Abstract
Previous studies indicated that the use of a phage cocktail, composed of bacteriophages vB_SenM-2 and vB_Sen-TO17, is effective in killing cells of Salmonella enterica serovars Typhimurium and Enteritidis in vitro and in the Galleria mellonella animal model as efficiently as antibiotics (enrofloxacin or colistin) and induced fewer deleterious changes in immune responses. Here, we investigated the effects of this phage cocktail on the hematological parameters and selected biochemical markers in chickens infected with S. enterica serovar Typhimurium, in comparison to those caused by enrofloxacin or colistin. We found that treatment with antibiotics (especially with enrofloxacin) caused nonbeneficial effects on red blood cell parameters, including hematocrit, MCV, MCH, and MCHC. However, Salmonella-induced changes in the aforementioned parameters were normalized by the use of the phage cocktail. Importantly, hepatotoxicity was suggested to be induced by both antibiotics on the basis of increased alanine transaminase (ALT) and aspartate aminotransferase (AST) activities, in contrast to the phage cocktail, which did not influence these enzymes. We conclude that phage therapy with the cocktail of vB_SenM-2 and vB_Sen-TO17 in Salmonella-infected chickens is not only as effective as antibiotics but also significantly safer for the birds than enrofloxacin and colistin.
1. Introduction
Salmonellosis is one of the most serious forms of infectious disease affecting poultry. Although Salmonella enterica is not the most dangerous pathogen to birds, it can lead to serious food-borne disease in humans [1,2,3]. Almost 100 million cases of salmonellosis are detected every year in humans; among which, more than 150 thousand are fatal cases. Therefore, when poultry infection with Salmonella is detected, the meat is disqualified from the market [4]. The appearance of S. enterica strains that are resistant to many antibiotics makes the problem even more serious [1,5,6,7]. In order to prevent or limit the development of antibiotic resistance, the use of antibiotics in livestock feed has been forbidden in the European Union, and there are partial restrictions in the United States [4,8,9]. For this reason, the development of novel therapies and approaches to protect poultry from S. enterica infection is required [5,10,11,12,13].
Our research group has previously contributed to the development of novel therapies and/or preventive treatments against S. enterica by creating a cocktail of the bacteriophages vB_SenM-2 and vB_Sen-TO17, which infect serovars Typhimurium and Enteritidis [14]. The cocktail was characterized in vitro to be effective at killing S. enterica cells and reducing the bacterial biofilm. Moreover, safety was initially assessed by the limulus (LAL) test and by determining the viability of chicken fibroblasts. The treatment of S. enterica-infected Galleria mellonella larvae with the cocktail improved their survival rate [14]. These results provided a basis to perform additional studies using experimental infection on chickens with S. enterica serovar Typhimurium. Overall, phage therapy was found to be as effective as therapy with either enrofloxacin or colistin and appeared less deleterious, inducing fewer changes in the microbiome than antibiotics [15]. This conclusion was corroborated in subsequent analyses in which the levels of several pro- and anti-inflammatory cytokines were measured, and the relative levels of the stress hormones were determined. The analyses showed, for the first time, that the phage cocktail did not disturb the immune homeostasis in chickens, while treatment with antibiotics (enrofloxacin or colistin) caused cytokine imbalance, changes in proportions between immune cell subpopulations, and stress axis hyperactivity [16]. Thus, antibiotics (especially enrofloxacin) may cause serious adverse effects in poultry, while phage therapy appears safer [17].
To further assess the safety of phage therapy vs. antibiotics (enrofloxacin and colistin) on S. enterica-infected chickens, we measured the hematological parameters and levels of selected biochemical markers in blood samples withdrawn from tested birds. The main goal of these experiments was to further determine, compare, and detect any potential adverse effects of these two kinds of antibacterial therapies. Such studies should facilitate the identification of the most efficacious and safe methods for the treatment of infected poultry.
2. Results
2.1. Changes in the Counts of Blood Morphotic Elements of Chickens Receiving Phage Cocktail or Antibiotics
Whether bacteriophages may affect the number of blood-morphotic elements has been examined; therefore, a comprehensive analysis of this phenomenon was completed. The absolute monocyte count, absolute eosinophiles count, erythrocyte count, hemoglobin level, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count were examined.
The chickens treated with enrofloxacin (group 4) had significantly decreased erythrocyte counts (Figure 1) and hematocrit (Figure 2) throughout the experiment compared to the uninfected control groups. The increased erythrocyte counts in group 3, as compared to groups 1 and 2, were evident throughout the experiment, especially in comparison with group 1. It was observed that the red blood cell count in the phage-treated group one day after infection (group 6) was slightly decreased when compared to the phage-treated control group. However, this value normalized at termination 2, termination 3, and termination 4.
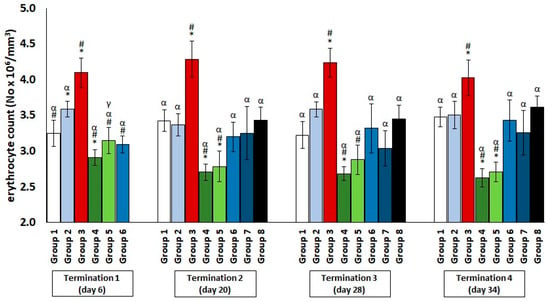
Figure 1.
