Prevalence of Antibiotic-Resistant E. coli Strains in a Local Farm and Packing Facilities of Honeydew Melon in Hermosillo, Sonora, Mexico
Abstract
1. Introduction
2. Results and Discussion
2.1. Identified Strains of E. coli
2.2. Antibiotic Susceptibility of the Isolated E. coli Strains
2.3. Risk Analysis at Different E. coli Sampling Sites
3. Conclusions
4. Materials and Methods
4.1. Collection of Samples
4.2. Isolation and Biochemical Characterization of E. coli
4.3. Genomic DNA Extraction
4.4. Molecular Identification of E. coli
4.5. Antibiotic Resistance
4.6. Molecular Identification of Extended Spectrum β-Lactamases (ESBLs) and Non-ESBL Genes
4.7. Disposal of Microorganisms and Reagents
4.8. Statistical Analysis
4.9. Ethical Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- CDC. CDC Reports of Selected E. coli Outbreak Investigations; CDC: Atlanta, GA, USA, 2022.
- Khan, F.M.; Gupta, R. Escherichia coli (E. coli) as an indicator of fecal contamination in Groundwater: A Review. In International Conference on Sustainable Development of Water and Environment; Springer: Cham, Switzerland, 2020; pp. 225–235. [Google Scholar]
- Rojas-Lopez, M.; Monterio, R.; Pizza, M.; Desvaux, M.; Rosini, R. Intestinal pathogenic Escherichia coli: Insights for vaccine development. Front. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Ariyama, H.A.; García-Heredia, A.; Heredia, N.; García, S.; León, J.; Jaykus, L.; Solís-Soto, L. Phylogroups, pathotypes, biofilm formation and antimicrobial resistance of Escherichia coli isolates in farms and packing facilities of tomato, jalapeño pepper and cantaloupe from Northern Mexico. Int. J. Food Microbiol. 2019, 290, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jiménez, D.; García-Meniño, I.; Herrera, A.; Lestón, L.; Mora, A. Microbiological risk assessment of Turkey and chicken meat for consumer: Significant differences regarding multidrug resistance, mcr or presence of hybrid aEPEC/ExPEC pathotypes of E. coli. Food Control 2021, 123, 107713. [Google Scholar] [CrossRef]
- Vidal, A.; Aguirre, L.; Seminati, C.; Tello, M.; Redondo, N.; Martín, M.; Darwich, L. Antimicrobial resistance profiles and characterization of Escherichia coli strains from cases of neonatal diarrhea in spanish pig farms. Vet. Sci. 2020, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.S.; Heuzenroeder, M.W.; Barton, M.D. A Study of the transfer of tetracycline resistance genes between Escherichia coli in the intestinal tract of a mouse and a chicken model. J. Vet. Med. Ser. B 2006, 53, 333–340. [Google Scholar] [CrossRef]
- Araújo, S.; Silva, I.A.T.; Tacão, M.; Patinha, C.; Alves, A.; Henriques, I. Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 2017, 257, 192–200. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the Risk Posed by Pathogens in Food of Non-Animal Origin. EFSA J. 2013, 11, 3025. [Google Scholar]
