Combination Therapy versus Monotherapy in the Treatment of Stenotrophomonas maltophilia Infections: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Definition and Outcome Measures
2.5. Statistical Analysis
2.6. Subgroup and Sensitivity Analysis
3. Results
3.1. Search Results and Included Study Characteristics
3.2. Quality Assessment
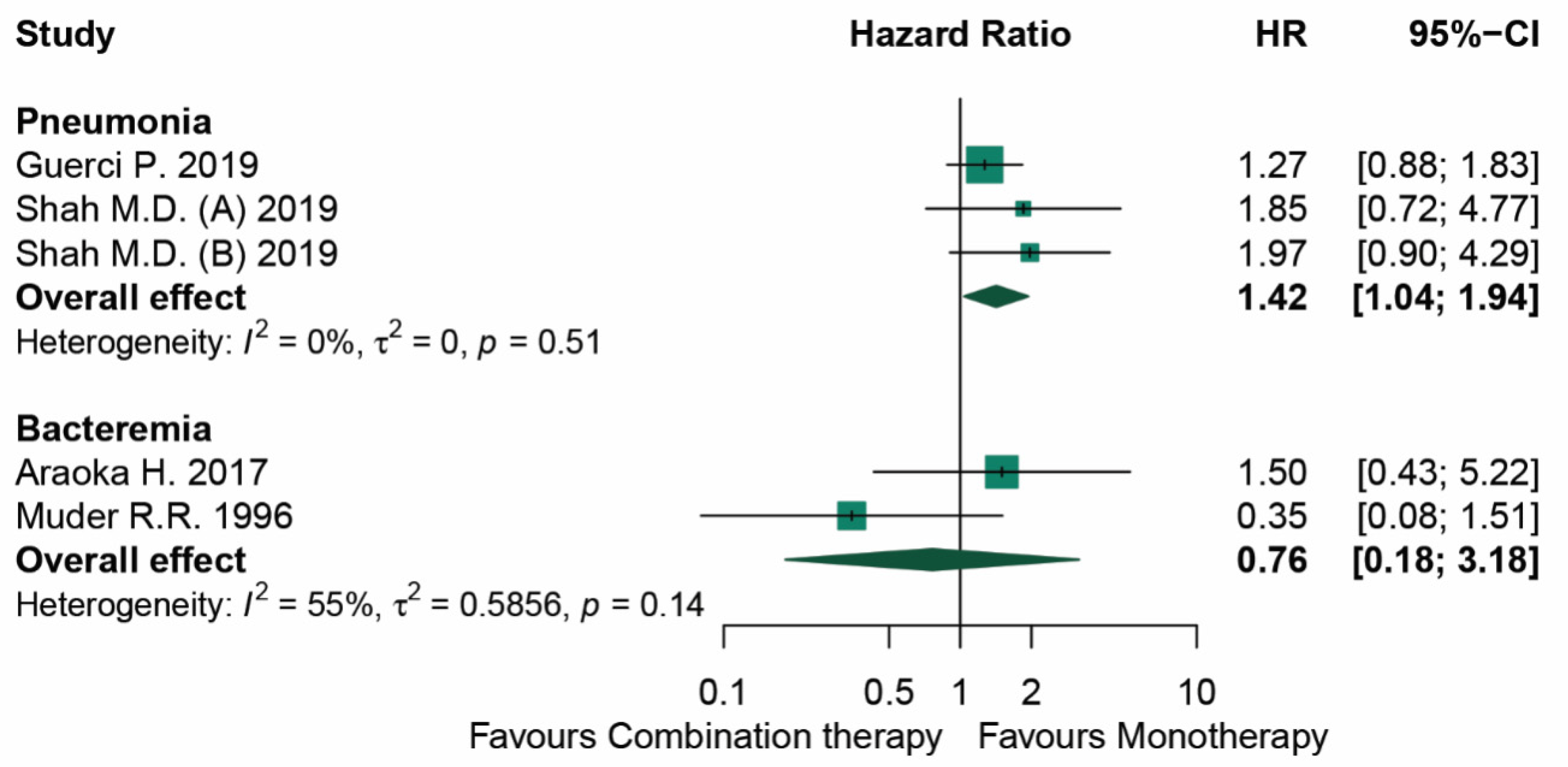
3.3. Mortality
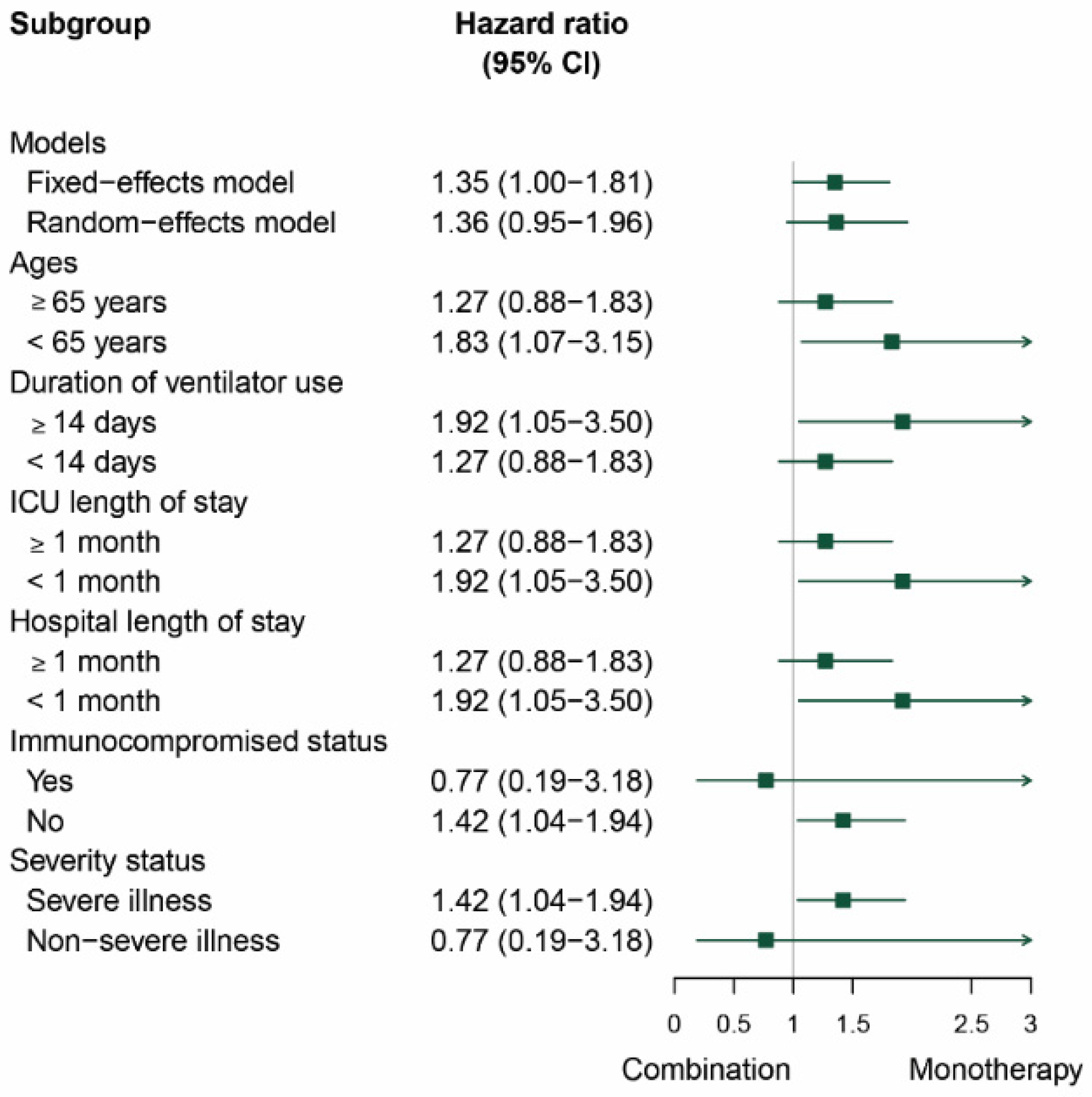
3.4. Subgroup and Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic Pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Lin, C.Y.; Chen, Y.H.; Hsueh, P.R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 2015, 6, 893. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Chong, Y.; Miyake, N.; Akahoshi, T.; Yasuda, M.; Shimono, N.; Shimoda, S.; Maehara, Y.; Akashi, K. Risk factors associated with Stenotrophomonas maltophilia bacteremia: A matched case-control study. PLoS ONE 2015, 10, e0133731. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gil, T.; Martínez, J.L.; Blanco, P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: A review of current knowledge. Expert Rev. Anti Infect. Ther. 2020, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Gibb, J.; Wong, D.W. Antimicrobial Treatment Strategies for Stenotrophomonas maltophilia: A Focus on Novel Therapies. Antibiotics 2021, 10, 1226. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Prevalence and Antibiotic Resistance of Stenotrophomonas maltophilia in Respiratory Tract Samples: A 10-Year Epidemiological Snapshot. Health Serv. Res. Manag. Epidemiol. 2019, 15, 2333392819870774. [Google Scholar] [CrossRef]
- Coppola, N.; Maraolo, A.E.; Onorato, L.; Scotto, R.; Calò, F.; Atripaldi, L.; Borrelli, A.; Corcione, A.; De Cristofaro, M.G.; Durante-Mangoni, E.; et al. Epidemiology, Mechanisms of Resistance and Treatment Algorithm for Infections Due to Carbapenem-Resistant Gram-Negative Bacteria: An Expert Panel Opinion. Antibiotics 2022, 11, 1263. [Google Scholar] [CrossRef]