Changes in the erythrocyte count number in the blood of chickens receiving phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SD. Statistical analyses were performed by ANOVA and post hoc Tukey’s test. The significance of differences between controls and particular treated groups are observed and marked by asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3); (γ) vs. termination 2.
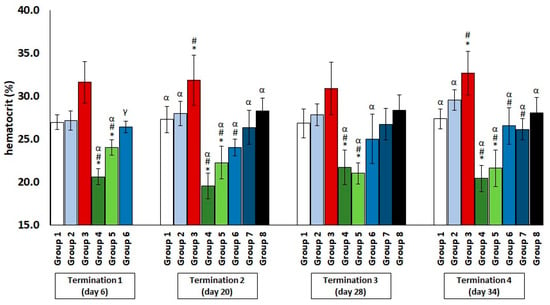
Figure 2.
Changes in the hematocrit level in the blood of chickens receiving phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SD. Statistical analyses were performed by the Kruskal–Wallis test and post hoc Dunn’s test. The significance of differences between controls and particular treated groups are observed and marked as follows: asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3); (γ) vs. termination 2.
Regarding the platelet count (Figure 3), a slight decrease was observed in the infected control group and the colistin-treated group compared to the saline-treated control group, but these values normalized later (termination 2 and termination 3).
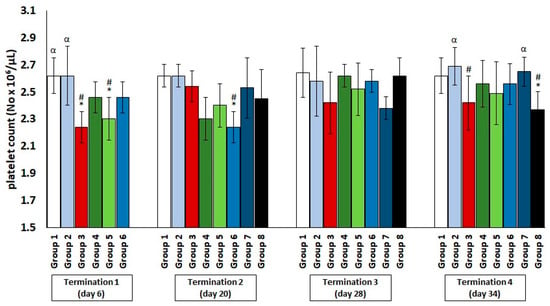
Figure 3.
Changes in the platelets count number in the blood of chickens receiving phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SD. Statistical analyses were performed by ANOVA and post hoc Tukey’s test. The significance of differences between controls and particular treated groups are observed and marked by asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3).
The values of the absolute monocyte count, absolute eosinophilia count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) are shown in Table 1. In the case of group 3, a drastic increase in the total monocyte count and the eosinophil count was noted during the experiment. Interestingly, the values of these two parameters in the groups treated with antibiotics (group 4 and group 5) were drastically reduced as compared to the uninfected control groups throughout the experiment. On the other hand, in animals that were treated with a phage cocktail one day after infection (group 6), as well as two days after the detection of bacteria in the feces (group 7), no statistically significant changes were observed relative to the uninfected phage groups. However, it was noted that, in the case of the group treated with the phage cocktail (group 8), the final counts of monocytes and eosinophils were significantly elevated relative to the uninfected control groups, while they were significantly lower than the values obtained in the infected control group (group 3). Moreover, in the groups treated with antibiotics (group 4 and group 5), the Mean Corpuscular Volume (MCV) values were initially (termination 1 and termination 2) not significantly different from the uninfected control groups, but at further stages of the experiment, they started to decrease and became statistically significantly different from the uninfected control groups. In the case of groups 6 and 7, the MCV levels were not significantly different from the uninfected control groups, contrary to group 8, which, from termination 2 to the end of the experiment, significantly increased in comparison to the control group receiving saline.

Table 1.
Changes in the absolute monocyte count, absolute eosinophil count, Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC) of chickens receiving the phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments.
The Mean Corpuscular Hemoglobin (MCH) values were significantly decreased throughout the experiment in the infected control group. A drastic increase was observed in the enrofloxacin-treated group compared to the saline-treated control group at termination 1, termination 3, and termination 4. Interestingly, in colistin-treated animals, despite the initial lack of difference from the uninfected control groups, an increase was observed compared to the saline-treated control group (group 1) at the end of the experiment (termination 4). Although no significant differences were observed in group 6 relative to the uninfected control groups, such differences were observed in groups 7 and 8 at termination 2 and termination 4. Additionally, it was observed that the Mean Corpuscular Hemoglobin Concentration (MCHC) values in group 8, although not significantly different from the uninfected control groups at the beginning of the experiment, significantly decreased at termination 3 and termination 4. No significant changes were observed in group 6 and group 7 compared to the uninfected control groups throughout the experiment. Interestingly, the MCHC in the colistin-treated group, although initially significantly increased at termination 1 and termination 2 relative to the uninfected control groups, normalized at the end of the experiment (termination 4) and was not significantly different from the uninfected control groups. The enrofloxacin-treated group noted elevated MCHC as compared to the uninfected control groups at termination 1, termination 2, and termination 4, contrary to the infected control group, whose values were significantly decreased.
2.2. Blood Biochemical Parameters in Plasma of Chickens Subjected to Phage Therapy and Antibiotic Therapy
The levels of alanine transaminase (ALT), aspartate aminotransferase (AST), and c-reactive protein (CRP) in the blood plasma of chickens that were treated with phage therapy or antibiotic therapy were investigated.
