Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Treatment
2.2. Bacterial Culture
2.3. Experimental Animals
2.4. Experiment Design and Sampling
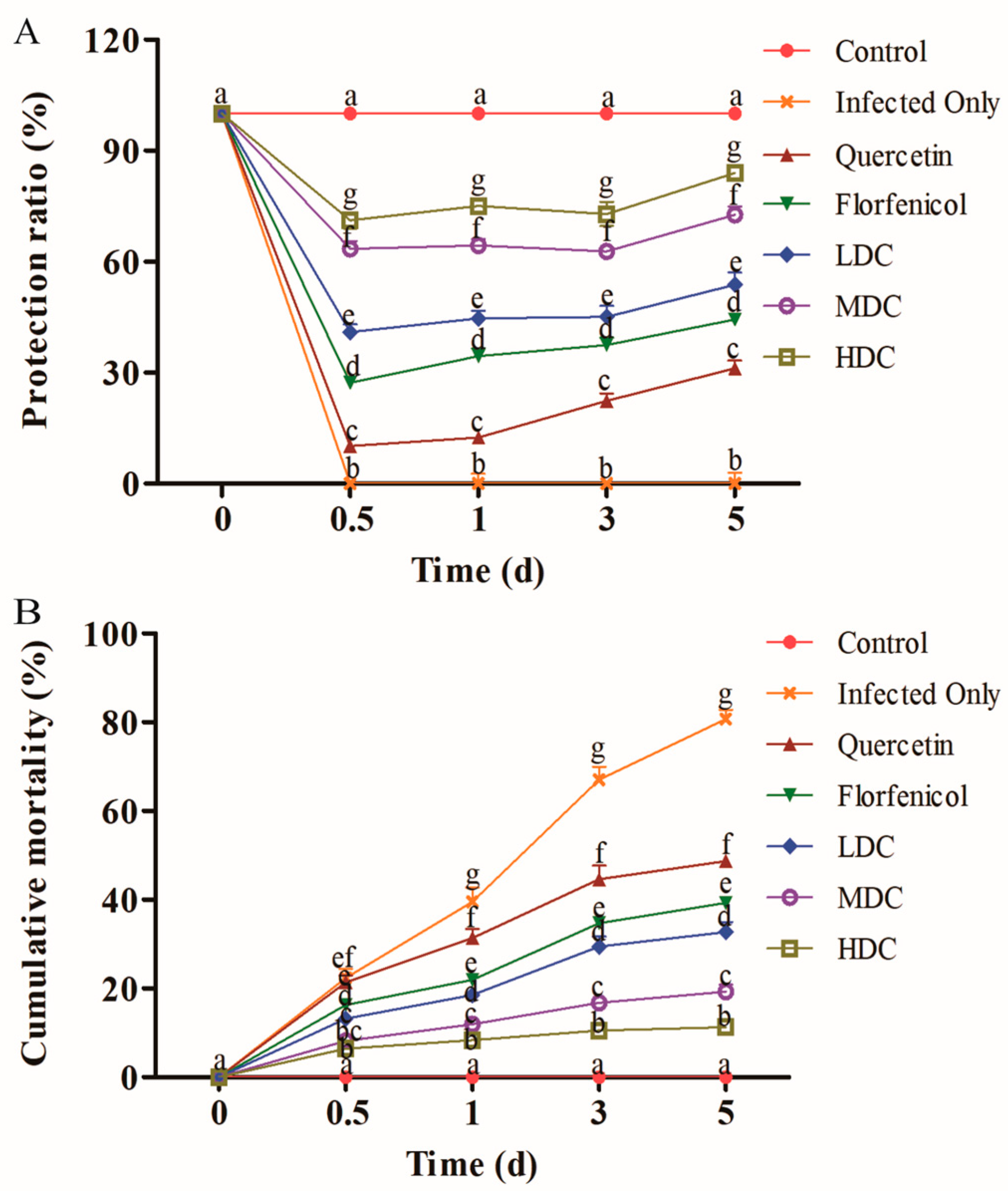
2.5. Shrimp Survival Assay
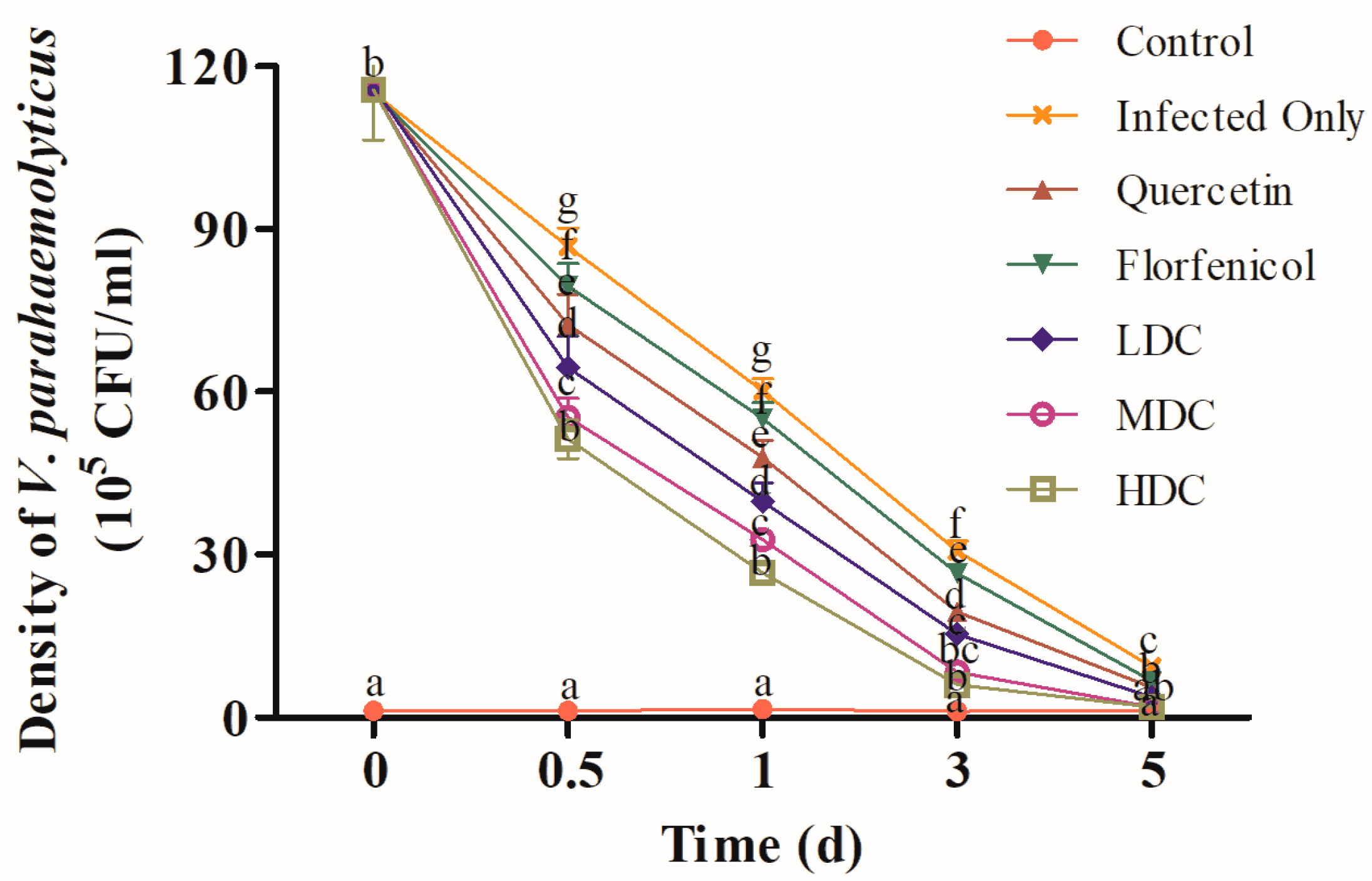
2.6. The Density of VPAHPND in Hepatopancreas
2.7. Immune Parameters Assessment
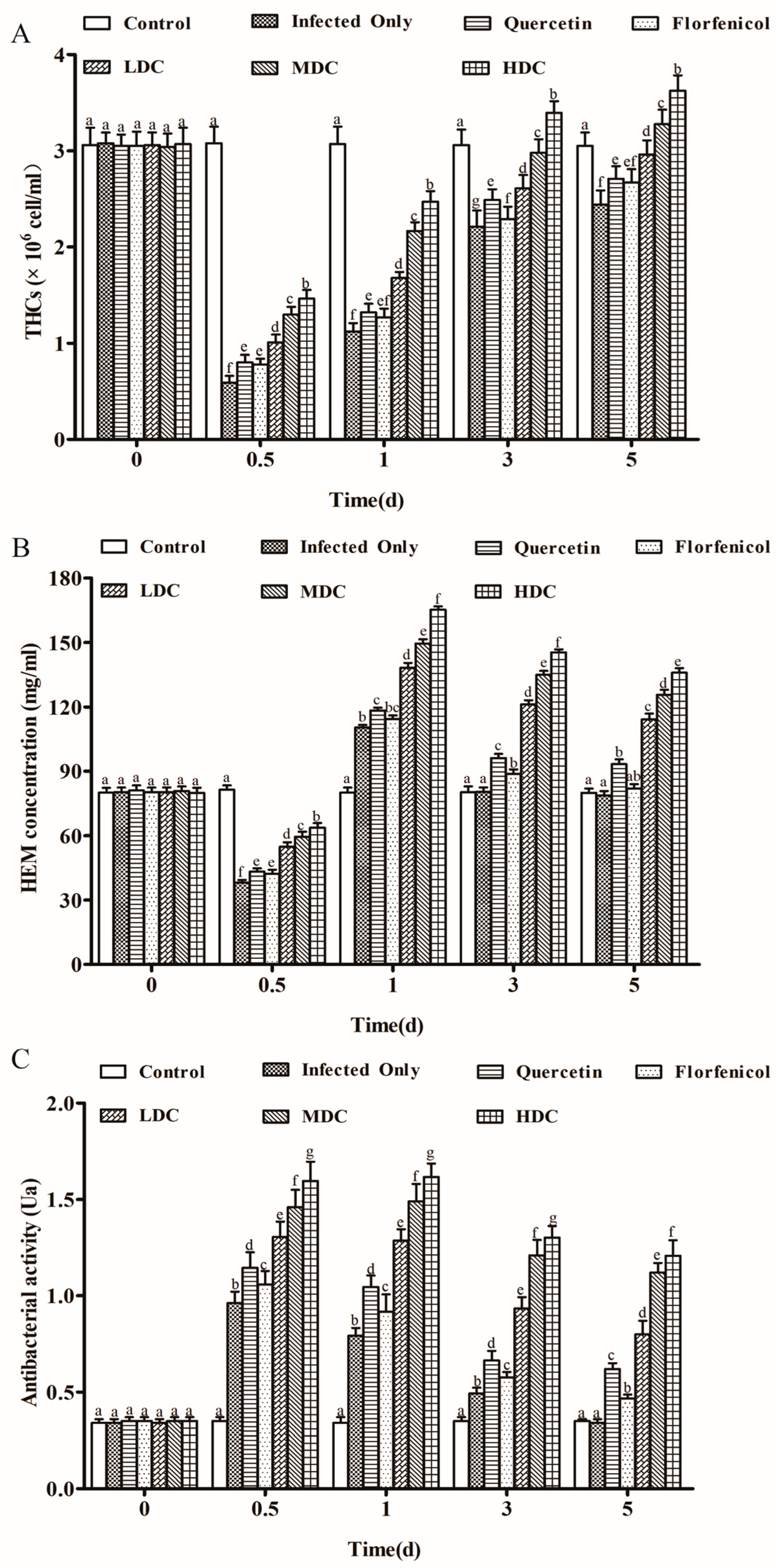
2.7.1. Total Hemocyte Counts
2.7.2. Hemocyanin Determination
2.7.3. Antibacterial Activity
2.7.4. Activities of Immunity-Related Enzymes
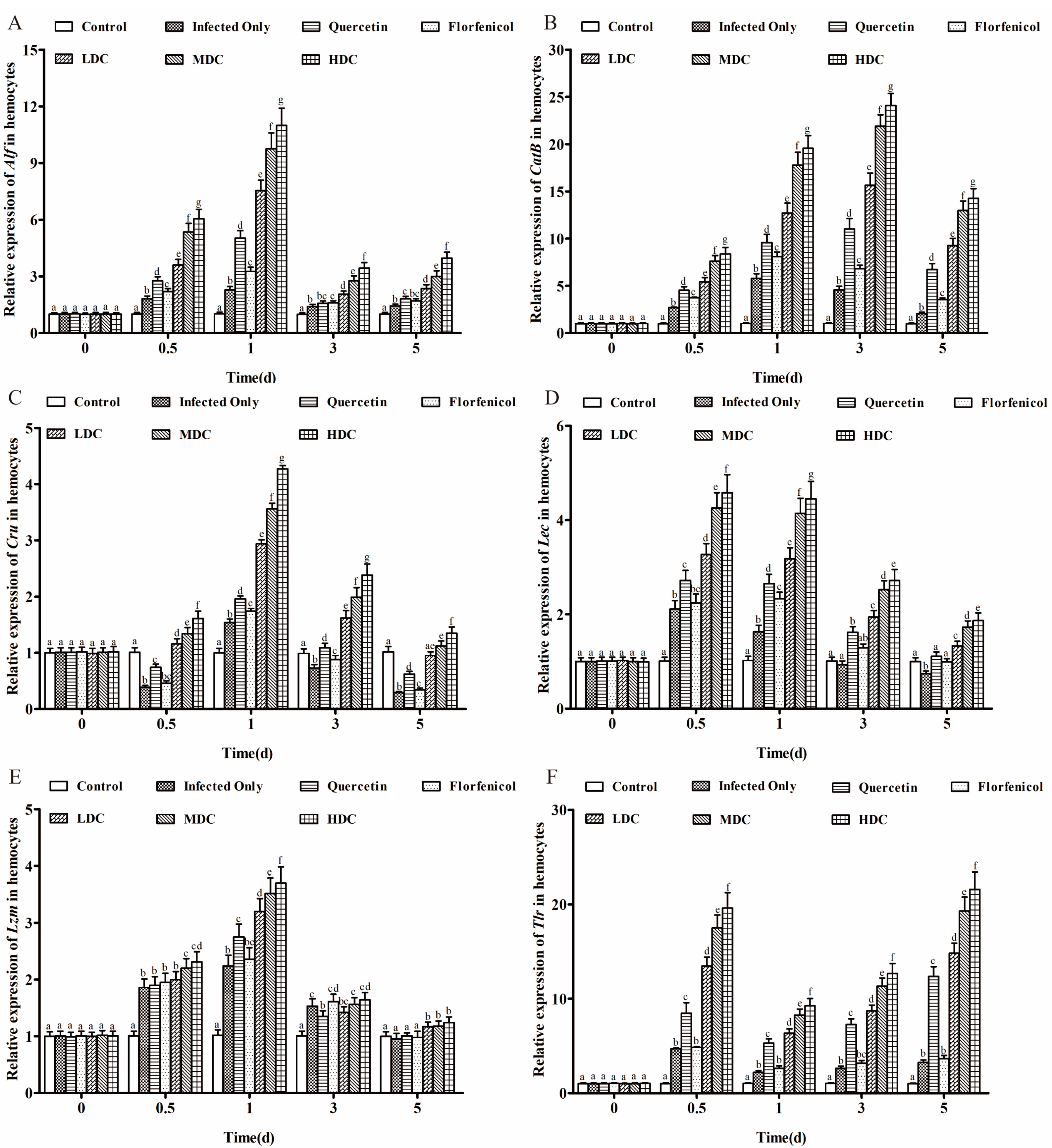
2.7.5. Expression of Immunity-Related Genes
2.8. HE Staining
2.9. Statistical Analysis
3. Results
3.1. Survival and RPS of Shrimp
3.2. Hepatopancreatic VPAHPND Clearance
3.3. THC and HEM Levels and Antimicrobial Activity in Shrimp
3.4. Immunity-Related Enzyme Activities in Acellular Hemolymph
3.5. Immunity-Related Gene Expression Profiles in Hemocytes
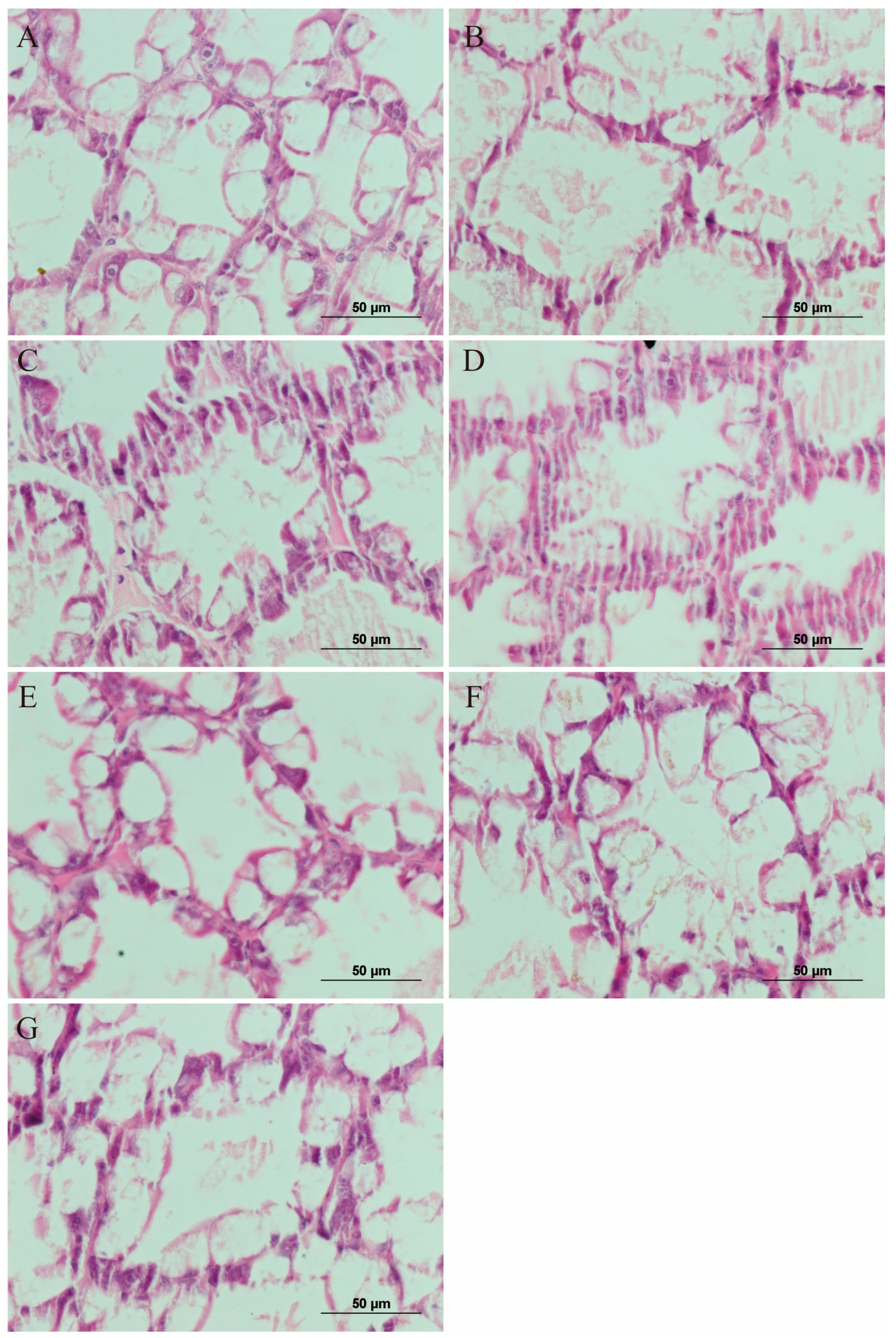
3.6. Analysis of the Hepatopancreas Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.H.; Xiang, J.H. Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immunol. 2013, 34, 973–980. [Google Scholar] [CrossRef]
- MOA (Fisheries Bureau, Ministry of Agriculture). China Fishery Statistics Yearbook 2021; China Agriculture Press: Beijing, China, 2021; pp. 21–36. [Google Scholar]
- Han, J.E.; Tang, K.F.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.M.; Henriksson, P.J.G.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; Van den Brink, P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412–413, 231–243. [Google Scholar] [CrossRef]
- Reilly, A.; Käferstein, F. Food safety hazards and the application of the principles of the hazard analysis and critical control point (HACCP) system for their control in aquaculture production. Aquac. Res. 1997, 28, 735–752. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Dharaneedharan, S.; Moon, Y.G.; Kim, M.C.; Kim, J.S.; Heo, M.S. Effect of plant active compounds on immune response and disease resistance in Cirrhina mrigala infected with fungal fish pathogen, Aphanomyces invadans. Aquac. Res. 2009, 40, 1170–1181. [Google Scholar] [CrossRef]