- Oh, S.-S.; Song, J.; Kim, J.; Shin, J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef]
- Erb, A.; Stürmer, T.; Marre, R.; Brenner, H. Prevalence of antibiotic resistance in Escherichia coli: Overview of geographical, temporal, and methodological variations. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 83–90. [Google Scholar] [CrossRef]
- Pagadala, S.; Marine, S.C.; Micallef, S.A.; Wang, F.; Pahl, D.M.; Melendez, M.V.; Kline, W.L.; Oni, R.A.; Walsh, C.S.; Everts, K.L.; et al. Assessment of region, farming system, irrigation source and sampling time as food safety risk factors for tomatoes. Int. J. Food Microbiol. 2015, 196, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodriguez, E.; Adhikari, A. Preharvest farming practices impacting fresh produce safety. Microbiol. Spectr. 2018, 6, 19–46. [Google Scholar] [CrossRef]
- Peng, J.-J.; Balasubramanian, B.; Ming, Y.-Y.; Niu, J.-L.; Yi, C.-M.; Ma, Y.; Liu, W.-C. Identification of antimicrobial resistance genes and drug resistance analysis of Escherichia coli in the animal farm environment. J. Infect. Public Health 2021, 14, 1788–1795. [Google Scholar] [CrossRef]
- Muhterem-Uyar, M.; Dalmasso, M.; Bolocan, A.S.; Hernandez, M.; Kapetanakou, A.E.; Kuchta, T.; Manios, S.G.; Melero, B.; Minarovičová, J.; Nicolau, A.I.; et al. Environmental sampling for Listeria monocytogenes control in food processing facilities reveals three contamination scenarios. Food Control 2015, 51, 94–107. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Millner, P.; Turner, E.; Feng, H. Assessment of Escherichia coli O157:H7 transference from soil to iceberg lettuce via a contaminated field coring harvesting knife. Int. J. Food Microbiol. 2012, 153, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.R. Regulatory Action criteria for filth and other extraneous materials. Regul. Toxicol. Pharmacol. 1998, 28, 199–211. [Google Scholar] [CrossRef]
- Butler, J.F.; Garcia-Maruniak, A.; Meek, F.; Maruniak, J.E. Wild Florida house flies (Musca domestica) as carriers of pathogenic bacteria. Florida Entomol. 2010, 93, 218–223. [Google Scholar] [CrossRef]
- Collinet-Adler, S.; Babji, S.; Francis, M.; Kattula, D.; Premkumar, P.S.; Sarkar, R.; Mohan, V.R.; Ward, H.; Kang, G.; Balraj, V.; et al. Environmental factors associated with high fly densities and diarrhea in Vellore, India. Appl. Environ. Microbiol. 2015, 81, 6053–6058. [Google Scholar] [CrossRef]
- Erickson, M.C.; Habteselassie, M.Y.; Liao, J.; Webb, C.C.; Mantripragada, V.; Davey, L.E.; Doyle, M.P. Examination of factors for use as potential predictors of human enteric pathogen survival in soil. J. Appl. Microbiol. 2014, 116, 335–349. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Wei, G.; Wu, L.; Wu, J.; Xu, J. Survival of Escherichia coli O157:H7 in soils under different land use types. Environ. Sci. Pollut. Res. 2014, 21, 518–524. [Google Scholar] [CrossRef]