The results showed a significant increase in the ALT levels in group 3, which were infected with Salmonella but not treated throughout the experiment (Figure 4). Interestingly, an even greater deviation from the reference value manifested in the significantly elevated level of the enzyme, which indicates liver dysfunction or even damage, was observed in group 5, who were receiving colistin. Furthermore, a hepatotoxic effect was also evident in group 4, in which enrofloxacin was administered. In contrast, when the phage cocktail was used, as well as in the Salmonella-infected groups where it was applied 24 h after infection (group 6) or after 2 days (group 7), the level of the tested enzyme remained low, within the normal range throughout the experiment. Only in group 8, in which phage cocktail administration was implemented 4 days after the development of bacterial infection, the level of the examined parameter was significantly increased. An analogous direction of change was also observed for the second indicator of liver dysfunction, AST (Figure 5).
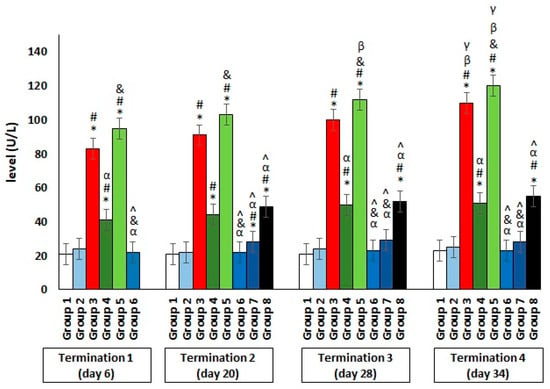
Figure 4.
Changes in the alanine transaminase (ALT) level in the blood of chickens receiving the phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SD. Statistical analyses were performed by the Kruskal–Wallis test and post hoc Dunn’s test. The significance of the differences between controls and particular treated groups are observed and marked by asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3); (&) vs. group 4; (^) vs. group 5; (β) vs. termination 1.
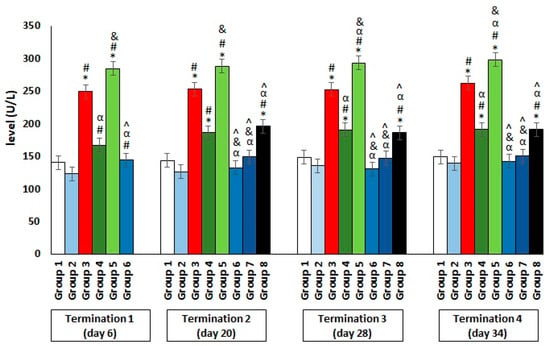
Figure 5.
Changes in the aspartate aminotransferase (AST) level in the blood of chickens receiving the phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SD. Statistical analyses were performed by the Kruskal–Wallis test and post hoc Dunn’s test. The significance of the differences between controls and particular treated groups are observed and marked by asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3); (&) vs. group 4; (^) vs. group 5.
With regard to CRP (Figure 6), its significantly elevated level was shown only in Salmonella-infected group 3 and group 8, where the phage cocktail was implemented later. The increase in CRP release by the liver into the peripheral blood is an indicator of the development of severe inflammation in the organism.
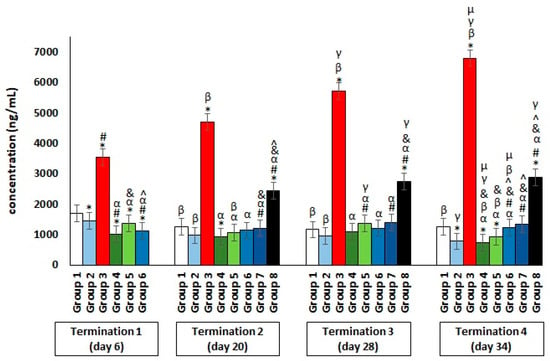
Figure 6.
Changes in the C-reactive protein (CRP) concentration in the blood of chickens receiving the phage cocktail or antibiotics after 6, 20, 28, and 34 days of experiments. Results are presented as mean values ± SDs. Statistical analyses were performed by the Kruskal–Wallis test and post hoc Dunn’s test. The significance of differences between controls and particular treated groups are observed and marked by asterisks (*) vs. saline control; (#) vs. phage control (group 2); (α) vs. infected control (group 3); (&) vs. group 4; (^) vs. group 5; (β) vs. termination 1; (γ) vs. termination 2; (µ) vs. termination 3.
3. Discussion
Phage therapy is considered to be a possible alternative to the use of antibiotics to combat pathogenic bacteria [18]. Although the introduction of this method as an officially approved therapy to treat patients requires both intensive studies and changes in the current law, it appears that the use of bacteriophages in the treatment of infected animals might be possible significantly earlier, especially due to differences in regulations between medical and veterinary rules [19]. Nevertheless, before formal recommendations to use phage therapy to treat animals can be issued, both the efficacy and safety of this method should be confirmed.
Our previous studies led to the development of a phage cocktail, composed of bacteriophages vB_SenM-2 and vB_Sen-TO17, which is effective in eliminating S. enterica serovars Typhimurium and Enteritidis and safe to the G. mellonella animal model, as well as in cell culture tests [14]. Moreover, this cocktail was as effective in the treatment of S. enterica-infected chickens as antibiotics (enrofloxacin or colistin) while causing significantly less adverse effects than those drugs, as estimated by changes in the gut microbiome [15] or disturbances in the immune response [16]. On the other hand, the effects of these two methods of treatment were not previously compared in light of changes in the hematological parameters and biochemical markers. Thus, this work was conducted to fill this gap in our knowledge.
