An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals
Abstract
1. Introduction
2. Mechanisms of Antibacterial Activity and Resistance
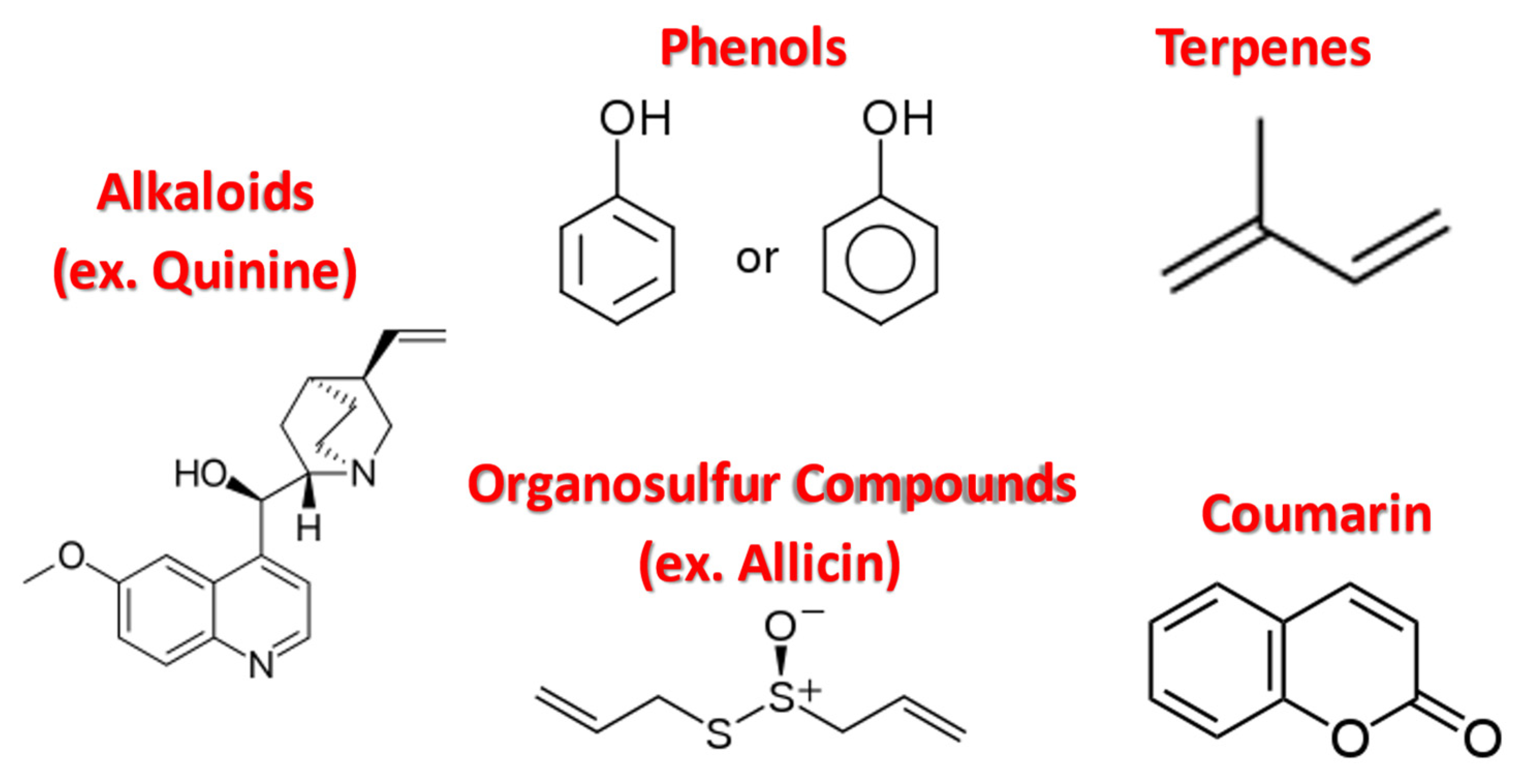
2.1. Alkaloids
2.2. Organosulfur Compounds
2.3. Phenolic Compounds
2.4. Flavonoids
2.5. Non-Flavonoids
3. Clinical Trial Assessment of Phytochemicals against Microbes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Peláez, F. The historical delivery of antibiotics from microbial natural products—Can history repeat? Biochem. Pharmacol. 2006, 71, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.-C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M.; CDC Prevention Epicenters Program. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated with Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef] [PubMed]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Harper, L.; Shopsin, B.; Torres, V.J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. 2017, 75, ftx005. [Google Scholar] [CrossRef] [PubMed]
- Sabir, N.; Ikram, A.; Zaman, G.; Satti, L.; Gardezi, A.; Ahmed, A.; Ahmed, P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control. 2017, 45, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Rana, R.; Sharma, R.; Kumar, A. Repurposing of Existing Statin Drugs for Treatment of Microbial Infections: How Much Promising? Infect. Disord.—Drug Targets 2019, 19, 224–237. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Hintz, T.; Matthews, K.K.; Di, R. The Use of Plant Antimicrobial Compounds for Food Preservation. BioMed Res. Int. 2015, 2015, 246264. [Google Scholar] [CrossRef]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. ISBN 978-94-007-3926-0. [Google Scholar]
- Dahiya, P.; Purkayastha, S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012, 74, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Bazzaz, B.S.F. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sharifi, M.J.; Bazzaz, B.S.F.; Emami, A.; Soheili, V.; Sahebkar, A.; Asili, J. Bioautography Detection of Antimicrobial Compounds from the Essential Oil of Salvia Pachystachys. Curr. Bioact. Compd. 2018, 14, 80–85. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An Update of the Sanguinarine and Benzophenanthridine Alkaloids’ Biosynthesis and Their Applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef]
- Obiang-Obounou, B.W.; Kang, O.-H.; Choi, J.-G.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Shin, D.-W.; Kim, K.W.; Park, C.-B.; Kim, Y.-G.; et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Hubert, D.; Réglier-Poupet, H.; Sermet-Gaudelus, I.; Ferroni, A.; Le Bourgeois, M.; Burgel, P.-R.; Serreau, R.; Dusser, D.; Poyart, C.; Coste, J. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J. Cyst. Fibros. 2013, 12, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Lafrance, M.; Boulanger, S.; Séguin, D.L.; Guay, I.; Gattuso, M.; Marsault, E.; Bouarab, K.; Malouin, F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2011, 67, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Guay, I.; Boulanger, S.; Isabelle, C.; Brouillette, E.; Chagnon, F.; Bouarab, K.; Marsault, E.; Malouin, F. Tomatidine and analog FC04-100 possess bactericidal activities against Listeria, Bacillus and Staphylococcus spp. BMC Pharmacol. Toxicol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Dorsaz, S.; Snäkä, T.; Favre-Godal, Q.; Maudens, P.; Boulens, N.; Furrer, P.; Ebrahimi, S.N.; Hamburger, M.; Allémann, E.; Gindro, K.; et al. Identification and Mode of Action of a Plant Natural Product Targeting Human Fungal Pathogens. Antimicrob. Agents Chemother. 2017, 61, e00829-17. [Google Scholar] [CrossRef]
