Outcomes of Beta-Lactam Allergic and Non-Beta-Lactam Allergic Patients with Intra-Abdominal Infection: A Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Isolated Pathogens, Antimicrobial Resistance, and Antibiotic Treatments
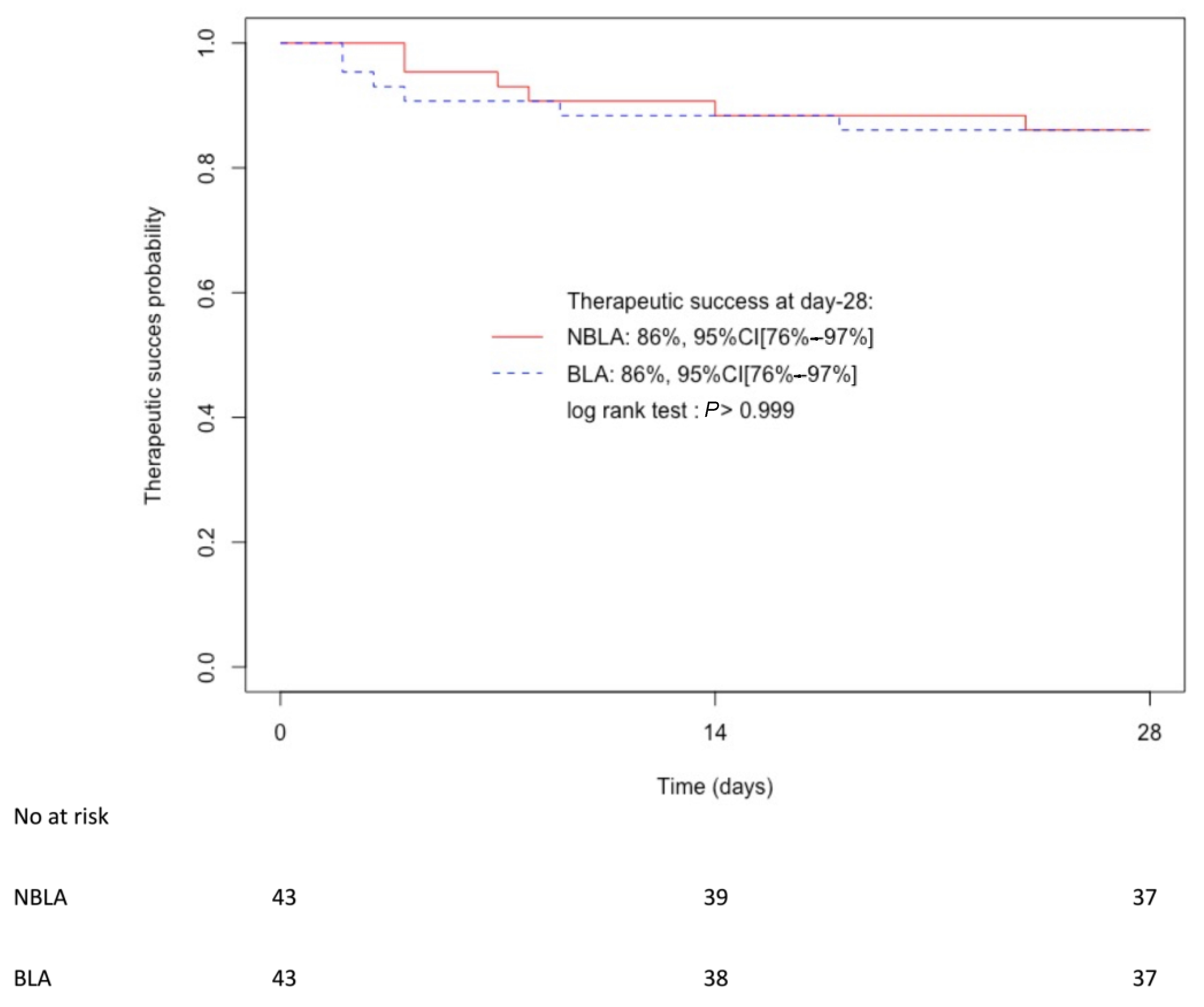
2.3. Outcomes
3. Discussion
4. Materials and Methods
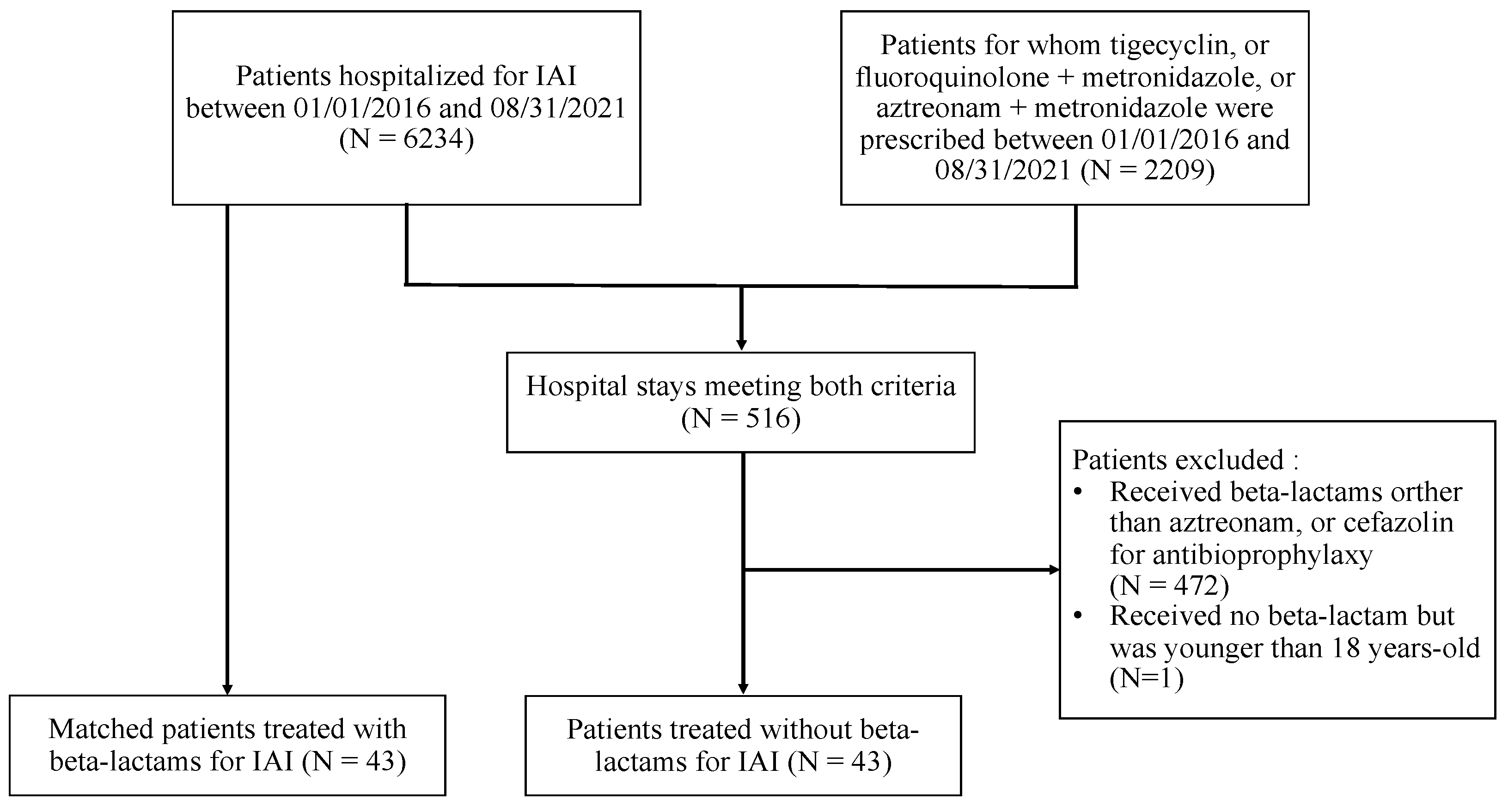
4.1. Study Design and Settings
4.2. Patients
4.3. Study Definitions
4.4. Data Collection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Brismar, B.; Nord, C.E. Monobactams and Carbapenems for Treatment of Intraabdominal Infections. Infection 1999, 27, 136–147. [Google Scholar] [CrossRef]
- Vogelaers, D.; Blot, S.; Van den Berge, A.; Montravers, P. Abdominal Sepsis Study (‘AbSeS’) Group on behalf of the Trials Group of the European Society of Intensive Care Medicine Antimicrobial Lessons from a Large Observational Cohort on Intra-Abdominal Infections in Intensive Care Units. Drugs 2021, 81, 1065–1078. [Google Scholar] [CrossRef]
- Montravers, P.; Dupont, H.; Leone, M.; Constantin, J.-M.; Mertes, P.-M.; Société Française D’anesthésie et de Réanimation (Sfar); Société de Réanimation de Langue Française (SRLF); Laterre, P.-F.; Misset, B.; Société de Pathologie Infectieuse de Langue Française (SPILF); et al. Guidelines for Management of Intra-Abdominal Infections. Anaesth. Crit. Care Pain Med. 2015, 34, 117–130. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-Abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Tessier, J.M.; May, A.K.; Sawyer, R.G.; Nadler, E.P.; Rosengart, M.R.; Chang, P.K.; O’Neill, P.J.; Mollen, K.P.; Huston, J.M.; et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg. Infect. 2017, 18, 1–76. [Google Scholar] [CrossRef]
