Antibiotic Use in European Pig Production: Less Is More
Abstract
:1. Introduction
2. The Ban on Growth Promotors in Europe
3. Systematic Monitoring of Antibiotic Sales Data in Animals in Europe
4. Quantitative Insights in Antimicrobial Use in Pigs in Europe
4.1. Surveys on Antibiotic Use in Pigs in Europe
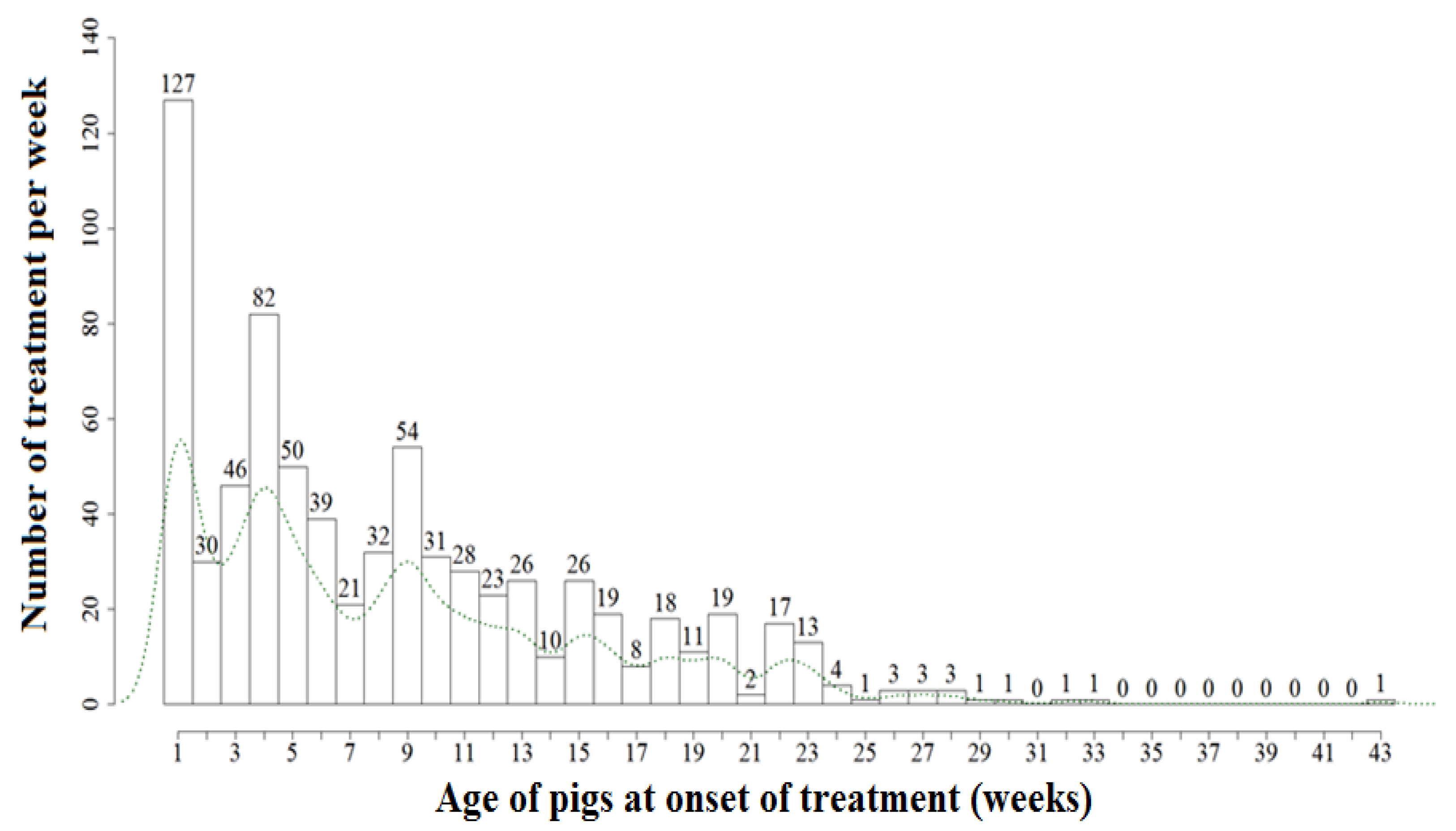
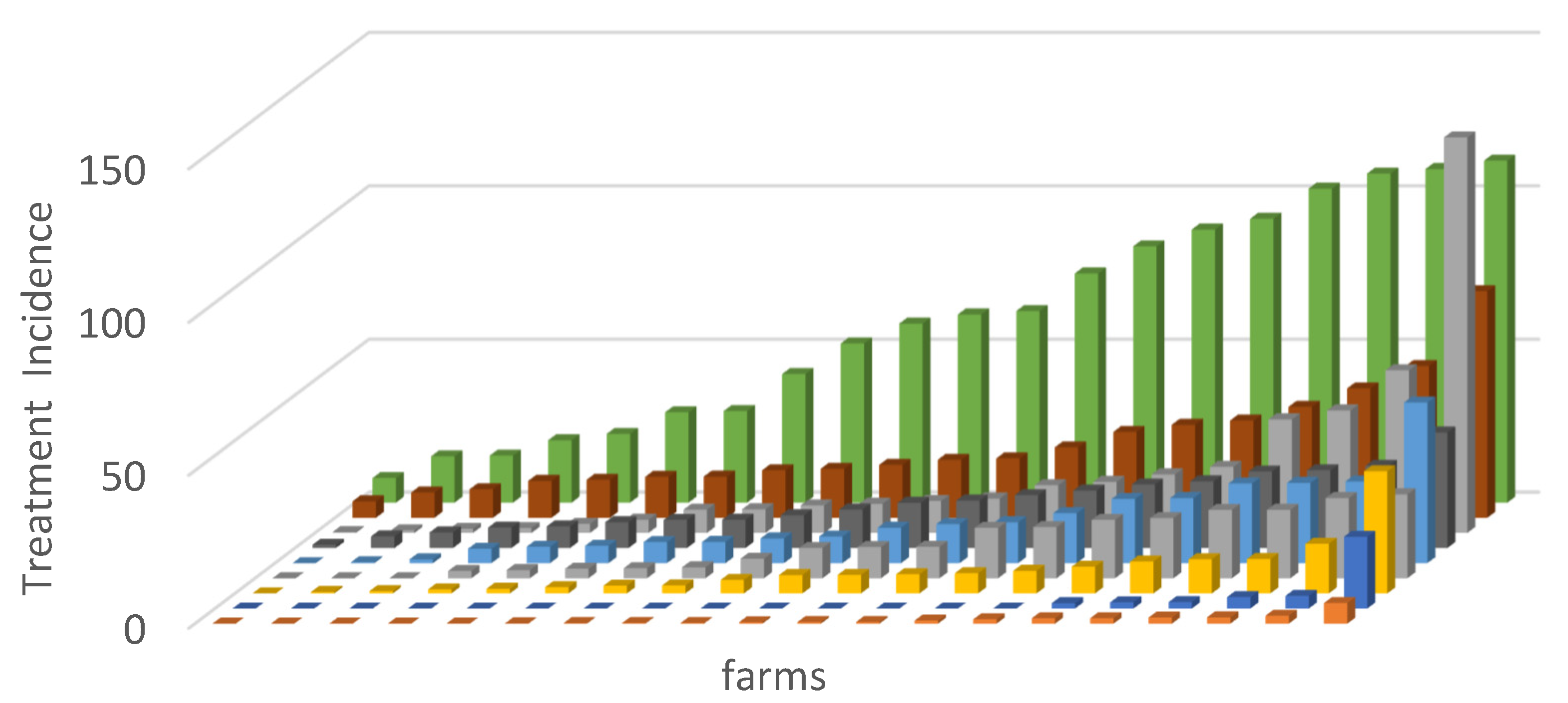
4.2. Herd Level Monitoring of Use Data
5. Antimicrobial Use in Pig Production in Europe: The Way Forward
5.1. Better Health Management and Biosecurity
5.2. Towards Zero Antimicrobial Use
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw-Taylor, L. An introduction to the history of infectious diseases, epidemics and the early phases of the long-run decline in mortality. Econ. Hist. Rev. 2020, 73, E1–E19. [Google Scholar] [CrossRef]
- Cars, O.; Hogberg, L.D.; Murray, M.; Nordberg, O.; Sivaraman, S.; Lundborg, C.S.; So, A.D.; Tomson, G. Meeting the challenge of antibiotic resistance. BMJ-Brit. Med. J. 2008, 337, a1438. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, A.M. Animals and antibiotics. Int. J. Antimicrob. Agents 2001, 18, 291–294. [Google Scholar] [CrossRef]
- Schwarz, S.; Chaslus-Dancla, E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef]