Hematocrit is an indicator that determines the volume ratio of erythrocytes to whole blood. A decrease in this index, as well as an insufficient erythrocyte count, can indicate a number of abnormalities, such as gastrointestinal bleeding, bone marrow disturbances, kidney damage, or, most commonly, the onset of anemia resulting from iron deficiency. Iron determines the supply of adequate amounts of oxygen, electron transport, and the proper functioning of enzymes. It is important for high-metabolic rate cells [17]. Hematocrit is of particular importance in the assessment of animal physiology. Some mammals, such as dogs or horses, can store erythrocytes in the spleen and thus modulate the hematocrit or hemoglobin levels, depending on the intensity of the exercise. Birds, on the other hand, which do not have this regulatory mechanism, can lower their hematocrit values by hemodilution in response to intensive exercise or exposure to aversive factors [18]. When considering the effect of the administered substances on erythrocyte indices, including the hematocrit value, physiological factors such as age, gender and hormone levels, should also be taken into account [19]. Other indicators also play an important role when it comes to assessing the organism’s overall condition. The MCV provides information on the average volume of a single erythrocyte and allows for the early detection of anemia. Its value depends on plasma osmolarity and the rate of erythrocyte division. The MCH is an indicator of the average mass of hemoglobin in a single red blood cell. Together with the MCHC value, it is useful in distinguishing between different types of anemia [19]. Changes in hematological parameters are useful for assessing the organism’s adaptation to adverse conditions or stressors. This is particularly important in the case of industrial poultry-rearing for meat and eggs. Erythrocyte indices change significantly depending on the husbandry conditions and nutritional status. Overly nutrient-poor feed and prolonged exposure of poultry to stress factors result in lower erythrocyte counts and hemoglobin levels, leading to erythrocytopenia and reduced organism performance. An increase in ambient temperature results in the loss of a large amount of liquid through the respiratory system, which then leads to a decrease in the plasma volume and an increase in the hematocrit level. Similarly, in the case of dehydration by the evaporation process, there is a significant increase in the hematocrit values. In contrast, a non-physiological reduction in the hematocrit levels through the hemodilution process occurs, with severe stressors, especially heat stress exposure [20]. As indicated by the results shown in this report, antibiotic therapy, which, in poultry, is sometimes administered prophylactically for chicken growth promotion or therapeutically [21] can be a type of negative stressor that causes a number of disturbances in the organism, especially if we consider the immune system [16] and, in particular, erythrocyte indices. This is important, because it has a number of negative consequences, including economic ones, due to deteriorations in the quality of the meat, the lower number of eggs laid, or the welfare of consumers (adverse effects on the intestinal microbiome and the development of drug-resistant bacteria) [21]. The search for alternative methods to control bacterial infections, therefore, needs to be addressed in depth, especially regarding infections with Salmonella strains, which pose serious challenges to the immune system of animals destined for consumption [22]. It was demonstrated that Salmonella enterica serotype Enteritidis leads, in chickens, to the slowed growth of key immune organs, changes in the profile of important immune cell types, reduced antibody production, increased levels of pro-inflammatory cytokines, and excessive activation of the stress axis, with consequent effects on the hematological parameters [23]. This was also confirmed by our observations, which showed, among other things, a non-physiological increase in the erythrocyte count, hematocrit level, and MCV value. This type of deviation from reference values is most often observed in situations of severe dehydration, which result in blood thickening. Although the literature contains significant reports on the negative effects of antibiotic therapy on hematological parameters in poultry, there are papers indicating a number of different side effects of the administration of popular veterinary antibiotics, such as enrofloxacin [24]. Only a few previously published reports addressed the problem investigated in this work. Nevertheless, it appears that the results of all these studies are quite similar [25,26]. A comparison of the efficacy of the phages and antibiotics against acute pneumonia in a mouse model was previously described [27]. In that work, two phages (536_P1 and LM33_P1) and three antibiotics (ceftriaxone, cefoxitin, and imipenem–cilastatin) were used. The phages significantly and rapidly reduced the number of bacterial cells and restored the normal blood counts, which were otherwise disrupted by the development of an abrupt bacterial infection [28]. It should also be emphasized that the rapid lysis of bacterial cells does not induce an increase in inflammatory markers as compared to antibiotic therapy [29]. Our studies indicated that there was no significant change in the number of immunocompetent cells (lymphocytes, monocytes, or neutrophils) in the serum after the administration of bacteriophages vB_SenM-2 and vB_Sen-TO17. Analogous results were previously reported by others regarding changes in the number of macrophages, T and B lymphocytes, and dendritic cells in peripheral organs (spleen, liver, and lymph nodes) after the administration of other phages [30,31,32]. Our results also confirmed that, after the administration of a phage cocktail, the analyzed hematological parameters were not significantly different from the results obtained in the control groups. The normalization of the erythrocyte counts following the phage cocktail had tangible physiological benefits, as it avoided disturbances in the hematological parameters induced by bacterial infection, which led to changes and had a negative impact on many organs, particularly the liver and spleen. However, it should be emphasized that only administration immediately or up to two days after detection of the presence of bacteria in the feces guarantees the effective action of the phage cocktail. When it is administered after a longer period, the bacterial infection is already developed enough to cause a number of negative changes that disrupt the functioning of many systems and organs, including, but not limited to, the spleen and liver, resulting in hepatosplenomegaly [33].