- Chang, J. Medicinal herbs: Drugs or dietary supplements? Biochem. Pharmacol. 2000, 59, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, V.; Babu, M.M.; Immanuel, G.; Murugadass, S.; Citarasu, T.; Marian, M.P. Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquaculture 2004, 237, 9–20. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current research on the use of plant-derived products in farmed fish. Aquac. Res. 2015, 46, 513–551. [Google Scholar] [CrossRef]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Schoeberl, B.; Nielsen, U.B.; Sorger, P.K. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2006, 2, 458–466. [Google Scholar] [CrossRef]
- Plumb, D. Florfenicol, in Plumb D (Ed.): Veterinary Drug Handbook, 5th ed.; Iowa State Press: Ames, IA, USA, 2004; pp. 335–336. [Google Scholar]
- Gaunt, P.; Endris, R.; Khoo, L.; Leard, A.T.; Jack, S.; Santucci, T.; Katz, T.; Radecki, S.V.; Simmons, R. Preliminary Assessment of the Tolerance and Efficacy of Florfenicol against Edwardsiella ictaluri Administered in Feed to Channel Catfish. J. Aquat. Anim. Health 2003, 15, 239–247. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; García-Mediavilla, M.V.; Gutiérrez, B.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008, 100, 968–976. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Borah, A.; Das, S. Quercetin-induced amelioration of deltamethrin stress in freshwater teleost, Channa punctata: Multiple biomarker analysis. Comp. Biochem. Phys. C 2020, 227, 108626. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Pradhan, L.K.; Aparna, S.; Agarwal, K.; Banerjee, A.; Das, S.K. Quercetin abrogates bisphenol A induced altered neurobehavioral response and oxidative stress in zebrafish by modulating brain antioxidant defence system. Environ. Toxicol. Phar. 2020, 80, 103483. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, C.N.; Zhang, J.L.; Xie, J.; Xing, Y.F.; Li, Z.F. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- ADCC (Agriculture Department Compiling Committee). Handbook of Fishery Drugs; China Agriculture Press: Beijing, China, 2005; pp. 208–209. (In Chinese) [Google Scholar]
- Li, J.; Liu, S.L.; Shi, Q.C.; Zhai, S.W. Effects of dietary quercetin on growth performance, serum biochemical indices, non-specific immune indices of Juvenile Tilapia (GIFT Oreochromis niloticus). Feed Ind. 2014, 35, 21–25. (In Chinese) [Google Scholar] [CrossRef]