- Gustafson, R.H.; Bowen, R.E. Antibiotic use in animal agriculture. J. Appl. Microbiol. 1997, 83, 531–541. [Google Scholar] [CrossRef]
- Steinfeld, H. The livestock revolution—A global veterinary mission. Vet. Parasitol. 2004, 125, 19–41. [Google Scholar] [CrossRef]
- Nielsen, C.L.; Kongsted, H.; Sorensen, J.T.; Krogh, M.A. Antibiotic and medical zinc oxide usage in Danish conventional and welfare-label pig herds in 2016–2018. Prev. Vet. Med. 2021, 189, 105283. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fevre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlstrom, R.C. The effect of high level antibiotic supplementation during part or all of the growing-fattening period of swine. J. Anim. Sci. 1956, 15, 1059–1066. [Google Scholar] [CrossRef]
- Hanson, L.E.; Ferrin, E.F.; Anderson, P.A.; Aunan, W.J. Growth and Carcass Characteristics of Pigs Fed Antibiotics for Part or All of the Growing-Fattening Period. J. Anim. Sci. 1955, 14, 30–42. [Google Scholar] [CrossRef]
- Barber, R.S.; Braude, R.; Mitchell, K.G. Comparison of the Growth-Promoting Effects of a Proprietary Trimethylalkylammonium Stearate and a Proprietary Antibiotic for Fattening Pigs. Chem. Ind.-Lond 1958, 7, 18–19. [Google Scholar]