- Medina, J.M.; Rodrigues, J.C.F.; De Souza, W.; Atella, G.C.; Barrabin, H. Tomatidine promotes the inhibition of 24-alkylated sterol biosynthesis and mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitology 2012, 139, 1253–1265. [Google Scholar] [CrossRef]
- Desmond, E.; Gribaldo, S. Phylogenomics of Sterol Synthesis: Insights into the Origin, Evolution, and Diversity of a Key Eukaryotic Feature. Genome Biol. Evol. 2009, 1, 364–381. [Google Scholar] [CrossRef]
- Simons, V.; Morrissey, J.P.; Latijnhouwers, M.; Csukai, M.; Cleaver, A.; Yarrow, C.; Osbourn, A. Dual Effects of Plant Steroidal Alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2006, 50, 2732–2740. [Google Scholar] [CrossRef]
- Khan, I.A.; Mirza, Z.M.; Kumar, A.; Verma, V.; Qazi, G.N. Piperine, a Phytochemical Potentiator of Ciprofloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 810–812. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Atashbeyk, D.G.; Bazzaz, B.S.F.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 2014, 41, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Lobkovsky, E.B.; Gange, A.C.; Singh, S.K.; Prakash, S. Piperine production by endophytic fungus Periconia sp. Isolated from Piper longum L. J. Antibiot. 2011, 64, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, J.; Guo, N.; Feng, H.; Li, L.; Liang, J.; Sun, K.; Wu, X.; Wang, X.; Liu, M.; et al. The plant alkaloid piperine as a potential inhibitor of ethidium bromide efflux in Mycobacterium smegmatis. J. Med. Microbiol. 2011, 60, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kalia, N.P.; Suden, P.; Chauhan, P.S.; Kumar, M.; Ram, A.B.; Khajuria, A.; Bani, S.; Khan, I.A. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis 2014, 94, 389–396. [Google Scholar] [CrossRef]
- Iwasa, K.; Moriyasu, M.; Yamori, T.; Turuo, T.; Lee, D.-U.; Wiegrebe, W. In Vitro Cytotoxicity of the Protoberberine-Type Alkaloids. J. Nat. Prod. 2001, 64, 896–898. [Google Scholar] [CrossRef]
- Yi, Z.-B.; Yu, Y.; Liang, Y.-Z.; Zeng, B. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J. Pharm. Biomed. Anal. 2007, 44, 301–304. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine Targets Assembly of Escherichia coli Cell Division Protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; He, C.; et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217–5223. [Google Scholar]
- Leng, B.-F.; Qiu, J.-Z.; Dai, X.-H.; Dong, J.; Wang, J.-F.; Luo, M.-J.; Li, H.-E.; Niu, X.-D.; Zhang, Y.; Ai, Y.-X.; et al. Allicin Reduces the Production of α-Toxin by Staphylococcus aureus. Molecules 2011, 16, 7958–7968. [Google Scholar] [CrossRef]
- Khodavandi, A.; Alizadeh, F.; Aala, F.; Sekawi, Z.; Chong, P.P. In Vitro Investigation of Antifungal Activity of Allicin Alone and in Combination with Azoles Against Candida Species. Mycopathologia 2009, 169, 287–295. [Google Scholar] [CrossRef]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef]