- Zhai, Q.Q.; Chang, Z.Q.; Li, J.T.; Li, J. Effects of combined florfenicol and chlorogenic acid to treat acute hepatopancreatic necrosis disease in Litopenaeus vannamei caused by Vibrio parahaemolyticus. Aquaculture 2022, 47, 737462. [Google Scholar] [CrossRef]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.F.; Yao, C.L.; Wang, Z.Y. Immune response and gene expression in shrimp (Litopenaeus vannamei) hemocytes and hepatopancreas against some pathogen-associated molecular patterns. Fish Shellfish Immunol. 2009, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.Q.; Li, J. Effectiveness of traditional Chinese herbal medicine, San-Huang-San, in combination with enrofloxacin to treat AHPND-causing strain of Vibrio parahaemolyticus infection in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef]
- Lai, H.C.; Ng, T.H.; Ando, M.; Lee, C.T.; Chen, I.T.; Chuang, J.C.; Mavichak, R.; Chang, S.H.; Yeh, M.D.; Chiang, Y.A. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish Immunol. 2015, 47, 1006–1014. [Google Scholar] [CrossRef]
- Umbetova, A.K.; Beyatli, A.; Seitimova, G.A.; Yeskaliyeva, B.K.; Burasheva, G.S. Flavonoids from the Plant Atraphaxis virgata. Chem. Nat. Compd. 2021, 57, 531–533. [Google Scholar] [CrossRef]
- Ozga, J.A.; Saeed, A.; Wismer, W.; Reinecke, D.M. Characterization of cyanidin- and quercetin-derived flavonoids and other phenolics in mature Saskatoon fruits (Amelanchier alnifoliaNutt.). J. Agric. Food Chem. 2007, 55, 10414–10424. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin. Monograph. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar]
- Wang, X.W.; Wang, J.X. Diversity and multiple functions of lectins in shrimp immunity. Dev. Comp. Immunol. 2013, 39, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.Q.; Li, J.; Li, J.T.; Wang, J.J.; Li, Z.D. Immune response of Exopalaemon carinicauda infected with an AHPND-causing strain of Vibrio parahaemolyticus. Fish Shellfish Immunol. 2018, 74, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, H.; Zhang, H. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2006, 20, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Pholdaeng, K.; Pongsamart, S. Studies on the immunomodulatory effect of polysaccharide gel extracted from Durio zibethinus in Penaeus monodon shrimp against Vibrio harveyi and WSSV. Fish Shellfish Immunol. 2010, 28, 555–561. [Google Scholar] [CrossRef]
- Garcia-Carreno, F.L.; Cota, K.; Navarrete del Toro, M.A. Phenoloxidase activity of hemocyanin in whiteleg shrimp Penaeus vannamei: Conversion, characterization of catalytic properties, and role in postmortem melanosis. J. Agric. Food Chem. 2008, 56, 6454–6459. [Google Scholar] [CrossRef]