- Sacco, K.A.; Bates, A.; Brigham, T.J.; Imam, J.S.; Burton, M.C. Clinical Outcomes Following Inpatient Penicillin Allergy Testing: A Systematic Review and Meta-Analysis. Allergy 2017, 72, 1288–1296. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Macy, E.; Rowe, T.; Blumenthal, K.G. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019, 321, 188–199. [Google Scholar] [CrossRef]
- Wilhelm, N.B.; Bonsall, T.J.; Miller, C.L. The Effect of Beta-Lactam Allergy Status on the Rate of Surgical Site Infections: A Retrospective Cohort Study. Ann. Surg. 2022, 275, 208–212. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Ryan, E.E.; Li, Y.; Lee, H.; Kuhlen, J.L.; Shenoy, E.S. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin. Infect. Dis. 2018, 66, 329–336. [Google Scholar] [CrossRef]
- Kuriakose, J.P.; Vu, J.; Karmakar, M.; Nagel, J.; Uppal, S.; Hendren, S.; Englesbe, M.J.; Ravikumar, R.; Campbell, D.A.; Krapohl, G.L. β-Lactam vs Non-β-Lactam Antibiotics and Surgical Site Infection in Colectomy Patients. J. Am. Coll. Surg. 2019, 229, 487–496.e2. [Google Scholar] [CrossRef]
- Lam, P.W.; Tarighi, P.; Elligsen, M.; Gunaratne, K.; Nathens, A.B.; Tarshis, J.; Leis, J.A. Self-Reported Beta-Lactam Allergy and the Risk of Surgical Site Infection: A Retrospective Cohort Study. Infect. Control Hosp. Epidemiol. 2020, 41, 438–443. [Google Scholar] [CrossRef]
- Jeffres, M.N.; Narayanan, P.P.; Shuster, J.E.; Schramm, G.E. Consequences of Avoiding β-Lactams in Patients with β-Lactam Allergies. J. Allergy Clin. Immunol. 2016, 137, 1148–1153. [Google Scholar] [CrossRef]
- Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 23 August 2022).