In summary, our results confirmed a favorable safety profile for the use of phage therapy (particularly, the phage cocktail consisting of vB_SenM-2 and vB_Sen-TO17) in Salmonella-infected chickens. Moreover, this study indicated serious adverse effects of enrofloxacin and colistin on the hematological parameters and ALT and AST activities in these birds. Therefore, phage therapy with bacteriophages vB_SenM-2 and vB_Sen-TO17 may be further considered as an alternative method to either treat or prevent chicken infections with S. enterica serovar Typhimurium.
Conclusions
Our results indicate significantly fewer adverse effects as a result of the phage cocktail relative to the tested antibiotics. The latter agents caused deleterious changes in the red blood cells parameters, including hematocrit, MCV, MCH, and MCHC. However, Salmonella-induced changes in the aforementioned parameters were normalized by the use of the phage cocktail. Furthermore, bacteriophages administered either immediately or two days after infection did not significantly affect the number of lymphocytes, monocytes, and neutrophils in the serum. Administration of the tested antibiotics also caused increased activities of ALT and AST, suggesting the hepatotoxicity of these compounds. This was in contrast to the phage cocktail, which did not influence the activities of these enzymes.
4. Materials and Methods
4.1. Animals
Details of the animals and experimental conditions were described previously [15,16]. In brief, the experiment was conducted on nongenetically modified chickens (Gallus gallus domesticus) that were purchased from a licensed breeder (registration number PL28036602, Poland). The Experimental Infection Pavilon at the Department of Bird Diseases (Faculty of Veterinary Medicine, University of Warmia and Mazury, Olsztyn, Poland) was divided into 8 m2 boxes that held 25 chickens each. Living conditions were strictly controlled and monitored, with an average humidity of 75% under conditions of regular light–dark cycles (12 h day/12 h night, at light intensity 10 lx) and forced ventilation with 17 air changes per hour). Temperature was reduced from 33 °C (beginning of the experiment) to 22 °C (the end of the experiment). The chickens had unlimited access to forage and water. To reduce the risk of contamination, the entire research complex was equipped with a high-efficiency particulate-absorbing (HEPA) filter system and automation to maintain a cascade of pressures in the sanitary corridors, boxes, and locks. All the experiments were approved by the Local Ethics Committee for Experimental Animals in Olsztyn (permission no. 62/2019, dated 30 July, 2019).
4.2. Bacteriophages and Bacterial Strain
The bacteriophages vB_Sen-TO17 and vB_SenM-2, used for the phage cocktail, were characterized previously [34,35], and their safety was confirmed using in vitro experiments with the chicken fibroblast model (UMNSAH/DF-1) and in vivo studies with the Galleria mellonella animal model [14]. Salmonella enterica serovar Typhimurium (strain KOS 13) was obtained from the National Salmonella Center at the Medical University of Gdansk (Poland), and Salmonella enterica serovar Heidelberg came from the Collection of the Department of Molecular Biology, University of Gdansk. Isolation of S. Typhimurium in chicken fecal samples and cloacal swabs was conducted in accordance with ISO 65791:2017 standards and the previously described procedure [16].
4.3. The Preparation of Phage Pocktail
The preparation of the phage lysates included in the cocktail used in the experiment was carried out in accordance with the previously published protocols [15]. In brief, an overnight culture of S. enterica (S. Heidelberg for phage vB_SenM-2 and S. Typhimurium for vB_SenTO17) after inoculation into fresh LB medium (BioShop, Burlington, ON, Canada) at the ratio (v/v) of 1:100 and incubation at 37 °C with shaking (150 rpm) until OD600 = 0.15 (1.5 × 108 CFU/mL) was infected with the appropriate bacteriophage at a multiplicity of infection (m.o.i) of 0.5 and incubated at 37 °C until lysis. To purify the phage lysate, polyethylene glycol 8000 (PEG8000) (BioShop, Burlington, ON, Canada) was added to a final concentration of 10%, and the lysate was then incubated with shaking overnight at 4 °C, using a magnetic stirrer (Carl Roth, Karlsruhe, Germany). Then, the lysate was centrifuged at 10,000× g for 30 min at 4 °C (Avanti JXN-26, rotor JLA-8.100, Beckman Coulter, Indianapolis, IN, USA), and the obtained precipitate was suspended in 0.89% NaCl (Alchem, Torun, Poland). To remove PEG8000 completely, 2 mL of chloroform was added to the lysate, which was subjected to double-centrifugation at 4000× g for 15 min at 4 °C (Avanti JXN-26, rotor JS-13.1, Beckman Coulter, Indianapolis, IN, USA). In the next step, the lysate was subjected to ultracentrifugation in a sucrose gradient (Sigma Aldrich, St. Louis, MO, USA) at 95,000× g (Optima XPN-100, rotor SW32.1 Ti, Beckman Coulter, Indianapolis, IN, USA) for 2.5 h at 10 °C. To remove residual sucrose, the lysate was dialyzed against 0.89% NaCl for 7 days at 4 °C. In order to exclude the possibility of contamination with bacterial endotoxin, the Purified Thermo ScientificTM LAL Chromogenic Endotoxin Quantitation Kit (Catalog No.: 12117850, Thermo Fisher Scientific Inc., Paisley, UK) was used. The obtained lysates were used to prepare a phage cocktail, which was administered to the chickens. The purified and checked lysates of bacteriophages vB_SenM-2 and vB_Sen-TO17 were mixed in a 1:1 ratio (1 × 109 PFU/mL of each phage). Finally, the cocktail was suspended in 20 mM of CaCO3.