- Hornsey, M.; Longshaw, C.; Phee, L.; Wareham, D.W. In vitro activity of telavancin in combination with colistin versus Gram-negative bacterial pathogens. Antimicrob. Agents Chemother. 2012, 56, 3080–3085. [Google Scholar] [CrossRef]
- Ko, J.H.; Kang, C.I.; Cornejo-Jußrez, P.; Yeh, K.M.; Wang, C.H.; Cho, S.Y.; Kim, S.H.; Hsueh, P.R.; Sekiya, N.; Matsumura, Y.; et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 546–554. [Google Scholar] [CrossRef]
- Khanum, I.; Ilyas, A.; Ali, F. Stenotrophomonas maltophilia Meningitis—A Case Series and Review of the Literature. Cureus 2020, 12, e11221. [Google Scholar] [CrossRef]
- Payen, D.; Faivre, V.; Miatello, J.; Leentjens, J.; Brumpt, C.; Tissières, P.; Dupuis, C.; Pickkers, P.; Lukaszewicz, A.C. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 2019, 19, 931. [Google Scholar] [CrossRef]
- Subhani, S.; Patnaik, A.N.; Barik, R.; Nemani, L. Case Reports Infective endocarditis caused by Stenotrophomonas maltophilia: A report of two cases and review of literature. Indian Heart J. 2016, 68, s267–s270. [Google Scholar] [CrossRef][Green Version]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Shor, E.; Roelfs, D.; Vang, Z.M. The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc. Sci. Med. 2017, 186, 20–33. [Google Scholar] [CrossRef]
- Munter, R.G.; Yinnon, A.M.; Schlesinger, Y.; Hershko, C. Infective Endocarditis due to Stenotrophomonas (Xanthomonas) maltophilia. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 353–356. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.W.; Kang, H.R.; Bae, G.B.; Park, J.H.; Nam, E.J.; Kang, Y.M.; Lee, J.M.; Kim, N.S. Two Episodes of Stenotrophomonas maltophilia Endocarditis of Prosthetic Mitral Valve: Report of a Case and Review of the Literature. J. Korean Med. Sci. 2002, 7, 263–265. [Google Scholar] [CrossRef]
- Wood, G.C.; Underwood, E.L.; Croce, M.A.; Swanson, J.M.; Fabian, T.C. Treatment of Recurrent Stenotrophomonas maltophilia Ventilator-Associated Pneumonia with Doxycycline and Aerosolized Colistin. Ann. Pharmacother. 2010, 44, 1665–1668. [Google Scholar] [CrossRef]