- Winfield, M.D.; Groisman, E.A. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Xuan, X.-T.; Li, J.; Chen, S.-G.; Liu, D.-H.; Ye, X.-Q.; Shi, J.; Xue, S.J. Disinfection efficacy and mechanism of slightly acidic electrolyzed water on Staphylococcus aureus in pure culture. Food Control 2016, 60, 505–510. [Google Scholar] [CrossRef]
- James, J. Overview of Microbial Hazards in Fresh Fruit and Vegetables Operations. In Microbial Hazard Identification in Fresh Fruit and Vegetables; John Wiley & Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 1–27. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Martinez de la Peña, C.F.; Rocha-Gracia, R.d.C.; Lozano-Zarain, P.; Navarro-Ocaña, A.; Martínez-Laguna, Y.; de la Rosa-López, R. Virulence and resistance determinants of uropathogenic Escherichia coli strains isolated from pregnant and non-pregnant women from two states in Mexico. Infect. Drug Resist. 2020, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [PubMed]
- Bester, L.; Essack, S. Observational study of the prevalence and antibiotic resistance of Campylobacter spp. from different poultry production systems in KwaZulu-Natal, South Africa. J. Food Prot. 2012, 75, 154–159. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Gillings, M.R. Evolutionary consequences of antibiotic use for the resistome, mobilome and microbial pangenome. Front. Microbiol. 2013, 4, 4. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.; Martinez, J. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011, 2011, 312956. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Ardiyati, T.; Rifa’i, M. Widodo Detection of class 1 integron-associated gene cassettes and tetracycline resistance genes in Escherichia coli isolated from ready to eat vegetables. Ann. Med. Surg. 2020, 55, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Zurita, J. Escherichia coli hyperepidemic clone ST410-A harboring blaCTX-M-15 isolated from fresh vegetables in a municipal market in Quito-Ecuador. Int. J. Food Microbiol. 2018, 280, 41–45. [Google Scholar] [CrossRef]
- Badi, S.; Salah Abbassi, M.; Snoussi, M.; Werheni, R.; Hammami, S.; Maal-Bared, R.; Hassen, A. High rates of antibiotic resistance and biofilm production in Escherichia coli isolates from food products of animal and vegetable origins in Tunisia: A real threat to human health. Int. J. Environ. Health Res. 2020, 32, 406–416. [Google Scholar] [CrossRef]
- Taylor, P.; Reeder, R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- FAO. El Plan de Acción de la FAO Sobre la Resistencia a Los Antimicrobianos 2016–2020; FAO: Rome, Italy, 2016. [Google Scholar]