- Song, Y.L.; Yu, C.I.; Lien, T.W.; Huang, C.C.; Lin, M.N. Haemolymph parameters of Pacific white shrimp (Litopenaeus vannamei) infected with Taura syndrome virus. Fish Shellfish Immunol. 2003, 14, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Peng, J.; Wang, Y.; Zhao, S.; Fang, W.; Hu, K.; Shen, J.Y.; Yao, J. Pharmacokinetics and pharmacodynamics of sulfamethoxazole and trimethoprim in swimming crabs (Portunus trituberculatus) and in vitro antibacterial activity against Vibrio: PK/PD of SMZ-TMP in crabs and antibacterial activity against Vibrio. Environ. Toxicol. Pharmacol. 2016, 46, 45–54. [Google Scholar] [CrossRef]
- Li, C.C.; Yeh, S.T.; Chen, J.C. The immune response of white shrimp Litopenaeus vannamei following Vibrio alginolyticus injection. Fish Shellfish Immunol. 2008, 25, 853–860. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Ge, Q.Q.; Sun, M.; Wang, Q.; Lv, H.Y.; Li, J. Immune responses by dietary supplement with Astragalus polysaccharides in the Pacific white shrimp, Litopenaeus vannamei. Aquacult. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Feng, N.N.; Yu, Y.M.; Wen, R.; Zhang, C.R.; Li, F.H. Analysis on the activity of immune related enzymes in survived Exopalaemon carinicauda from WSSV infection. Mar. Sci. 2014, 38, 75–79. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, J.X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Jaramillo, L.; Lascurain, R.; Cooper, E.L.; Rosas, P.; Zenteno, E. Bacterial agglutination by the sialic acid specific serum lectin from Macrobrachium rosenbergii. Comp. Biochem. Phys. B 1996, 113, 355–359. [Google Scholar] [CrossRef]
- Marques, M.F.; Barracco, M.A. Lectins, as non-self-recognition factors, in crustaceans. Aquaculture 2000, 191, 23–44. [Google Scholar] [CrossRef]
- Funami, K.; Matsumoto, M.; Oshiumi, H.; Akazawa, T.; Yamamoto, A.; Seya, T. The cytoplasmic ‘linker region’ in Toll-like receptor 3 controls receptor localization and signalling. Int. Immunol. 2004, 16, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Burge, E.J.; Madigan, D.J.; Burnett, L.E.; Burnett, K.G. Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol. 2007, 22, 327–339. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Li, F.; Xiang, J. Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2014, 46, 349–355. [Google Scholar] [CrossRef]