- Strehlow, B.; Bakowsky, U. A Novel Microparticulate Formulation with Allicin In Situ Synthesis. J. Pharm. Drug Deliv. Res. 2016, 5, 10-4172. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dockery, C.R.; Crosby, M.; Chavarria, K.; Patterson, B.; Giedd, M. Antibacterial Activities of Wasabi against Escherichia coli O157:H7 and Staphylococcus aureus. Front. Microbiol. 2016, 7, 1403. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Saavedra, M.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.; Rosa, E.; Simões, M. Antimicrobial Activity of Phenolics and Glucosinolate Hydrolysis Products and their Synergy with Streptomycin against Pathogenic Bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Prati, D.; Bossdorf, O. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am. J. Bot. 2004, 91, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.; Saavedra, M.J.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Drobnica, L.; Zemanová, M.; Nemec, P.; Antoš, K.; Kristián, P.; Martvoň, A.; Závodská, E. Antifungal Activity of Isothiocyanates and Related Compounds. Appl. Environ. Microbiol. 1968, 16, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; de Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.; de Souza, E. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Babu, K.S.; Babu, T.H.; Srinivas, P.; Sastry, B.; Kishore, K.H.; Murty, U.; Rao, J.M. Synthesis and in vitro study of novel 7-O-acyl derivatives of Oroxylin A as antibacterial agents. Bioorganic Med. Chem. Lett. 2005, 15, 3953–3956. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Ávila, H.P.; Smânia, E.D.F.A.; Monache, F.D.; Smânia, A. Structure-activity relationship of antibacterial chalcones. Bioorganic Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Boesen, T.; Larsen, M.; Schønning, K.; Kromann, H. Antibacterial chalcones—bioisosteric replacement of the 4′-hydroxy group. Bioorganic Med. Chem. 2004, 12, 3047–3054. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z.; Kędzia, B.; Schroeder, G. Synthesis, physicochemical properties and antimicrobial evaluation of new (E)-chalcones. Eur. J. Med. Chem. 2008, 43, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef]
- Alcaráz, L.; Blanco, S.; Puig, O.; Tomás, F.; Ferretti, F. Antibacterial Activity of Flavonoids Against Methicillin-Resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Otsuka, N.; Liu, M.-H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Anti-Methicillin Resistant Staphylococcus aureus (MRSA) Compounds Isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef]
- Mughal, E.U.; Ayaz, M.; Hussain, Z.; Hasan, A.; Sadiq, A.; Riaz, M.; Malik, A.; Hussain, S.; Choudhary, M.I. Synthesis and antibacterial activity of substituted flavones, 4-thioflavones and 4-iminoflavones. Bioorganic Med. Chem. 2006, 14, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.; Hamilton, V.; Chapman, D.; Taylor, P.; Lamb, A. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]
- Lin, R.-D.; Chin, Y.-P.; Hou, W.-C.; Lee, M.-H. The Effects of Antibiotics Combined with Natural Polyphenols against Clinical Methicillin-Resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 840–846. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of Synergy between Tetracycline and Quercetin against Antibiotic Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Yi, S.; Wang, W.; Bai, F.; Zhu, J.; Li, J.; Li, X.; Xu, Y.; Sun, T.; He, Y. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2013, 30, 451–460. [Google Scholar] [CrossRef]