- Laxminarayan, R.; Van Boeckel, T.; Teillant, A. The economic costs of withdrawing antimicrobial growth promoters from the livestock sector. OECD Food Agric. Fish. Pap. 2015, 78, 78. [Google Scholar] [CrossRef]
- Swedish Ministry of Agriculture. Report from the Commission on Antimicrobial Feed Additives; Government Official Reports; Swedish Ministry of Agriculture: Stockholm, Sweden, 1997. Available online: http://www.regeringskansliet.se/rattsdokument/statens-offentliga-utredningar/1997/01/sou-1997132/ (accessed on 12 September 2022).
- Bos, M.E.; Taverne, F.J.; van Geijlswijk, I.M.; Mouton, J.W.; Mevius, D.J.; Heederik, D.J.; Authority, N.V.M. Consumption of antimicrobials in pigs, veal calves, and broilers in the Netherlands: Quantitative results of nationwide collection of data in 2011. PLoS ONE 2013, 8, e77525. [Google Scholar] [CrossRef]
- Grave, K.; Jensen, V.F.; Odensvik, K.; Wierup, M.; Bangen, M. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev. Vet. Med. 2006, 75, 123–132. [Google Scholar] [CrossRef]
- Grave, K.; Kaldhusdal, M.; Kruse, H.; Harr, L.M.F.; Flatlandsmo, K. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev. Vet. Med. 2004, 62, 59–72. [Google Scholar] [CrossRef]
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf (accessed on 12 September 2022).