- Holifield, K.; Lazzaro, D.R. Case Report: Spontaneous Stenotrophomonas maltophilia Keratitis in a Diabetic Patient. Eye Contact Lens. 2011, 37, 326–327. [Google Scholar] [CrossRef]
- Mori, M.; Tsunemine, H.; Imada, K.; Ito, K.; Kodaka, T.; Takahashi, T. Life-threatening hemorrhagic pneumonia caused by Stenotrophomonas maltophilia in the treatment of hematologic diseases. Ann. Hematol. 2014, 93, 901–911. [Google Scholar] [CrossRef]
- Reynaud, Q.; Weber, E.; Gagneux-Brunon, A.; Suy, F.; Romeyer-Bouchard, C.; Lucht, F.; Botelho-Nevers, E. Late Stenotrophomonas maltophilia pacemaker infective endocarditis Endocardite infectieuse sur pacemaker à Stenotrophomonas maltophilia. Médecine Mal. Infect. 2015, 45, 95–97. [Google Scholar] [CrossRef]
- Mojica, M.F.; Ouellette, C.P.; Leber, A.; Becknell, M.B.; Ardura, M.I.; Perez, F.; Shimamura, M.; Bonomo, R.A.; Aitken, S.L.; Shelburne, S.A. Successful Treatment of Bloodstream Infection Due to Metallo-beta-Lactamase-Producing Stenotrophomonas maltophilia in a Renal Transplant Patient. Antimicrob. Agents Chemother. 2016, 60, 5130–5134. [Google Scholar] [CrossRef] [PubMed]
- Kaito, S.; Sekiya, N.; Najima, Y.; Sano, N.; Horiguchi, S.; Kakihana, K.; Hishima, T.; Ohashi, K. Fatal Neutropenic Enterocolitis Caused by Stenotrophomonas maltophilia: A Rare and Underrecognized Entity. Intern. Med. 2018, 57, 3667–3671. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Ghiaur, A.; Brezeanu, L.; Martac, C.; Nicolau, A.; Droc, G.; Droc, G. Successful treatment of pulmonary haemorrhage and acute respiratory distress syndrome caused by fulminant Stenotrophomonas maltophilia respiratory infection in a patient with acute lymphoblastic leukaemia—Case report. BMC Infect. Dis. 2020, 20, 658. [Google Scholar] [CrossRef] [PubMed]
- Petca, R.C.; Dănău, R.A.; Popescu, R.I.; Damian, D.; Mareș, C.; Petca, A.; Jinga, V. Xanthogranulomatous Pyelonephritis Caused by Stenotrophomonas maltophilia-The First Case Report and Brief Review. Pathogens 2022, 11, 81. [Google Scholar] [CrossRef]