4.4. Animal Groups and the Schedule of the Treatment
The detailed course of the experiment was described in two previously published papers [15,16]. In brief, two hundred seven-day-old chickens were randomly divided into eight experimental groups: group 1, receiving saline, and group 2, receiving a phage cocktail from day 1 to day 15; these were the controls and were not infected with bacteria. For the former group, the aim was to see if the administration procedure could have a significant effect on the studied parameters, while the latter group was used to test the potential impact of the phage cocktail. Group 3 was the positive control; there were 25 Salmonella-infected chickens receiving saline until day 15 of the experiment. At day 0 of the experiment, groups 3–8 were infected by administering 1 mL of S. Typhimurium (106 CFU/mL) suspended in 0.89% NaCl into the beak. Twenty-four hours after infection (day 1 of the experiment), the chickens in group 4 started receiving enrofloxacin (Scanflox, Scanvet, Warsaw, Poland; dose 10 mg/kg per day), while 25 animals in group 5 were given colistin (Colisol, Ceva Animal Health, Warsaw, Poland; dose 120.000 IU/kg per day). For both groups, administration was continued for 5 days. Groups 6, 7, and 8 received the phage cocktail for 14 days. In the case of group 6, administration began analogously to the antibiotics 24 h after infection, while the animals in groups 7 and 8 started receiving the phage cocktail two and four days after confirmation of the bacteria in feces, respectively. The blood samples were taken at four timepoints, while some of the animals from each group were sacrificed in a CO2 chamber. At day 6 of the experiment, after the end of the antibiotic treatment, 5 mL of blood was collected from five chickens of each group (termination 1). At day 20 of the experiment, following the completion of phage therapy in group 6, blood was collected from another 5 chickens (in groups 1–6) and 10 chickens (in groups 7 and 8; termination 2). Subsequent blood sampling and sacrifice were performed at day 28 of the experiment (5 chickens from each group; termination 3) and day 34 of the experiment (10 chickens from each group; termination 4).
4.5. Blood Collection
The blood sampling methodology was consistent with the previously published protocols [16]. In brief, blood samples of 5 mL were collected from each chicken. To prevent blood clotting, the heparinized syringes tipped with a 25-gauge, 1-in-long needle, and tubes containing sodium heparin were used. During the procedure, the animals were gently immobilized by holding, and the needle was inserted into the brachial wing vein at a shallow angle (approximately 10–20°). Each blood sample was immediately divided according to the course of further determination: 1 mL of whole blood was used for morphological analyses (monocytes, eosinophils, and red blood cell parameters) and flow cytometry (results of flow cytometric analyses were already published by Grabowski et al. [16]), while the remaining blood was centrifuged (1800× g for 15 min at 4 °C) to obtain plasma, which was subjected to deep freezing (−80 °C) until further analysis.
4.6. Analysis of Selected Blood Morphological Parameters
The morphological analysis of the collected whole blood sample (200 µL) was performed in the Horiba ABX Micros ES 60 automatic analyzer (Horiba Medical. Japan). Linearity specifications were determined by analyzing dilutions of a commercially available linearity control material that contains no interfering substances. In order to avoid meaningless results due to incorrect counts, the linearity range used for these particular parameters was: 0–99.9 K/µL for WBC; 0–8 M/µL for RBC; 0–24 g/dL for HGB; 50–200 fL for MCV; 0–2000 K/µL for PLT; 5–18 fL for MPV; and 0–30% for RETIC%. The automatic analyzer used is commonly applied for the evaluation of hematological parameters in various animal species [36,37,38]. The following parameters were monitored: absolute number of monocytes and eosinophils, as well as red blood cell system indexes: erythrocyte count, hematocrit (HCT) level, mean red cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and platelet number.
4.7. Determination of Alanine Transaminase (ALT) and Aspartate Aminotransferase (AST) Concentrations in Peripheral Blood Plasma
Levels of the blood biochemical parameters, such as alanine transaminase (ALT) and aspartate aminotransferase (AST), were determined using an automated Architect c8000 Abbott biochemical analyzer (Abbott, Chicago, IL, USA). This is a fully automated system that performs sample processing using potentiometric and photometric methods. The relevant calibration parameters and chicken-dedicated reference values were configured into the system and validated prior to the main analysis. For the determination of the above parameters, the following reference interval was used: 19–21 (IU/L) for ALT and 131–486 (IU/L) for AST, respectively. This was defined on the basis of the Merck Veterinary Manual (2011).