- Yoda, Y.; Hu, Z.-Q.; Shimamura, T.; Zhao, W.-H. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hara, Y.; Taylor, P.W. Potentiation of Catechin Gallate-Mediated Sensitization of Staphylococcus aureus to Oxacillin by Nongalloylated Catechins. Antimicrob. Agents Chemother. 2006, 50, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Steric Effects on Interaction of Tea Catechins with Lipid Bilayers. Biosci. Biotechnol. Biochem. 2001, 65, 2638–2643. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Effects of External Factors on the Interaction of Tea Catechins with Lipid Bilayers. Biosci. Biotechnol. Biochem. 2002, 66, 2330–2335. [Google Scholar] [CrossRef]
- Kubo, I.; Xiao, P.; Fujita, K. Anti-MRSA activity of alkyl gallates. Bioorganic Med. Chem. Lett. 2001, 12, 113–116. [Google Scholar] [CrossRef]
- Bernal, P.; Zloh, M.; Taylor, P.W. Disruption of d-alanyl esterification of Staphylococcus aureus cell wall teichoic acid by the β-lactam resistance modifier (−)-epicatechin gallate. J. Antimicrob. Chemother. 2009, 63, 1156–1162. [Google Scholar] [CrossRef]
- Yam, T.S.; Hamilton-Miller, J.M.; Shah, S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2’ synthesis, and beta-lactamase production in Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 211–216. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin Resistance in Staphylococcus aureus: Mechanisms and Modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef]
- Bernal, P.; Lemaire, S.; Pinho, M.; Mobashery, S.; Hinds, J.; Taylor, P.W. Insertion of Epicatechin Gallate into the Cytoplasmic Membrane of Methicillin-resistant Staphylococcus aureus Disrupts Penicillin-binding Protein (PBP) 2a-mediated β-Lactam Resistance by Delocalizing PBP2. J. Biol. Chem. 2010, 285, 24055–24065. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Jones, P. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Donald, P.R.; Lamprecht, J.H.; Freestone, M.; Albrecht, C.F.; Bouic, P.J.; Kotze, D.; van Jaarsveld, P.P. A randomised place-bo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 1997, 1, 518–522. [Google Scholar] [PubMed]
- Raicht, R.F.; I Cohen, B.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403–405. [Google Scholar]
- Yamada, H.; Yoshino, M.; Matsumoto, T.; Nagai, T.; Kiyohara, H.; Cyong, J.-C.; Nakagawa, A.; Tanaka, H.; Omura, S. Effects of phytosterols on anti-complementary activity. Chem. Pharm. Bull. 1987, 35, 4851–4855. [Google Scholar] [CrossRef]
- Berges, R.R.; Windeler, J.; Trampisch, H.J.; Senge, T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet 1995, 345, 1529–1532. [Google Scholar] [CrossRef]
- Bouic, P.J.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; Van Jaarsveld, P.P. Beta-Sitosterol and beta-sitosterol glu-coside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Ahmed, S.; Ullah, N.; Parveen, S.; Javed, I.; Jalil, N.A.C.; Das Murtey, M.; Sheikh, I.S.; Khan, S.; Ojha, S.C.; Chen, K. Effect of Silymarin as an Adjunct Therapy in Combination with Sofosbuvir and Ribavirin in Hepatitis C Patients: A Miniature Clinical Trial. Oxid. Med. Cell. Longev. 2022, 2022, 9199190. [Google Scholar] [CrossRef]
- Rahim, A.; Seo, H.; Kim, S.; Jeong, Y.K.; Tajdozian, H.; Kim, M.; Lee, S.; Song, H.-Y. A Clinical Trial to Evaluate the Efficacy of α-Viniferin in Staphylococcus aureus—Specific Decolonization without Depleting the Normal Microbiota of Nares. Pol. J. Microbiol. 2021, 70, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Nawarathne, N.W.; Wijesekera, K.; Wijayaratne, W.M.D.G.B.; Napagoda, M. Development of Novel Topical Cosmeceutical Formulations from Nigella sativa L. with Antimicrobial Activity against Acne-Causing Microorganisms. Sci. World J. 2019, 2019, 5985207. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the In Vitro and In Vivo Antimicrobial Activity on Salivary Streptococci and Lactobacilli and Chemical Characterisation of the Phenolic Content of a Plantago lanceolata Infusion. BioMed Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef]