- Postma, M.; Speksnijder, D.C.; Jaarsma, A.D.C.; Verheij, T.J.M.; Wagenaar, J.A.; Dewulf, J. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Vet. Rec. 2016, 179, 68. [Google Scholar] [CrossRef]
- Laanen, M.; Maes, D.; Hendriksen, C.; Gelaude, P.; De Vliegher, S.; Rosseel, Y.; Dewulf, J. Pig, cattle and poultry farmers with a known interest in research have comparable perspectives on disease prevention and on-farm biosecurity. Prev. Vet. Med. 2014, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stygar, A.H.; Chantziaras, I.; Toppari, I.; Maes, D.; Niemi, J.K. High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Animal 2020, 14, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Vet. Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; Emborg, H.D.; Aarestrup, F.M. Indications and patterns of therapeutic use of antimicrobial agents in the Danish pig production from 2002 to 2008. J. Vet. Pharmacol. Ther. 2012, 35, 33–46. [Google Scholar] [CrossRef]
- Moreno, M.A. Survey of quantitative antimicrobial consumption per production stage in farrow-to-finish pig farms in Spain. Vet. Rec. Open 2014, 1, e000002. [Google Scholar] [CrossRef] [Green Version]
- Hemme, M.; Ruddat, I.; Hartmann, M.; Werner, N.; van Rennings, L.; Kasbohrer, A.; Kreienbrock, L. Antibiotic use on German pig farms—A longitudinal analysis for 2011, 2013 and 2014. PLoS ONE 2018, 13, e0199592. [Google Scholar] [CrossRef]
- van Rennings, L.; von Munchhausen, C.; Ottilie, H.; Hartmann, M.; Merle, R.; Honscha, W.; Kasbohrer, A.; Kreienbrock, L. Cross-Sectional Study on Antibiotic Usage in Pigs in Germany. PLoS ONE 2015, 10, e0119114. [Google Scholar] [CrossRef] [Green Version]
- Sjolund, M.; Backhans, A.; Greko, C.; Emanuelson, U.; Lindberg, A. Antimicrobial usage in 60 Swedish farrow-to-finish pig herds. Prev. Vet. Med. 2015, 121, 257–264. [Google Scholar] [CrossRef]
- Hemonic, A.; Chauvin, C.; Delzescaux, D.; Verliat, F.; Correge, I.; antimicrobia, F.W.G. Reliable estimation of antimicrobial use and its evolution between 2010 and 2013 in French swine farms. Porc. Health Manag. 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Scali, F.; Santucci, G.; Maisano, A.M.; Giudici, F.; Guadagno, F.; Tonni, M.; Amicabile, A.; Formenti, N.; Giacomini, E.; Lazzaro, M.; et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Scoppetta, F.; Sensi, M.; Franciosini, M.P.; Capuccella, M. Evaluation of antibiotic usage in swine reproduction farms in Umbria region based on the quantitative analysis of antimicrobial consumption. Ital. J. Food Saf. 2017, 6, 112–119. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.; da Costa, M.R.; Leonard, F.C.; Gibbons, J.; Diaz, J.A.C.; McCutcheon, G.; Manzanilla, E.G. Quantification, description and international comparison of antimicrobial use on Irish pig farms. Porc. Health Manag. 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Sali, V.; Nykaesenoja, S.; Heikinheimo, A.; Haelli, O.; Tirkkonen, T.; Heinonen, M. Antimicrobial Use and Susceptibility of Indicator Escherichia coli in Finnish Integrated Pork Production. Front. Microbiol. 2021, 12, 754894. [Google Scholar] [CrossRef]
- Yun, J.; Muurinen, J.; Nykasenoja, S.; Seppa-Lassila, L.; Sali, V.; Suomi, J.; Tuominen, P.; Joutsen, S.; Hamalainen, M.; Olkkola, S.; et al. Antimicrobial use, biosecurity, herd characteristics, and antimicrobial resistance in indicator Escherichia coli in ten Finnish pig farms. Prev. Vet. Med. 2021, 193, 105408. [Google Scholar] [CrossRef]
- Echtermann, T.; Muentener, C.; Sidler, X.; Kuemmerlen, D. Antimicrobial Usage Among Different Age Categories and Herd Sizes in Swiss Farrow-to-Finish Farms. Front. Vet. Sci. 2020, 7, 566529. [Google Scholar] [CrossRef]