- Shah, M.D.; Coe, K.E.; El Boghdadly, Z.; Wardlow, L.C.; Dela-Pena, J.C.; Stevenson, K.B.; Reed, E.E. Efficacy of combination therapy versus monotherapy in the treatment of Stenotrophomonas maltophilia pneumonia. J. Antimicrob. Chemother. 2019, 74, 2055–2059. [Google Scholar] [CrossRef]
- Guerci, P.; Bellut, H.; Mokhtari, M.; Gaudefroy, J.; Mongardon, N.; Charpentier, C.; Louis, G.; Tashk, P.; Dubost, C.; Ledochowski, S.; et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: A nationwide retrospective study. Crit. Care 2019, 23, 371. [Google Scholar] [CrossRef]
- Araoka, H.; Baba, M.; Okada, C.; Abe, M.; Kimura, M.; Yoneyama, A. Evaluation of trimethoprim-sulfamethoxazole based combinationtherapy against Stenotrophomonas maltophilia: In vitro effects andclinical efficacy in cancer patients. Int. J. Infect. Dis. 2017, 58, 18–21. [Google Scholar] [CrossRef]
- Muder, R.R.; Harris, A.P.; Muller, S.; Edmond, M.; Chow, J.W.; Papadakis, K.; Wagener, M.W.; Bodey, G.P.; Steckelberg, J.M. Bacteremia Due to Stenotrophomonas (Xanthomonas) maltophilia: A Prospective, Multicenter Study of 91 Episodes. Clin. Infect. Dis. 1996, 22, 508–512. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
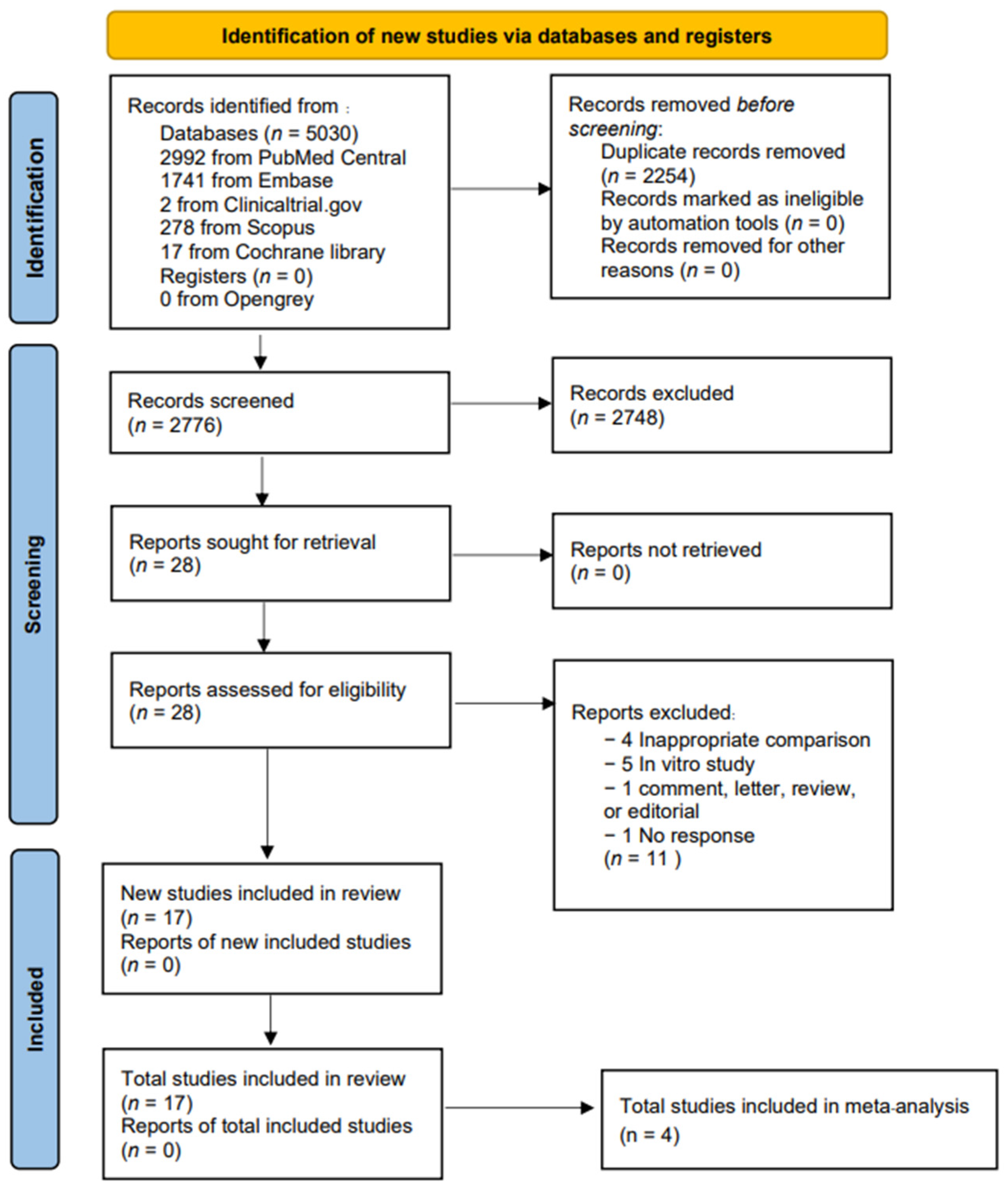
| Author (Year) | Region | Design | Sample Size | Infection | Treatment Duration (Days) | Follow Up Time (Days) (n) | Outcome |
|---|---|---|---|---|---|---|---|
| Robert (1996) [28] | USA | Cohort | 18 | Bacteremia | N/A | N/A | Died 18% |
| Munter (1998) [15] | Isreael | Case report | 1 | Infective endocarditis | 40 | N/A | Died |
| Kim (2001) [16] | Korea | Case report | 1 | Infective endocarditis | 42 | 60 | Clinical response * |
| Wood (2010) [17] | USA | Case report | 1 | VAP | 14 | 33 | Clinical response * Microbiological response ** |
| Holifield (2011) [18] | USA | Case report | 1 | Keratitis | 10 | N/A | Clinical response * Microbiological response ** |
| Mori (2014) [19] | Japan | Case series | 8 | Hemorrhagic pneumonia | 1–16 | N/A | Died 100% |
| Reynaud (2015) [20] | France | Case report | 1 | Infective endocarditis | 2 | N/A | Died |