- Kerdar, T.; Rabienejad, N.; Alikhani, Y.; Moradkhani, S.; Dastan, D. Clinical, in vitro and phytochemical, studies of Scrophularia striata mouthwash on chronic periodontitis disease. J. Ethnopharmacol. 2019, 239, 111872. [Google Scholar] [CrossRef]
- Mergia, E.; Shibeshi, W.; Terefe, G.; Teklehaymanot, T. Antitrypanosomal activity of Verbascum sinaiticum Benth. (Scrophulariaceae) against Trypanosoma congolense isolates. BMC Complement. Altern. Med. 2016, 16, 362. [Google Scholar] [CrossRef]
- Askari, S.F.; Jahromi, B.N.; Dehghanian, A.; Zarei, A.; Tansaz, M.; Badr, P.; Azadi, A.; Mohagheghzadeh, A. Effect of a novel herbal vaginal suppository containing myrtle and oak gall in the treatment of vaginitis: A randomized clinical trial. DARU J. Pharm. Sci. 2020, 28, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Karumathil, D.P.; Surendran-Nair, M.; Venkitanarayanan, K. Efficacy of Trans-Cinnamaldehyde and Eugenol in Reducing Acinetobacter baumannii Adhesion to and Invasion of Human Keratinocytes and Controlling Wound Infection In Vitro. Phytother. Res. 2016, 30, 2053–2059. [Google Scholar] [CrossRef]
- Hanafiah, K.M.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Averhoff, F.M.; Glass, N.; Holtzman, D. Global Burden of Hepatitis C: Considerations for Healthcare Providers in the United States. Clin. Infect. Dis. 2012, 55, S10–S15. [Google Scholar] [CrossRef]
- Travasso, C. Indian government plans 10 regional laboratories to estimate hepatitis burden. BMJ 2014, 349, g5021. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Tsao, S.-M.; Liao, H.-H.; Chen, S.-C.; Lee, Y.-T. Treatment of chronic hepatitis C regiments containing with recombinant interferon in patients with sustained virological response predicts risk of hepatocellular carcinoma. Medicine 2020, 99, e22435. [Google Scholar] [CrossRef] [PubMed]
- Khezri, H.D.; Salehifar, E.; Kosaryan, M.; Aliasgharian, A.; Jalali, H.; Amree, A.H. Potential Effects of Silymarin and Its Flavonolignan Components in Patients with β-Thalassemia Major: A Comprehensive Review in 2015. Adv. Pharmacol. Sci. 2016, 2016, 3046373. [Google Scholar] [CrossRef]
- Amniattalab, A.; Malekinejad, H.; Rezabakhsh, A.; Rokhsartalab-Azar, S.; Alizade-Fanalou, S. Silymarin: A Novel Natural Agent to Restore Defective Pancreatic β Cells in Streptozotocin (STZ)-Induced Diabetic Rats. Iran. J. Pharm. Res. IJPR 2016, 15, 493–500. [Google Scholar]
- Gharagozloo, M.; Jafari, S.; Esmaeil, N.; Javid, E.N.; Bagherpour, B.; Rezaei, A. Immunosuppressive Effect of Silymarin on Mitogen-Activated Protein Kinase Signalling Pathway: The Impact on T Cell Proliferation and Cytokine Production. Basic Clin. Pharmacol. Toxicol. 2013, 113, 209–214. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Velardi, E.; Bruscoli, S.; Agostini, M.; Di Sante, M.; Donato, V.; Amirghofran, Z.; Riccardi, C. Silymarin suppress CD4+ T cell activation and proliferation: Effects on NF-κB activity and IL-2 production. Pharmacol. Res. 2010, 61, 405–409. [Google Scholar] [CrossRef]
- Balouchi, S.; Gharagozloo, M.; Esmaeil, N.; Mirmoghtadaei, M.; Moayedi, B. Serum levels of TGFβ IL-10, IL-17, and IL-23 cytokines in β-thalassemia major patients: The impact of silymarin therapy. Immunopharmacol. Immunotoxicol. 2014, 36, 271–274. [Google Scholar] [CrossRef]
