. 5.1). Available online: http://mpdb.nibiohn.go.jp/kconsort/kconsort.html (accessed on 1 September 2022).
- Buffington, C.A.; Blaisdell, J.L.; Komatsu, Y.; Kawase, K. Effects of Choreito and takushya consumption on in vitro and in vivo struvite solubility in cat urine. Am. J. Vet. Res. 1997, 58, 150–152. [Google Scholar]
- Wang, D.; Liu, M.; Cao, J.; Cheng, Y.; Zhuo, C.; Xu, H.; Tian, S.; Zhang, Y.; Zhang, J.; Wang, F. Effect of Colla corii asini (E’jiao) on D-Galactose induced aging mice. Biol. Pharm. Bull. 2012, 35, 2128–2132. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, X.; Han, L.; Wei, J.A.; Huang, H. Diuretic activity and kidney medulla AQP1, AQP2, AQP3, V2R expression of the aqueous extract of sclerotia of Polyporus umbellatus FRIES in normal rats. J. Ethnopharmacol. 2010, 128, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Azuhata, Y.; Matsuura, D.; Kameda, Y.; Yokota, M. Antitumor-promoting effect of Kampo formulations on rat urinary bladder carcinogenesis in a short-term test with concanavalin A. J. Tradit. Med. 1994, 11, 148–155. [Google Scholar]
- Hattori, T.; Hayashi, K.; Nagao, T.; Furuta, K.; Ito, M.; Suzuki, Y. Studies on antinephritic effects of plant components (3): Effect of pachyman, a main component of Poria cocos Wolf on original-type anti-GBM nephritis in rats and its mechanisms. Jpn. J. Pharmacol. 1992, 59, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Torimoto, K.; Gotoh, D.; Hori, S.; Nakai, Y.; Miyake, M.; Tokita, Y.; Kobayashi, R.; Aoki, K.; Fujimoto, K. The effects of choreito on a model of nocturnal polyuria using Dahl salt-sensitive rats. LUTS Low. Urin. Tract Symptoms 2022, 14, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Ito, Y.; Sekiya, Y.; Narita, A.; Okuno, Y.; Muramatsu, H.; Irie, M.; Hama, A.; Takahashi, Y.; Kojima, S. Choreito formula for BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015, 21, 319–325. [Google Scholar] [CrossRef]
- Horii, A.; Maekawa, M. [Clinical evaluation of chorei-to and chorei-to-go-shimotsu-to in patients with lower urinary tract symptoms]. Hinyokika Kiyo 1988, 34, 2237–2241. [Google Scholar]
- Kajiwara, M.; Mutaguchi, K. Clinical efficacy and tolerability of gosha-jinki-gan, Japanese traditional herbal medicine, in females with overactive bladder. Hinyokika kiyo. Acta Urol. Jpn. 2008, 54, 95–99. [Google Scholar]
- Imamura, T.; Ishizuka, O.; Aizawa, N.; Zhong, C.; Ogawa, T.; Nakayama, T.; Tanabe, T.; Nishizawa, O. Gosha-jinki-gan reduces transmitter proteins and sensory receptors associated with C fiber activation induced by acetic acid in rat urinary bladder. Neurourol. Urodyn. 2008, 27, 832–837. [Google Scholar] [CrossRef]
- Kawashima, N.; Deveaux, T.E.; Yoshida, N.; Matsumoto, K.; Kato, K. Choreito, a formula from Japanese traditional medicine (Kampo medicine), for massive hemorrhagic cystitis and clot retention in a pediatric patient with refractory acute lymphoblastic leukemia. Phytomedicine 2012, 19, 1143–1146. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442-442A. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, T.; Ishizuka, O. Status of urological Kampo medicine: A narrative review and future vision. Int. J. Urol. 2015, 22, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Seki, T.; Tsutani, K. Traditional Japanese medicine, Kampo: Its history and current status. Chin. J. Integr. Med. 2011, 17, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Arai, I.; Hyodo, I.; Tsutani, K. Current status of Kampo (Japanese herbal) medicines in Japanese clinical practice guidelines. Complement. Ther. Med. 2009, 17, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Karibe, J.; Saito, T.; Ishibashi, Y.; Usui, K.; Mori, K.; Kuroda, S.; Komeya, M.; Yumura, Y. Effect of keishibukuryogan combined with tocopherol nicotinate on sperm parameters in patients with a varicocele. Int. J. Urol. 2022, 29, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Naya, Y.; Kino, M.; Awa, Y.; Nagata, M.; Suzuki, H.; Yamaguchi, K.; Nozumi, K.; Ichikawa, T. Low dose tamsulosin for stone expulsion after extracorporeal shock wave lithotripsy: Efficacy in Japanese male patients with ureteral stone. Int. J. Urol. 2008, 15, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M. KAMPOmics: A framework for multidisciplinary and comprehensive research on Japanese traditional medicine. Gene 2022, 831, 146555. [Google Scholar] [CrossRef] [PubMed]
- Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Halder Tobler, R.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 pandemic on inpatient antibiotic consumption in Switzerland. Antibiotics 2022, 11, 792. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-term impact of the COVID-19 pandemic on in-hospital antibiotic consumption and antibiotic resistance: A time series analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.A.; Zahreddine, N.; Haddad, N.; Ahmadieh, R.; Hannun, A.; Bou Harb, S.; Haddad, S.F.; Zeenny, R.M.; Kanj, S.S. The Impact of antimicrobial stewardship and infection control interventions on Acinetobacter baumannii resistance rates in the ICU of a tertiary care center in Lebanon. Antibiotics 2022, 11, 911. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Vázquez-Cancela, O.; Piñeiro-Lamas, M.; Figueiras, A.; Zapata-Cachafeiro, M. Impact of the COVID-19 pandemic on antibiotic prescribing by dentists in Galicia, Spain: A quasi-experimental approach. Antibiotics 2022, 11, 1018. [Google Scholar] [CrossRef]
- Lamb, L.E.; Timar, R.; Wills, M.; Dhar, S.; Lucas, S.M.; Komnenov, D.; Chancellor, M.B.; Dhar, N. Long COVID and COVID-19-associated cystitis (CAC). Int. Urol. Nephrol. 2022, 54, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Suzuki, Y.; Ueno, K.; Okada, A.; Fujiu, K.; Matsuoka, S.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Association of Life’s Simple 7 with incident cardiovascular disease in 52,956 patients with cancer. Eur. J. Prev. Cardiol. 2022, zwac195. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Iwagami, M.; Sasabuchi, Y.; Yasunaga, H. Increased risk of infective endocarditis after traumatic skin wound. Heart 2021, 107, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Sato, T.; Ikeda, S.; Noda, M.; Nakayama, T. Development of a database of health insurance claims: Standardization of disease classifications and anonymous record linkage. J. Epidemiol. 2010, 20, 413–419. [Google Scholar] [CrossRef] [PubMed]
| Prescription for Acute Cystitis n (%), or Median (IQR) | p-Value | ||
|---|---|---|---|
| Antimicrobials n = 8515 | Antimicrobials with Choreito n = 319 | ||
| Female | 8515 (100) | 319 (100) | – |
| Age | 32 (24–42) | 34 (26–43) | 0.014 |