| Mojica (2016) [21] | USA | Case report | 1 | Bacteremia | 48 | N/A | Microbiological response ** |
| Subhani (2016) [12] | India | Case series | 28 | Infective endocarditis | 42 (1), N/A (27) | N/A (27), 60 (1) | Cured 67.86% Died 28.57% N/A 3.57% |
| Araoka (2017) [27] | Japan | Cohort | 14 | Bacteremia | N/A | 30 | Died 50% |
| Kaito (2018) [22] | Japan | Case report | 1 | Bacteremia, Pneumonia | 18 | N/A | Died |
| Payen (2019) [11] | France | Case series | 4 | Peritonitis VAP | 14 | 176 | Clinical response * 100% Microbiological response ** 100% |
| Shah (2019) [25] | USA | Cohort | 38 | Pneumonia | N/A | N/A | Died 39.47% |
| Guerci (2019) [26] | France | Cohort | 167 | Pneumonia | 7 | N/A | Died 37.72% |
| Andrei (2020) [23] | Romania | Case report | 1 | Severe pneumonia with pulmonary hemorrhage | 7 | 300 | Clinical response * Microbiological response ** |
| Khanum (2020) [10] | Pakistan | Case series | 2 | Meningitis | 21 | N/A | Clinical response * 100% CSF culture negative 100% |
| Petca (2022) [24] | Romaniav | Case report | 1 | Pyelonephritis | 14 | N/A | Microbiological response ** |
| Author (Year) | Region | Baseline Characteristics | Details of Antimicrobials | Effect Size (95% CI) | Severity Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Age of Exposure Group (Year) | Type of Infection | Immuno-Compromised Population (%) | Male (%) | Monotherapy | Combination Therapy | ||||
| Muder (1996) [28] | USA | 91 | N/A | Bacteremia | 97.8 | N/A | TMP-SMX Third-generation cephalosporin Extended-spectrum penicillin | Receiving more than 1 of monotherapy agents | 0.35 (0.08–3.18) | Severity score |
| Araoka (2017) [27] | Japan | 20 | 60.5 a | Bacteremia | 100 | 85.71 | TMP-SMX | TMP-SMX + fluoroquinolone | 1.5 (0.43–5.22) | Pitt score |
| Guerci (2019) [26] | France | 282 | 65 (±9) b | Pneumonia | 37.4 | 69.9 | TMP-SMX Levofloxacin Ciprofloxacin Ticarcillin/clavulanate Ceftazidime Minocycline Colistin Rifampicin Tigecycline | N/A | 1.27 (0.88–1.83) | SOFA score |