- Islam, Z.; Johannesen, T.B.; Lilje, B.; Urth, T.R.; Larsen, A.R.; Angen, Ø.; Larsen, J. Investigation of the human nasal microbiome in persons with long- and short-term exposure to methicillin-resistant Staphylococcus aureus and other bacteria from the pig farm environment. PLoS ONE 2020, 15, e0232456. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Chew, Y.L.; Chan, E.W.L.; Tan, P.L.; Lim, Y.Y.; Stanslas, J.; Goh, J.K. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complement. Altern. Med. 2011, 11, 12. [Google Scholar] [CrossRef]
- Seo, H.; Kim, M.; Kim, S.; Al Mahmud, H.; Islam, I.; Nam, K.-W.; Cho, M.-L.; Kwon, H.-S.; Song, H.-Y. In vitro activity of alpha-viniferin isolated from the roots of Carex humilis against Mycobacterium tuberculosis. Pulm. Pharmacol. Ther. 2017, 46, 41–47. [Google Scholar] [CrossRef]
- Budhiraja, A.; Dhingra, G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Lyles, J.T.; Li, T.; Saitta, A.; Addie-Noye, E.; Tyler, P.; Quave, C.L. Anti-Acne Activity of Italian Medicinal Plants Used for Skin Infection. Front. Pharmacol. 2016, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Elmarzugi, N.A.; Abu Ayyash, L.M.; Sawafta, M.N.; Daana, H.I. A Review on the Cosmeceutical and External Applications of Nigella sativa. J. Trop. Med. 2017, 2017, 7092514. [Google Scholar] [CrossRef] [PubMed]
- Campus, G.; Condò, S.G.; Di Renzo, G.; Ferro, R.; Gatto, R.; Giuca, M.R.; Giuliana, G.; Majorana, A.; Marzo, G.; Ottolenghi, L.; et al. National Italian Guidelines for caries prevention in 0 to 12 years-old children. Eur. J. Paediatr. Dent. 2007, 8, 153–159. [Google Scholar] [PubMed]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Seminario, A.; Broukal, Z.; Ivancakova, R.K. Mutans streptococci and the development of dental plaque. Prague Med. Rep. 2005, 106, 349–358. [Google Scholar]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant Polyphenols and Their Anti-Cariogenic Properties: A Review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and Scoring of Antimicrobial and Biological Activities of Italian Vulnerary Plants against Major Oral Pathogenic Bacteria. Evid.-Based Complement. Altern. Med. 2013, 2013, 316280. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Roberto, L.; Amato, I.; Cantile, T.; Sangianantoni, G.; Ingenito, A. Antimicrobial Properties of Green Tea Extract Against Cariogenic Microflora: An In Vivo Study. J. Med. Food 2011, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.; Martín-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of Plantago bellardii All. Phytother. Res. 2005, 19, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.; Martín-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant Activity of Methanol Extracts Obtained from Plantago Species. J. Agric. Food Chem. 2005, 53, 1927–1933. [Google Scholar] [CrossRef]
- Shahbazi, Y. Chemical Composition and In Vitro Antibacterial Activity of Mentha spicata Essential Oil against Common Food-Borne Pathogenic Bacteria. J. Pathog. 2015, 2015, 916305. [Google Scholar] [CrossRef]
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Feyera, T.; Terefe, G.; Shibeshi, W. Phytochemical Screening and In Vitro Antitrypanosomal Activity of the Aerial Parts of Artemisia abyssinica Against Trypanosoma congolense Field Isolate. Ethiop. Pharm. J. 2013, 29, 137–142. [Google Scholar] [CrossRef]