- Méndez-Moreno, E.; Caporal-Hernandez, L.; Mendez-Pfeiffer, P.A.; Enciso-Martinez, Y.; De la Rosa López, R.; Valencia, D.; Arenas-Hernández, M.M.P.; Ballesteros-Monrreal, M.G.; Barrios-Villa, E. Characterization of diarreaghenic Escherichia coli strains isolated from healthy donors, including a triple hybrid strain. Antibiotics 2022, 11, 833. [Google Scholar] [CrossRef]
- Sambrook, J. Molecular Cloning a Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Carreón León, E.A. Estudio Molecular de la Resistencia y Virulencia de Cepas de Escherichia coli Productoras de β-lactamasas de Espectro Extendido Aisladas de Vegetales Crudo. Master’s Thesis, Benemérita Universidad Autonóma de Puebla, Puebla, Mexico, 2019. [Google Scholar]
- Walker, D.I.; McQuillan, J.; Taiwo, M.; Parks, R.; Stenton, C.A.; Morgan, H.; Mowlem, M.C.; Lees, D.N. A highly specific Escherichia coli qPCR and its comparison with existing methods for environmental waters. Water Res. 2017, 126, 101–110. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Barrios-Villa, E.; Juarez, J.; Álvarez-Ainza, M.L.; Taboada, P.; De la Rosa-López, R.; Bolado-Martínez, E.; Valencia, D. Bacterial morphotypes as important trait for uropathogenic E. coli diagnostic; a virulence-phenotype-phylogeny study. Microorganisms 2021, 9, 2381. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-del-Valle, M.; Ponce-de-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug resistance phenotypes and genotypes in Mexico in representative gram-negative species: Results from the infivar network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, F.; Seyedpour, S. Prevalence of qnr and aac(6′)-Ib-cr genes in clinical isolates of Klebsiella pneumoniae from Imam Hussein hospital in Tehran. Iran J. Med. Sci. 2015, 40, 515–521. [Google Scholar] [PubMed]
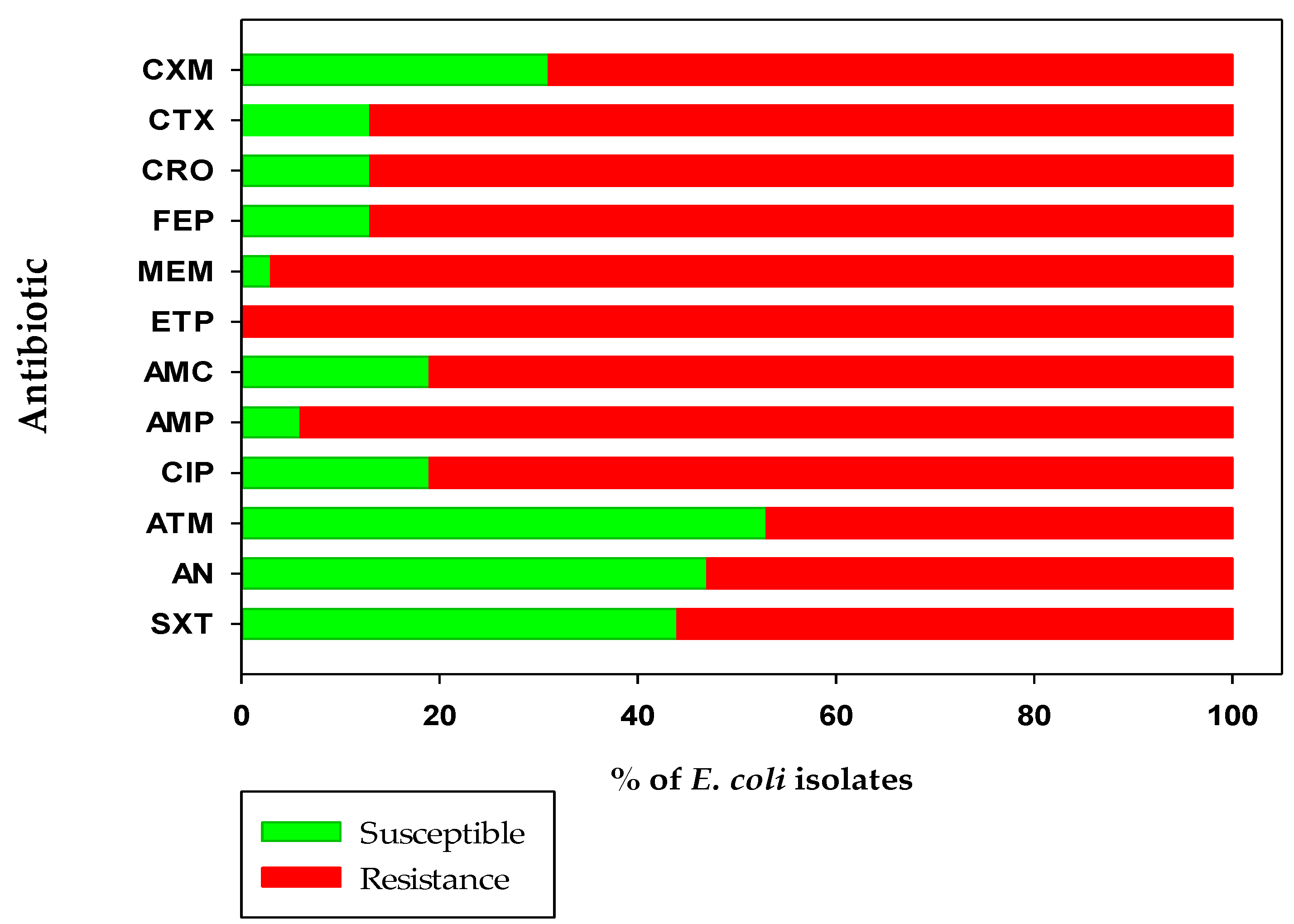
 irrigation water,
irrigation water,  harvested melon,
harvested melon,  packing workers’ hands,
packing workers’ hands,  cardboard packing boxes, and
cardboard packing boxes, and  discarded melon from the Honeydew melon farm and packing facility in Hermosillo, Sonora. Cefuroxime (CXM), cefotaxime (CTX), ceftriaxone (CRO), cefepime (FEP), meropenem (MEM), ertapenem (ETP), amoxicillin/clavulanic acid (AMP), ampicillin (AMP), ciprofloxacin (CIP), aztreonam (ATM), amikacin (AN), and sulfamethoxazole/trimethoprim (STX).
discarded melon from the Honeydew melon farm and packing facility in Hermosillo, Sonora. Cefuroxime (CXM), cefotaxime (CTX), ceftriaxone (CRO), cefepime (FEP), meropenem (MEM), ertapenem (ETP), amoxicillin/clavulanic acid (AMP), ampicillin (AMP), ciprofloxacin (CIP), aztreonam (ATM), amikacin (AN), and sulfamethoxazole/trimethoprim (STX).