- Collineau, L.; Belloc, C.; Stark, K.D.C.; Hemonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the Selection of Appropriate Indicators for Quantification of Antimicrobial Usage in Humans and Animals. Zoonoses Public Health 2017, 64, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Postma, M.; Vanderhaeghen, W.; Sarrazin, S.; Maes, D.; Dewulf, J. Reducing Antimicrobial Usage in Pig Production without Jeopardizing Production Parameters. Zoonoses Public Health 2017, 64, 63–74. [Google Scholar] [CrossRef]
- Postma, M.; Sjolund, M.; Collineau, L.; Losken, S.; Stark, K.D.C.; Dewulf, J.; Consortium, M. Assigning defined daily doses animal: A European multi-country experience for antimicrobial products authorized for usage in pigs. J. Antimicrob. Chemother. 2015, 70, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Sjolund, M.; Postma, M.; Collineau, L.; Losken, S.; Backhans, A.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stark, K.; Dewulf, J.; et al. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Prev. Vet. Med. 2016, 130, 41–50. [Google Scholar] [CrossRef]
- Visschers, V.H.M.; Postma, M.; Sjolund, M.; Backhans, A.; Collineau, L.; Loesken, S.; Belloc, C.; Dewulf, J.; Emanuelson, U.; Beilage, E.G.; et al. Higher perceived risks of antimicrobial use are related to lower usage among pig farmers in four European countries. Vet. Rec. 2016, 179, 490. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Dewulf, J.; Van Limbergen, T.; Klinkenberg, M.; Palzer, A.; Pineiro, C.; Moustsen, V.A.; Niemi, J.; Kyriazakis, I.; Maes, D. Factors associated with specific health, welfare and reproductive performance indicators in pig herds from five EU countries. Prev. Vet. Med. 2018, 159, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Sibila, M.; Pieters, M.; Haesebrouck, F.; Segalés, J.; Guilherme de Oliveira, L. Assessing respiratory tract lesions in pigs at slaughter: What does it (not) teach us? Vet. Res. 2022; submitted. [Google Scholar]
- Chantziaras, I.; Dewulf, J.; Van Limbergen, T.; Stadejek, T.; Niemi, J.; Kyriazakis, I.; Maes, D. Biosecurity levels of pig fattening farms from four EU countries and links with the farm characteristics. Livest. Sci. 2020, 237, 104037. [Google Scholar] [CrossRef]
- Caekebeke, N.; Ringenier, M.; Jonquiere, F.J.; Tobias, T.J.; Postma, M.; van den Hoogen, A.; Houben, M.A.M.; Velkers, F.C.; Sleeckx, N.; Stegeman, A.; et al. Coaching Belgian and Dutch Broiler Farmers Aimed at Antimicrobial Stewardship and Disease Prevention. Antibiotics 2021, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.; McKernan, C.; Benson, T.; Elliott, C.; Dean, M. Understanding farmers’ and veterinarians’ behavior in relation to antimicrobial use and resistance in dairy cattle: A systematic review. J. Dairy Sci. 2021, 104, 4584–4603. [Google Scholar] [CrossRef]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef]
- Collineau, L.; Rojo-Gimeno, C.; Leger, A.; Backhans, A.; Loesken, S.; Nielsen, E.O.; Postma, M.; Emanuelson, U.; Beilage, E.G.; Sjolund, M.; et al. Herd-specific interventions to reduce antimicrobial usage in pig production without jeopardising technical and economic performance. Prev. Vet. Med. 2017, 144, 167–178. [Google Scholar] [CrossRef]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Hog, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef]
- AACTING. Network on Quantification of Veterinary Antimicrobial Usage at Herd Level and Analysis, Communication and Benchmarking to Improve Responsible Usage. Available online: https://aacting.org/ (accessed on 12 September 2022).