4.8. Determination of C-Reactive Protein (CRP) Concentration (Using ELISA) in Peripheral Blood Plasma
The measurement of the CRP level was carried out using an ELISA immunoenzymatic assay, based on the formation of bonds between antigen and antibody, which are revealed by the color reaction with immunoglobulin-conjugated enzymes and their respective substrates. The procedure was performed according to the manual included in the set of commercially available reagents (Catalog No.: ELK2038, ELK Biotechnology Co., Ltd., Wuhan, China) and the previously described method [16]. All reagents and samples were brought to room temperature (20–25 °C) before use. A total of 100 uL of buffer, test samples, or standards were added in duplicate to each well of the titration plate (96-well Nunc plate) coated with CRP-specific monoclonal antibodies. The plate was covered and incubated at 37 °C for 80 min. Then, the plate contents were drained and washed with the prepared buffer three times to remove an excess of unbound antigens. Next, 100 µL of a solution of specific polyclonal biotinylated antibody, conjugated with the enzyme for CRP, were added. The plate was covered, incubated at 37 °C for 50 min, then drained again and washed three times. Subsequently, 100 µL of streptavidin-labeled horseradish peroxidase enzyme solution was added and incubated for 50 min at 37 °C. The plate was then drained and washed five times, and 90 µL of 3,3′5,5′-tetramethylbenzidine (a colored substrate for horseradish peroxidase) solution was added and incubated for 20 min in the dark at 37 °C. The reaction was stopped by adding 50 µL of the blocking solution, which changed the color of the product (from blue to yellow). Absorbance was measured at 10 min after stopping the reaction using a Multiskan FC microplate reader coupled with Skanlt 6.1.1. RE software (Thermo Fisher Scientific, Waltham, MA, USA), which analyzes the spectrophotometric color intensity, plots a standard curve based on the standards used, and reads the concentration values of the CRP in the tested plasma samples. The results were given in ng/mL. The minimum sensitivity for the test was 0.32–20 ng/mL.
4.9. Statistical Analysis
The results are presented as mean ± standard deviation (SD). For statistical analysis of the results, SPSS 21.0 (SPSS Inc., Armonk, NY, USA) software was used. The normality of the distribution of the variables was checked with the Kolmogorov–Smirnov test and the homogeneity of the variances with Levene’s test. If the assumptions of normality of distribution and/or homogeneity of variance were not met, the Kruskal–Wallis test and post hoc Dunn’s test were applied. Once both assumptions were met, the analysis was carried out on the basis of ANOVA and post hoc Tukey’s test. Statistically significant differences were considered when p < 0.05.
Author Contributions
Conceptualization, Ł.G. and M.P.; methodology, Ł.G. and M.P.; validation, Ł.G. and M.P.; formal analysis, Ł.G., A.W., G.W. and M.P.; investigation, Ł.G., A.W., G.W. and M.P.; writing—original draft preparation, Ł.G., A.W., G.W. and M.P.; writing—review and editing, Ł.G., A.W., G.W. and M.P.; visualization, Ł.G. and M.P.; supervision, A.W. and G.W.; project administration, A.W.; and funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre (Poland) within project grant no. 2017/27/B/NZ9/00393.
Institutional Review Board Statement
The animal study protocol was approved by the Local Ethics Committee for Experimental Animals in Olsztyn (protocol code: 62/2019, date of approval: 30 July 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the research team of the Pavilion of Experimental Birds Infections, University of Warmia and Mazury, Olsztyn, Poland, for their services during the experiments with the chickens.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Ahmad, S.M.; Bhat, S.A.; Ahmed, R.; Urwat, U.; Mumtaz, P.T.; Dar, T.A.; Shah, R.A.; Ganai, N.A. Salmonella Typhimurium in poultry: A review. Poult. Sci. J. 2017, 73, 345–354. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al.; EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel) Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control Salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.P.; de Melo, R.T.; Nalevaiko, P.C.; Monteiro, G.P.; Fonseca, B.B.; Galvão, N.N.; Giombelli, A.; Rossi, D.A. Spread of the serotypes and antimicrobial resistance in strains of Salmonella spp. isolated from broiler. Braz. J. Microbiol. 2019, 50, 515–522. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Perez, E.; Gonzalez-Ronquillo, M. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review. Animals 2021, 12, 60. [Google Scholar] [CrossRef]