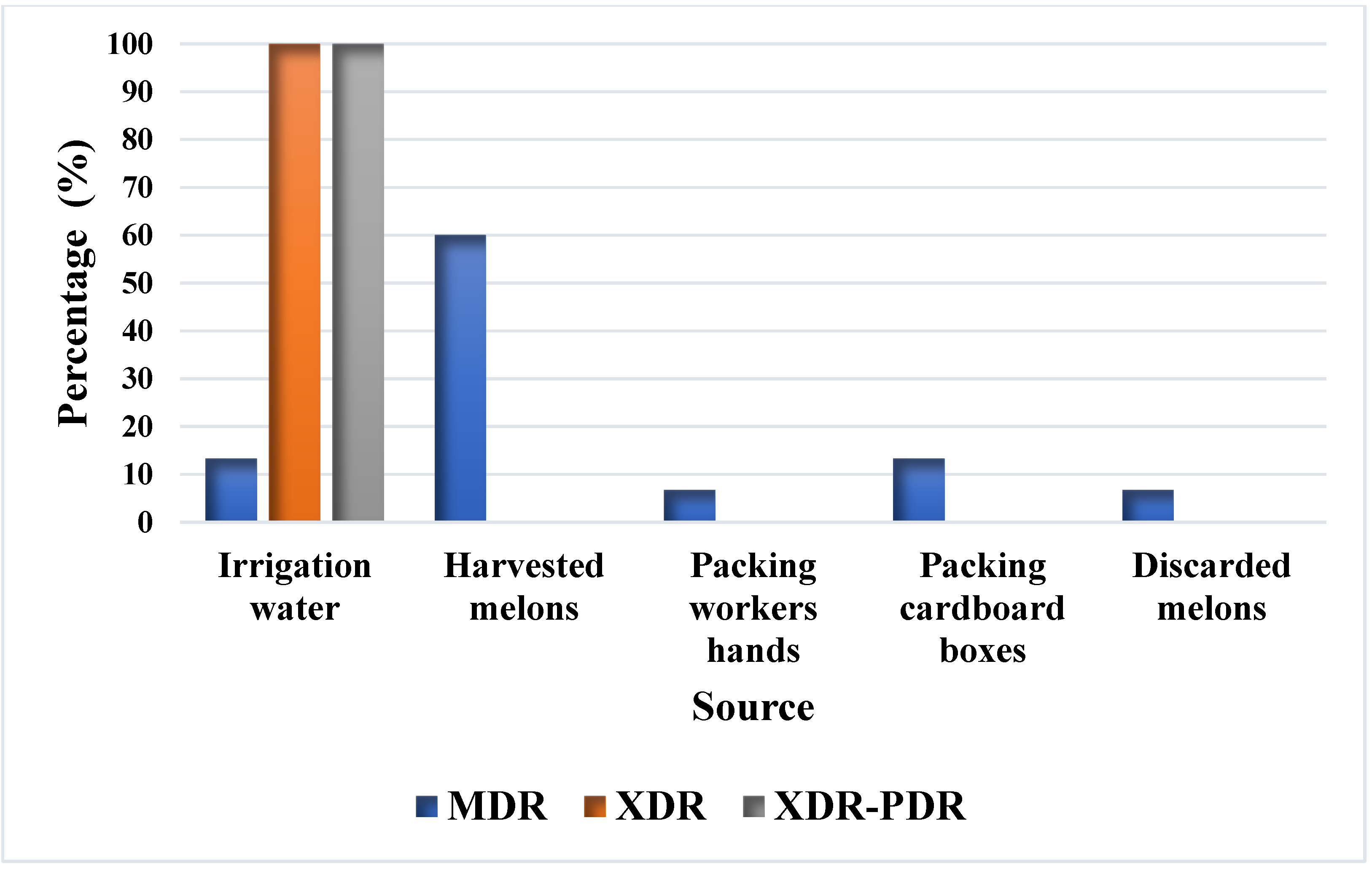
 irrigation water,
irrigation water,  harvested melon,
harvested melon,  packing workers’ hands,
packing workers’ hands,  cardboard packing boxes, and
cardboard packing boxes, and  discarded melon from the Honeydew melon farm and packing facility in Hermosillo, Sonora. Cefuroxime (CXM), cefotaxime (CTX), ceftriaxone (CRO), cefepime (FEP), meropenem (MEM), ertapenem (ETP), amoxicillin/clavulanic acid (AMP), ampicillin (AMP), ciprofloxacin (CIP), aztreonam (ATM), amikacin (AN), and sulfamethoxazole/trimethoprim (STX).