- Afewerk, Y.; Clausen, P.-H.; Abebe, G.; Tilahun, G.; Mehlitz, D. Multiple-drug resistant Trypanosoma congolense populations in village cattle of Metekel district, north-west Ethiopia. Acta Trop. 2000, 76, 231–238. [Google Scholar] [CrossRef]
- Mills, B.B. Vaginitis: Beyond the Basics. Obstet. Gynecol. Clin. N. Am. 2017, 44, 159–177. [Google Scholar] [CrossRef]
- Paladine, H.L.; Desai, U.A. Vaginitis: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 321–329. [Google Scholar]
- Esterly, J.S.; Griffith, M.; Qi, C.; Malczynski, M.; Postelnick, M.J.; Scheetz, M.H. Impact of Carbapenem Resistance and Receipt of Active Antimicrobial Therapy on Clinical Outcomes of Acinetobacter baumannii Bloodstream Infections. Antimicrob. Agents Chemother. 2011, 55, 4844–4849. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA Protein Plays a Role in Biofilm Formation on Abiotic Surfaces and in the Interaction of This Pathogen with Eukaryotic Cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Radji, M.; Agustama, R.A.; Elya, B.; Tjampakasari, C.R. Antimicrobial activity of green tea extract against isolates of methicil-lin-resistant Staphylococcus aureus and multi-drug resistant Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2013, 3, 663–666. [Google Scholar] [CrossRef] [PubMed]
| Author | Type of Study | Subjects | Phytochemical | Purpose | Results |
|---|---|---|---|---|---|
| Donald et al. [87] | Randomized Control Trial | Human | Beta-sitosterol | Treating Mycobacterium tuberculosis infection |
|
| Ahmed et al. [92] | Clinical Trial | Human | Silymarin | Treating hepatitis C infection |
|
| Rahim et al. [93] | Clinical Trial | Human—In Vitro | Alpha-Viniferin | Treating S. aureus from the nasal passages |
|
| Nawarathne et al. [94] | Clinical Trial | Human—In Vitro | Nigella sativa L. extract | Treating the Propionibacterium acnes infection |
|
| Ferrazzano et al. [95] | Randomized Control Trial | Human—In Vitro | Plantago lanceolata | Reducing oral streptococci and lactobacilli bacterial species |
|
| Kerdar et al. [96] | Randomized Control Trial | Human | Scrophularia striata | Treating periodontitis due to Streptococcus mutans |
|
| Mergia et al. [97] | Randomized Control Trial | Swiss Albino Murine Model | Verbascum sinaiticum | Treating Trypanosoma brucei species |
|
| Askari et al. [98] | Randomized Control Trial | Human Subjects | Myrtle and oak gall | Treating bacterial vaginosis |
|
| Karumathil et al. [99] | Randomized Control Trial | In Vitro Keratinocytes | Trans-cinnamaldehyde and Eugenol | Treating Acinetobacter baumannii wound infections |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopel, J.; McDonald, J.; Hamood, A. An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals. Antibiotics 2022, 11, 1838. https://doi.org/10.3390/antibiotics11121838
Kopel J, McDonald J, Hamood A. An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals. Antibiotics. 2022; 11(12):1838. https://doi.org/10.3390/antibiotics11121838
Chicago/Turabian StyleKopel, Jonathan, Julianna McDonald, and Abdul Hamood. 2022. "An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals" Antibiotics 11, no. 12: 1838. https://doi.org/10.3390/antibiotics11121838
APA StyleKopel, J., McDonald, J., & Hamood, A. (2022). An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals. Antibiotics, 11(12), 1838. https://doi.org/10.3390/antibiotics11121838