- Lv, X.; Li, S.; Liu, F.; Li, F.; Xiang, J. Identification and function analysis of an anti-lipopolysaccharide factor from the ridgetail prawn Exopalaemon carinicauda. Dev. Comp. Immunol. 2017, 70, 128–134. [Google Scholar] [CrossRef]
- Mizuochi, T.; Yee, S.T.; Kasai, M.; Kakiuchi, T.; Muno, D.; Kominami, E. Both cathepsin B and cathepsin D are necessary for processing of ovalbumin as well as for degradation of class II MHC invariant chain. Immunol. Lett. 1994, 43, 189–192. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Kong, J.; Luo, K.; Luan, S.; Cao, B.; Liu, N.; Pang, J.; Shi, X. Molecular cloning and characterization of a cathepsin B gene from the Chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2013, 35, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiravanichpaisal, P.; Soderhall, I.; Cerenius, L.; Soderhall, K. Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the crayfish Pacifastacus leniusculus. J. Virol. 2006, 80, 10365–10371. [Google Scholar] [CrossRef] [PubMed]
- Nunan, L.; Lightner, D.; Pantoja, C.; Gomez-Jimenez, S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Org. 2014, 111, 81–86. [Google Scholar] [CrossRef] [PubMed]

| Groups | |||||||
|---|---|---|---|---|---|---|---|
| Control | Infected Only | Quercetin | Florfenicol | LDC | MDC | HDC | |
| Ingredients | |||||||
| Fish meal a (g/kg) | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Wheat glutens a (g/kg) | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 |
| Wheat meal a (g/kg) | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Cellulose (g/kg) | 180.0 | 180 | 179.6 | 179.99 | 179.79 | 179.59 | 179.17 |
| Fish oil (g/kg) | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Soybean oil (g/kg) | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Soybean phospholipids (g/kg) | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Gelatin (g/kg) | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Choline chloride (g/kg) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Vitamin mix b (g/kg) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral mix c (g/kg) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Quercetin (mg/kg) | 0 | 0 | 400 | 0 | 200 | 400 | 800 |
| Florfenicol (mg/kg) | 0 | 0 | 0 | 15 | 7.0 | 15 | 30 |
| Proximate nutrient composition (as fed) | |||||||
| Crude protein (g/kg) | 431.0 | 431.0 | 431.0 | 431.0 | 431.0 | 431.0 | 431.0 |