discarded melon from the Honeydew melon farm and packing facility in Hermosillo, Sonora. Cefuroxime (CXM), cefotaxime (CTX), ceftriaxone (CRO), cefepime (FEP), meropenem (MEM), ertapenem (ETP), amoxicillin/clavulanic acid (AMP), ampicillin (AMP), ciprofloxacin (CIP), aztreonam (ATM), amikacin (AN), and sulfamethoxazole/trimethoprim (STX).
| E. coli Strains | |||
|---|---|---|---|
| Source | No. of Samples | Presumptive | Confirmed |
| Irrigation water | 50 | 44 (51%) | 19 (59%) |
| Soil | 50 | 6 (7%) | 0 (0%) |
| Workers’ hands during harvest | 25 | 3 (4%) | 0 (0%) |
| Harvested melons | 50 | 4 (5%) | 1(3%) |
| Washing water | 50 | 0 (0%) | 0 (0%) |
| Washed melons | 50 | 1 (1%) | 0 (0%) |
| Packing table surfaces | 50 | 4 (5%) | 0 (0%) |
| Packing workers’ hands | 20 | 3 (4%) | 2 (6%) |
| Cardboard packing boxes | 50 | 1 (1%) | 1 (3%) |
| Discarded melons | 50 | 20 (23%) | 9 (29%) |
| Total | 445 | 86 (19.3%) | 32 (7.2%) |
| Isolate | Source | Resistotype | ESBL Genes | ESBL Non-Genes |
|---|---|---|---|---|
| Ec -A4-2 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A21-2 | irrigation water | CTX, ATM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | blaCTX-M9, blaTEM | --- |
| Ec-A32 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, CIP, MEM, SXT, ETP | blaTEM | --- |
| Ec-A34 | irrigation water | CTX, CXM, CRO, AMP, FEP, AMC, CIP, MEM, SXT, ETP | blaCTX-M151 | --- |
| Ec-A35-2 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | blaTEM | --- |
| Ec-A36 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A37 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A38 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A39 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, ETP | blaCTX-M1-8, blaCTX-M151 | --- |
| Ec-A40 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A41 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A42 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AN, CIP, MEM, SXT, ETP | blaCTX-M1-8 | --- |
| Ec-A44 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | aac (6′)-lb-cr |
| Ec-A45 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A46 | irrigation water | CTX, ATM, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A47 | irrigation water | CTX, CXM, CRO, AMP, FEP, AMC, CIP, MEM, SXT, ETP | blaTEM | --- |
| Ec-A48 | irrigation water | CTX, CXM, CRO, AMP, FEP, AMC, CIP, MEM, SXT, ETP | --- | --- |
| Ec-A49 | irrigation water | CTX, CRO, FEP, AMC, AN, CIP, MEM, ETP | --- | --- |
| Ec-A51 | irrigation water | CTX, CRO, AMP, FEP, AMC, AN, CIP, MEM, SXT, ETP | blaCTX-M9 | --- |
| Ec-MR11 | discarded melon | CTX, CXM, CRO, AMP, FEP, AMC, AN, CIP, MEM, ETP | --- | --- |
| Ec-MR15 | discarded melon | CXM, AMP, FEP, AMC, MEM, SXT, ETP | --- | --- |
| Ec-MR16 | discarded melon | CRO, FEP, CIP, MEM, ETP | --- | --- |
| Ec-MR17 | discarded melon | CRO, AMP, AMC, AN, MEM, ETP | blaCTX-M1-8, blaTEM | --- |
| Ec-MR23 | discarded melon | CTX, AMP, FEP, MEM, ETP | --- | --- |