| Shah (2019) [25] | USA | 252 | 62 a | Pneumonia | 19.8 | 62.3 | TMP-SMX Levofloxacin Ciprofloxacin Moxifloxacin Minocycline Ceftazidime | TMP-SMX + Levofloxacin TMP-SMX + Ciprofloxacin TMP-SMX + Moxifloxacin TMP-SMX + Minocycline TMP-SMX + Ceftazidime Levofloxacin + Minocycline Levofloxacin + Ceftazidime Ciprofloxacin + Minocycline Ciprofloxacin + Ceftazidime Minocycline + Ceftazidime | (A) = 1.85 (0.75–4.98) (B) = 1.97 (0.96–4.55) | APACHE II score |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prawang, A.; Chanjamlong, N.; Rungwara, W.; Santimaleeworagun, W.; Paiboonvong, T.; Manapattanasatein, T.; Pitirattanaworranat, P.; Kitseree, P.; Kanchanasurakit, S. Combination Therapy versus Monotherapy in the Treatment of Stenotrophomonas maltophilia Infections: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1788. https://doi.org/10.3390/antibiotics11121788
Prawang A, Chanjamlong N, Rungwara W, Santimaleeworagun W, Paiboonvong T, Manapattanasatein T, Pitirattanaworranat P, Kitseree P, Kanchanasurakit S. Combination Therapy versus Monotherapy in the Treatment of Stenotrophomonas maltophilia Infections: A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(12):1788. https://doi.org/10.3390/antibiotics11121788
Chicago/Turabian StylePrawang, Abhisit, Naphatsawan Chanjamlong, Woranattha Rungwara, Wichai Santimaleeworagun, Taniya Paiboonvong, Thidarat Manapattanasatein, Prompiriya Pitirattanaworranat, Pongsakorn Kitseree, and Sukrit Kanchanasurakit. 2022. "Combination Therapy versus Monotherapy in the Treatment of Stenotrophomonas maltophilia Infections: A Systematic Review and Meta-Analysis" Antibiotics 11, no. 12: 1788. https://doi.org/10.3390/antibiotics11121788
APA StylePrawang, A., Chanjamlong, N., Rungwara, W., Santimaleeworagun, W., Paiboonvong, T., Manapattanasatein, T., Pitirattanaworranat, P., Kitseree, P., & Kanchanasurakit, S. (2022). Combination Therapy versus Monotherapy in the Treatment of Stenotrophomonas maltophilia Infections: A Systematic Review and Meta-Analysis. Antibiotics, 11(12), 1788. https://doi.org/10.3390/antibiotics11121788