| Crude fat (g/kg) | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 |
| Crude ash (g/kg) | 68.0 | 68.0 | 68.0 | 68.0 | 68.0 | 68.0 | 68.0 |
| Total energy (kJ/g) | 16.44 | 16.44 | 16.44 | 16.44 | 16.44 | 16.44 | 16.44 |
| Primer | Primer Sequence (5′-3′) | GenBank Accession Number |
|---|---|---|
| Tlr-F | TGAGAGATGCCCACTGCCTG | DQ923424.1 |
| Tlr-R | CGCTTGAAGGTTTGTGAGGGAG | |
| Alf-F | TGTTCCTGGTGGCACTCTTC | GQ227486.1 |
| Alf-R | GTCTCCTCGTTCCTCCACAG | |
| Cru-F | AACCAGAGACACCTGTTGGC | AY488497.1 |
| Cru-R | AGAATGAGGGAGGCTTGCAC | |
| Lzm-F | TCGAGTCGTCCTTCAACACG | AF425673.1 |
| Lzm-R | AGACGTTCTTGCCGTAGTCG | |
| Lec-F | CGGGATCCATGAAGTTCCTAGCGCCG | EF583939.1 |
| Lec-R | CGCTCGAGTATATTTCTTGAGGCAAAT | |
| CatB-F | CCTCTGTGGTTTTGGATGTA | GU571199.1 |
| CatB-R | GATGCTGTATGCTTTGCCTC | |
| β-actin-F | AGTAGCCGCCCTGGTTGT | AF300705.2 |
| β-actin-R | AGGATACCTCGCTTGCTCT |
| 0 d | 0.5 d | 1 d | 3 d | 5 d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| f | 0.005 | 0.196 | 93.267 | 0.000 | 84.533 | 0.003 | 270.506 | 0.002 | 597.818 | 0.000 | |
| Cumulative mortality | q | 0.030 | 0.188 | 37.363 | 0.000 | 51.956 | 0.001 | 166.258 | 0.001 | 367.430 | 0.000 |
| f × q | 0.050 | 0.185 | 6.039 | 0.003 | 0.894 | 0.009 | 2.860 | 0.014 | 6.320 | 0.027 | |
| f | 0.680 | 0.144 | 2.556 | 0.026 | 87.402 | 0.013 | 279.687 | 0.001 | 618.109 | 0.000 | |
| Protection ratio | q | 0.115 | 0.176 | 29.076 | 0.000 | 54.162 | 0.001 | 173.319 | 0.001 | 383.035 | 0.000 |
| f × q | 0.060 | 0.183 | 1.005 | 0.033 | 7.064 | 0.027 | 22.605 | 0.001 | 4.485 | 0.043 | |
| f | 0.009 | 0.847 | 254.365 | 0.000 | 126.111 | 0.000 | 336.524 | 0.000 | 9.680 | 0.010 | |
| Vibrio density | q | 0.001 | 0.888 | 101.898 | 0.000 | 43.015 | 0.000 | 88.474 | 0.000 | 4.044 | 0.062 |
| f × q | 0.000 | 0.908 | 16.471 | 0.003 | 10.322 | 0.009 | 19.093 | 0.002 | 0.225 | 0.575 | |
| f | 0.077 | 0.725 | 6.970 | 0.022 | 1.955 | 0.165 | 4.817 | 0.455 | 4.815 | 0.045 | |
| THCs | q | 0.077 | 0.725 | 79.297 | 0.000 | 0.575 | 0.408 | 13.967 | 0.004 | 7.194 | 0.021 |
| f × q | 0.187 | 0.816 | 2.741 | 0.110 | 7.625 | 0.018 | 1.552 | 0.207 | 0.951 | 0.305 | |
| f | 0.004 | 0.865 | 60.531 | 0.000 | 159.490 | 0.000 | 148.126 | 0.000 | 55.695 | 0.000 | |
| HEM | q | 0.298 | 0.561 | 185.007 | 0.000 | 454.173 | 0.000 | 731.756 | 0.000 | 393.622 | 0.000 |