| Charlson comorbidity index | |||
| 0 | 6321 (74.2) | 229 (71.8) | 0.543 |
| 1 | 1776 (20.9) | 71 (22.3) | |
| ≥2 | 418 (4.9) | 19 (6.0) | |
| The state of emergency against COVID-19 in Japan | |||
| Before (2018 April to 2020 March) | 5126 (60.2) | 179 (56.1) | 0.143 |
| After (2020 April to 2021 March) | 3389 (39.8) | 140 (43.9) | |
| OUTCOME | |||
| The number of clinic visits within 30 days | 1 (1–2) | 1 (1–2) | 0.885 |
| 1 | 5169 (60.7) | 194 (60.8) | 0.549 |
| 2 | 2898 (34.0) | 104 (32.6) | |
| ≥3 | 448 (5.3) | 21 (6.6) | |
| Total antibiotic prescription days | 5 (5–7) | 5 (4–7) | 0.002 |
| 1 to 3 days | 1021 (12.0) | 54 (16.9) | 0.041 |
| 4 to 5 days | 4587 (53.9) | 170 (53.3) | |
| 6 to 7 days | 2163 (25.4) | 68 (21.3) | |
| ≥8 days | 744 (8.7) | 27 (8.5) | |
| The number of antibiotic prescriptions within 30 days | 1 (1–1) | 1 (1–1) | 0.476 |
| 1 | 7667 (90.0) | 291 (91.2) | 0.488 |
| ≥2 | 848 (10.0) | 28 (8.8) | |
| Factors | Beta (95% CI) | p Value |
|---|---|---|
| Endpoint 1. The number of clinic visits within 30 days | ||
| Age, by 10 years | 1.127 (1.091 to 1.164) | <0.001 |
| Charlson comorbidity index (continuous) | 1.039 (0.995 to 1.085) | 0.085 |
| After the state of emergency against COVID-19 in Japan (vs. before) | 1.085 (1.019 to 1.155) | 0.011 |
| Choreito prescription (yes vs. no) | 1.041 (0.887 to 1.222) | 0.624 |
| Endpoint 2. Total antibiotic prescription days | ||
| Age, by 10 years | 1.015 (1.006 to 1.025) | 0.001 |
| Charlson comorbidity index (continuous) | 0.998 (0.984 to 1.011) | 0.713 |
| After the state of emergency against COVID-19 in Japan (vs. before) | 1.018 (1.000 to 1.036) | 0.049 |
| Choreito prescription (yes vs. no) | 0.950 (0.906 to 0.997) | 0.038 |
| Endpoint 3. The number of antibiotic prescriptions within 30 days | ||
| Age, by 10 years | 1.010 (0.990 to 1.032) | 0.326 |
| Charlson comorbidity index (continuous) | 1.002 (0.972 to 1.032) | 0.905 |
| After the state of emergency against COVID-19 in Japan (vs. before) | 1.008 (0.968 to 1.049) | 0.712 |
| Choreito prescription (yes vs. no) | 0.982 (0.882 to 1.092) | 0.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugihara, T.; Kamei, J.; Yasunaga, H.; Sasabuchi, Y.; Fujimura, T. Prescription of Choreito, a Japanese Kampo Medicine, with Antimicrobials for Treatment of Acute Cystitis: A Retrospective Cohort Study. Antibiotics 2022, 11, 1840. https://doi.org/10.3390/antibiotics11121840
Sugihara T, Kamei J, Yasunaga H, Sasabuchi Y, Fujimura T. Prescription of Choreito, a Japanese Kampo Medicine, with Antimicrobials for Treatment of Acute Cystitis: A Retrospective Cohort Study. Antibiotics. 2022; 11(12):1840. https://doi.org/10.3390/antibiotics11121840
Chicago/Turabian StyleSugihara, Toru, Jun Kamei, Hideo Yasunaga, Yusuke Sasabuchi, and Tetsuya Fujimura. 2022. "Prescription of Choreito, a Japanese Kampo Medicine, with Antimicrobials for Treatment of Acute Cystitis: A Retrospective Cohort Study" Antibiotics 11, no. 12: 1840. https://doi.org/10.3390/antibiotics11121840
APA StyleSugihara, T., Kamei, J., Yasunaga, H., Sasabuchi, Y., & Fujimura, T. (2022). Prescription of Choreito, a Japanese Kampo Medicine, with Antimicrobials for Treatment of Acute Cystitis: A Retrospective Cohort Study. Antibiotics, 11(12), 1840. https://doi.org/10.3390/antibiotics11121840