| Ec-MR25 | discarded melon | AMP, MEM, ETP | --- | --- |
| Ec-MR28 | discarded melon | CTX, CRO, AMP, FEP, AMC, CIP, MEM, ETP | blaTEM | --- |
| Ec-MR34 | discarded melon | CTX, CRO, AMP, FEP, CIP, MEM, ETP | --- | --- |
| Ec-MR35 | discarded melon | CTX, CXM, CRO, AMP, AMC, MEM, ETP | --- | --- |
| Ec-MC46 | harvested melon | CTX, CXM, AMP, FEP, AMC, CIP, MEM, ETP | blaTEM | qepA |
| Ec-MAE44 | packing workers hands | CTX, CXM, CRO, AMP, FEP, AMC, CIP, MEM, ETP | --- | --- |
| Ec-MAE45 | packing workers hands | CTX, ATM, CRO, AMP, AMC, CIP, MEM, ETP | --- | --- |
| Ec-C49 | melon packing boxes | CTX, CXM, CRO, AMP, FEP, AMC, ETP | --- | --- |
| Antibiotic | Sample | % Antibiotic Resistance of E. coli | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | RR | AR | ODD-E | ODD-NE | ODD-R | X2 | p-Value | ||
| ATM | Irrigation water | 73.7 | 26.3 | 9.5 | 0.65 | 2.8 | 0.08 | 33.6 | 13.4 | 0.001 |
| Others * | 7.7 | 92.3 | ||||||||
| CXM | Irrigation water | 84.2 | 15.8 | 1.8 | 0.38 | 5.3 | 0.85 | 6.2 | 5.2 | 0.023 |
| Others | 46.1 | 53.9 | ||||||||
| AN | Irrigation water | 78.9 | 21.1 | 5.1 | 0.63 | 3.7 | 0.18 | 20.6 | 12.5 | 0.001 |
| Others | 15.4 | 84.6 | ||||||||
| SXT | Irrigation water | 89.5 | 10.5 | 11.6 | 0.81 | 8.5 | 0.08 | 102 | 20.9 | 0.001 |
| Others | 7.7 | 92.3 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enciso-Martínez, Y.; Barrios-Villa, E.; Sepúlveda-Moreno, C.O.; Ballesteros-Monrreal, M.G.; Valencia-Rivera, D.E.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Prevalence of Antibiotic-Resistant E. coli Strains in a Local Farm and Packing Facilities of Honeydew Melon in Hermosillo, Sonora, Mexico. Antibiotics 2022, 11, 1789. https://doi.org/10.3390/antibiotics11121789
Enciso-Martínez Y, Barrios-Villa E, Sepúlveda-Moreno CO, Ballesteros-Monrreal MG, Valencia-Rivera DE, González-Aguilar GA, Martínez-Téllez MA, Ayala-Zavala JF. Prevalence of Antibiotic-Resistant E. coli Strains in a Local Farm and Packing Facilities of Honeydew Melon in Hermosillo, Sonora, Mexico. Antibiotics. 2022; 11(12):1789. https://doi.org/10.3390/antibiotics11121789
Chicago/Turabian StyleEnciso-Martínez, Yessica, Edwin Barrios-Villa, César O. Sepúlveda-Moreno, Manuel G. Ballesteros-Monrreal, Dora E. Valencia-Rivera, Gustavo A. González-Aguilar, Miguel A. Martínez-Téllez, and Jesús Fernando Ayala-Zavala. 2022. "Prevalence of Antibiotic-Resistant E. coli Strains in a Local Farm and Packing Facilities of Honeydew Melon in Hermosillo, Sonora, Mexico" Antibiotics 11, no. 12: 1789. https://doi.org/10.3390/antibiotics11121789
APA StyleEnciso-Martínez, Y., Barrios-Villa, E., Sepúlveda-Moreno, C. O., Ballesteros-Monrreal, M. G., Valencia-Rivera, D. E., González-Aguilar, G. A., Martínez-Téllez, M. A., & Ayala-Zavala, J. F. (2022). Prevalence of Antibiotic-Resistant E. coli Strains in a Local Farm and Packing Facilities of Honeydew Melon in Hermosillo, Sonora, Mexico. Antibiotics, 11(12), 1789. https://doi.org/10.3390/antibiotics11121789