- BelVetSac. Belgian Veterinary Surveillance of Antibacterial Consumption—National Consumption Report. 2021. Available online: https://belvetsac.ugent.be/belvetsac_SaniMed_rapport_2020.pdf (accessed on 12 September 2022).
- Postma, M.; Stark, K.D.; Sjolund, M.; Backhans, A.; Beilage, E.G.; Losken, S.; Belloc, C.; Collineau, L.; Iten, D.; Visschers, V.; et al. Alternatives to the use of antimicrobial agents in pig production: A multi-country expert-ranking of perceived effectiveness, feasibility and return on investment. Prev. Vet. Med. 2015, 118, 457–466. [Google Scholar] [CrossRef]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef]
- Raasch, S.; Postma, M.; Dewulf, J.; Stark, K.D.C.; Beilage, E.G. Association between antimicrobial usage, biosecurity measures as well as farm performance in German farrow-to-finish farms. Porc. Health Manag. 2018, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, N.; Diness, L.H.; Fertner, M.; Kristensen, C.S.; Stege, H. Antimicrobial reduction measures applied in Danish pig herds following the introduction of the “Yellow Card” antimicrobial scheme. Prev. Vet. Med. 2017, 138, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, W.; Dorado-Garcia, A.; Bonten, M.J.M.; Wagenaar, J.A.; Mevius, D.; Heederik, D.J.J. Risk factors for ESBL-producing Escherichia coli on pig farms: A longitudinal study in the context of reduced use of antimicrobials. PLoS ONE 2017, 12, e0174094. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Gimeno, C.; Postma, M.; Dewulf, J.; Hogeveen, H.; Lauwers, L.; Wauters, E. Farm-economic analysis of reducing antimicrobial use whilst adopting improved management strategies on farrow-to-finish pig farms. Prev. Vet. Med. 2016, 129, 74–87. [Google Scholar] [CrossRef] [PubMed]
- DANMAP. Use of Antimicrobial Agents and Occurence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2018. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/210256829/DANMAP_2018_1_.pdf (accessed on 12 September 2022).
- NSF. Raised without Antibiotics Certification. Available online: https://www.nsf.org/testing/food/food-beverage-product-certification/raised-without-antibiotics-certification (accessed on 12 September 2022).
- Bernaerdt, E.; Maes, D.; Van Limbergen, T.; Postma, M.; Dewulf, J. Determining the Characteristics of Farms That Raise Pigs without Antibiotics. Animals 2022, 12, 1224. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewulf, J.; Joosten, P.; Chantziaras, I.; Bernaerdt, E.; Vanderhaeghen, W.; Postma, M.; Maes, D. Antibiotic Use in European Pig Production: Less Is More. Antibiotics 2022, 11, 1493. https://doi.org/10.3390/antibiotics11111493
Dewulf J, Joosten P, Chantziaras I, Bernaerdt E, Vanderhaeghen W, Postma M, Maes D. Antibiotic Use in European Pig Production: Less Is More. Antibiotics. 2022; 11(11):1493. https://doi.org/10.3390/antibiotics11111493
Chicago/Turabian StyleDewulf, Jeroen, Philip Joosten, Ilias Chantziaras, Elise Bernaerdt, Wannes Vanderhaeghen, Merel Postma, and Dominiek Maes. 2022. "Antibiotic Use in European Pig Production: Less Is More" Antibiotics 11, no. 11: 1493. https://doi.org/10.3390/antibiotics11111493
APA StyleDewulf, J., Joosten, P., Chantziaras, I., Bernaerdt, E., Vanderhaeghen, W., Postma, M., & Maes, D. (2022). Antibiotic Use in European Pig Production: Less Is More. Antibiotics, 11(11), 1493. https://doi.org/10.3390/antibiotics11111493