- Jerab, J.; Jansen, W.; Blackwell, J.; van Hout, J.; Palzer, A.; Lister, S.; Chantziaras, I.; Dewulf, J.; De Briyne, N. Real-World Data on Antibiotic Group Treatment in European Livestock: Drivers, Conditions, and Alternatives. Antibiotics 2022, 11, 1046. [Google Scholar] [CrossRef]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.; Fuentes, J.A. One for All: Functional Transfer of OMV-Mediated Polymyxin B Resistance From Salmonella enterica sv. Typhi Δ tolR and Δ degS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Rahman, S.R. Use of Phages to Treat Antimicrobial-Resistant Salmonella Infections in Poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Int. Food Res. J. 2019, 119, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Stasiłojć, M.; Grabowski, Ł.; Zdrojewska, K.; Wegrzyn, G.; Wegrzyn, A. Efficacy and safety of phage therapy against Salmonella enterica serovars Typhimurium and Enteritidis estimated by using a battery of in vitro tests and the Galleria mellonella animal model. Microbiol. Res. 2022, 261, 127052. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Podlacha, M.; Grabowski, Ł.; Stasiłojć, M.; Nowak-Zaleska, A.; Ciemińska, K.; Cyske, Z.; Dydecka, A.; Gaffke, L.; Mantej, J.; et al. Biological aspects of phage therapy versus antibiotics against Salmonella enterica serovar Typhimurium infection of chickens. Front. Cell. Infect. Microbiol. 2022, 12, 941867. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Węgrzyn, G.; Wegrzyn, A.; Podlacha, M. Highly different effects of phage therapy and antibiotic therapy on immunological responses of chickens infected with Salmonella enterica serovar Typhimurium. Front. Immunol. 2022, 13, 956833. [Google Scholar] [CrossRef]
- Saleem, H.M.; Muhammed, T.M.; Al-Hetty, H.R.A.K.; Salman, D.A. Physiological, hematological and some biochemical alterations during pregnancy. Int. J. Health Sci. 2022, 6, 7156–7169. [Google Scholar] [CrossRef]
- Yap, K.N.; Dick, M.F.; Guglielmo, C.G.; Williams, T.D. Effects of experimental manipulation of hematocrit on avian flight performance in high- and low-altitude conditions. J. Exp. Biol. 2018, 221, jeb191056. [Google Scholar] [CrossRef]
- Buta, A.; Spătariu, S.; Ovidiu, O.; Daradics, Z.; Tamas-Krumpe, O.; Ognean, L. Current data regarding the evolution of hematological profile in Broiler chickens: A review. Lucr. Știnţifice 2019, 62, 172–179. [Google Scholar]
- Ribeiro, M.N.; Ribeiro, N.L.; Bozzi, R.; Costa, R.G. Physiological and biochemical blood variables of goats subjected to heat stress—A review. J. Appl. Anim. Res. 2018, 46, 1036–1041. [Google Scholar] [CrossRef]
- Alkhalf, A.; Alhaj, M.; Al-Homidan, I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 2010, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in chicken meat: Consumption, outbreaks, characteristics, current control methods and the potential of bacteriophage use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.A.; Shawrang, P.; Shakorzadeh, S. Immune Response of Salmonella Challenged Broiler Chickens Fed Diets Containing Gallipro®, a Bacillus subtilis Probiotic. Probiotics Antimicrob. Proteins 2015, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Węgrzyn, G.; Węgrzyn, A. Enrofloxacin–the ruthless killer of eukaryotic cells or the last hope in the fight against bacterial infections? Int. J. Mol. Sci. 2022, 23, 3648. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. The contribution of phage therapy to medical knowledge. J. Glob. Antimicrob. Resist. 2022, 28, 238–240. [Google Scholar] [CrossRef]
- Dufour, N.; Delattre, R.; Chevallereau, A.; Ricard, J.D.; Debarbieux, L. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob. Agents Chemother. 2019, 63, e00379-19. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Villa, G.T.; Sieiro, C. Phage therapy, lysin therapy, and antibiotics: A trio due to come. Antibiotics 2020, 9, 604. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Animal models in the evaluation of the effectiveness of phage therapy for infections caused by gram-negative bacteria from the ESKAPE group and the reliability of its use in humans. Microorganisms 2021, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Bibi, F.; Archer, A.C. Bacteriophage therapy: Recent developments and applications of a renaissant weapon. Res. Microbiol. 2021, 172, 103863. [Google Scholar] [CrossRef]
- Saraf, S.; Jafra, B.S.; Ray, P.; Rawat, A.; Verma, S. Multidrug-Resistant Nontyphoidal Salmonella Associated with Invasive Disease in an Immunocompetent Child. Indian J. Pediat. 2021, 88, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Ciemińska, K.; Grabski, M.; Grabowski, Ł.; Górniak, M.; Jurczak-Kurek, A.; Węgrzyn, G.; Węgrzyn, A. Characteristics of a series of three bacteriophages infecting Salmonella enterica strains. Int. J. Mol. Sci. 2020, 21, 6152. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Grabowski, Ł.; Grabski, M.; Kaszubski, M.; Górniak, M.; Jurczak-Kurek, A.; Węgrzyn, G.; Węgrzyn, A. Bacteriophages vB_Sen-TO17 and vB_Sen-E22, Newly Isolated Viruses from Chicken Feces, Specific for Several Salmonella enterica Strains. Int. J. Mol. Sci. 2020, 21, 8821. [Google Scholar] [CrossRef]
- Neut, D.; Dijkstra, R.J.B.; Thompson, J.I.; Kavanagh, C.; van der Mei, H.C.; Busscher, H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cells Mater. 2015, 29, 42–56. [Google Scholar] [CrossRef]
- Roch-Lefèvre, S.; Martin-Bodiot, C.; Grégoire, E.; Desbrée, A.; Roy, L.; Barquinero, J.F. A mouse model of cytogenetic analysis to evaluate caesium137 radiation dose exposure and contamination level in lymphocytes. Radiat. Environ. Biophys. 2016, 55, 61–70. [Google Scholar] [CrossRef]
- Aouni, R.; Ben Attia, M.; Jaafoura, M.H.; Bibi-Derbel, A.; Haouari, M. Effects of the hydro-ethanolic extract of Marrubium vulgare in female rats. Asian Pac. J. Trop. Med. 2017, 10, 160–164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).