| f × q | 0.024 | 0.805 | 18.015 | 0.002 | 27.887 | 0.001 | 8.339 | 0.015 | 26.103 | 0.001 | |
| f | 0.207 | 0.615 | 11.409 | 0.007 | 0.007 | 0.846 | 45.589 | 0.000 | 37.403 | 0.000 | |
| Antibacterial activity | q | 0.207 | 0.615 | 9.973 | 0.010 | 1.385 | 0.229 | 199.579 | 0.000 | 702.337 | 0.000 |
| f × q | 0.207 | 0.615 | 6.273 | 0.027 | 0.561 | 0.414 | 15.581 | 0.003 | 25.974 | 0.001 | |
| f | 0.001 | 0.890 | 0.360 | 0.497 | 1.125 | 0.271 | 13.069 | 0.005 | 7.017 | 0.022 | |
| PO activity | q | 0.007 | 0.853 | 18.273 | 0.002 | 22.375 | 0.001 | 82.919 | 0.000 | 42.334 | 0.000 |
| f × q | 0.011 | 0.841 | 1.504 | 0.213 | 0.115 | 0.665 | 4.471 | 0.039 | 0.204 | 0.589 | |
| f | 0.150 | 0.656 | 0.225 | 0.575 | 7.653 | 0.018 | 82.949 | 0.000 | 27.931 | 0.001 | |
| SOD activity | q | 0.025 | 0.802 | 3.792 | 0.068 | 10.656 | 0.008 | 182.416 | 0.000 | 38.814 | 0.000 |
| f × q | 0.013 | 0.834 | 1.172 | 0.263 | 10.821 | 0.008 | 0.126 | 0.654 | 3.592 | 0.075 | |
| f | 0.021 | 0.812 | 16.397 | 0.003 | 17.254 | 0.002 | 94.145 | 0.000 | 114.238 | 0.000 | |
| GSH-Px activity | q | 0.008 | 0.852 | 25.453 | 0.001 | 34.565 | 0.000 | 187.856 | 0.000 | 221.828 | 0.000 |
| f × q | 0.050 | 0.762 | 10.680 | 0.008 | 14.396 | 0.004 | 5.835 | 0.032 | 2.126 | 0.150 | |
| f | 0.006 | 0.858 | 9.263 | 0.012 | 18.371 | 0.002 | 13.245 | 0.005 | 26.161 | 0.001 | |
| LZM activity | q | 0.000 | 0.898 | 20.739 | 0.001 | 36.595 | 0.000 | 31.422 | 0.000 | 102.075 | 0.000 |
| f × q | 0.007 | 0.853 | 1.969 | 0.163 | 7.939 | 0.016 | 11.225 | 0.007 | 11.276 | 0.007 | |
| f | 0.000 | 0.897 | 12.535 | 0.006 | 112.852 | 0.000 | 161.979 | 0.000 | 110.784 | 0.000 | |
| ACP activity | q | 0.000 | 0.897 | 35.606 | 0.000 | 349.793 | 0.000 | 710.563 | 0.000 | 499.029 | 0.000 |
| f × q | 0.008 | 0.850 | 11.229 | 0.007 | 27.126 | 0.001 | 55.475 | 0.000 | 53.592 | 0.000 | |
| f | 0.002 | 0.879 | 23.603 | 0.001 | 44.391 | 0.000 | 75.945 | 0.000 | 71.283 | 0.000 | |
| AKP activity | q | 0.019 | 0.818 | 68.846 | 0.000 | 112.016 | 0.000 | 150.743 | 0.000 | 170.969 | 0.000 |
| f × q | 0.002 | 0.879 | 16.289 | 0.003 | 18.241 | 0.002 | 52.947 | 0.000 | 39.405 | 0.000 | |
| f | 0.116 | 0.686 | 54.434 | 0.000 | 310.115 | 0.000 | 55.365 | 0.000 | 55.063 | 0.000 | |
| Alf expression | q | 0.116 | 0.686 | 101.094 | 0.000 | 1168.187 | 0.000 | 168.119 | 0.000 | 124.353 | 0.000 |
| f × q | 0.013 | 0.834 | 37.731 | 0.000 | 238.163 | 0.000 | 41.080 | 0.000 | 39.807 | 0.000 | |
| f | 0.052 | 0.758 | 95.303 | 0.000 | 94.525 | 0.000 | 138.370 | 0.000 | 95.035 | 0.000 | |
| CatB expression | q | 0.052 | 0.758 | 528.251 | 0.000 | 214.873 | 0.000 | 513.988 | 0.000 | 389.612 | 0.000 |
| f × q | 0.000 | 0.909 | 92.325 | 0.000 | 84.915 | 0.000 | 127.617 | 0.000 | 89.241 | 0.000 | |
| f | 0.052 | 0.758 | 69.401 | 0.000 | 108.255 | 0.000 | 68.537 | 0.000 | 69.328 | 0.000 | |
| Cru expression | q | 0.052 | 0.758 | 200.568 | 0.000 | 179.874 | 0.000 | 199.446 | 0.000 | 135.883 | 0.000 |
| f × q | 0.000 | 0.909 | 39.513 | 0.000 | 52.957 | 0.000 | 40.079 | 0.000 | 46.409 | 0.000 | |
| f | 0.000 | 0.909 | 55.345 | 0.000 | 66.504 | 0.000 | 70.715 | 0.000 | 82.324 | 0.000 | |
| Lec expression | q | 0.000 | 0.909 | 106.820 | 0.000 | 245.946 | 0.000 | 297.324 | 0.000 | 227.333 | 0.000 |
| f × q | 0.052 | 0.758 | 40.208 | 0.000 | 52.042 | 0.000 | 57.150 | 0.000 | 66.246 | 0.000 | |
| f | 0.013 | 0.833 | 8.292 | 0.015 | 8.615 | 0.014 | 5.177 | 0.040 | 1.989 | 0.161 | |
| Lzm expression | q | 0.116 | 0.685 | 7.275 | 0.020 | 65.155 | 0.000 | 12.109 | 0.005 | 0.405 | 0.476 |
| f × q | 0.116 | 0.685 | 3.732 | 0.070 | 4.846 | 0.043 | 1.791 | 0.180 | 1.108 | 0.275 | |
| f | 0.000 | 0.909 | 73.174 | 0.000 | 140.643 | 0.000 | 71.193 | 0.000 | 49.602 | 0.000 | |
| Tlr expression | q | 0.045 | 0.767 | 1291.659 | 0.000 | 1349.896 | 0.000 | 516.204 | 0.000 | 474.346 | 0.000 |
| f × q | 0.045 | 0.767 | 67.100 | 0.000 | 93.076 | 0.000 | 40.728 | 0.000 | 36.800 | 0.000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Q.; Chang, Z.; Li, J.; Li, J. Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei. Antibiotics 2022, 11, 1784. https://doi.org/10.3390/antibiotics11121784
Zhai Q, Chang Z, Li J, Li J. Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei. Antibiotics. 2022; 11(12):1784. https://doi.org/10.3390/antibiotics11121784
Chicago/Turabian StyleZhai, Qianqian, Zhiqiang Chang, Jitao Li, and Jian Li. 2022. "Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei" Antibiotics 11, no. 12: 1784. https://doi.org/10.3390/antibiotics11121784
APA StyleZhai, Q., Chang, Z., Li, J., & Li, J. (2022). Protective Effects of Combined Utilization of Quercetin and Florfenicol on Acute Hepatopancreatic Necrosis Syndrome Infected Litopenaeus vannamei. Antibiotics, 11(12), 1784. https://doi.org/10.3390/antibiotics11121784