- Mavros, M.N.; Theochari, N.A.; Kyriakidou, M.; Economopoulos, K.P.; Sava, J.A.; Falagas, M.E. Fluoroquinolone-Based versus β-Lactam-Based Regimens for Complicated Intra-Abdominal Infections: A Meta-Analysis of Randomised Controlled Trials. Int J. Antimicrob. Agents 2019, 53, 746–754. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Reinhart, H.H.; Dellinger, E.P.; Bohnen, J.M.; Rotstein, O.D.; Vogel, S.B.; Simms, H.H.; Hill, C.S.; Bjornson, H.S.; Haverstock, D.C.; et al. Results of a Randomized Trial Comparing Sequential Intravenous/Oral Treatment with Ciprofloxacin plus Metronidazole to Imipenem/Cilastatin for Intra-Abdominal Infections. The Intra-Abdominal Infection Study Group. Ann. Surg. 1996, 223, 303–315. [Google Scholar] [CrossRef]
- Cohn, S.M.; Lipsett, P.A.; Buchman, T.G.; Cheadle, W.G.; Milsom, J.W.; O’Marro, S.; Yellin, A.E.; Jungerwirth, S.; Rochefort, E.V.; Haverstock, D.C.; et al. Comparison of Intravenous/Oral Ciprofloxacin Plus Metronidazole Versus Piperacillin/Tazobactam in the Treatment of Complicated Intraabdominal Infections. Ann. Surg. 2000, 232, 254–262. [Google Scholar] [CrossRef]
- Malangoni, M.A.; Song, J.; Herrington, J.; Choudhri, S.; Pertel, P. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann. Surg. 2006, 244, 204–211. [Google Scholar] [CrossRef]
- Wacha, H.; Warren, B.; Bassaris, H.; Nikolaidis, P. Intra-Abdominal Infections Study Group Comparison of Sequential Intravenous/Oral Ciprofloxacin plus Metronidazole with Intravenous Ceftriaxone plus Metronidazole for Treatment of Complicated Intra-Abdominal Infections. Surg. Infect. 2006, 7, 341–354. [Google Scholar] [CrossRef]
- Solomkin, J.; Zhao, Y.-P.; Ma, E.-L.; Chen, M.-J.; Hampel, B. DRAGON Study Team Moxifloxacin Is Non-Inferior to Combination Therapy with Ceftriaxone plus Metronidazole in Patients with Community-Origin Complicated Intra-Abdominal Infections. Int. J. Antimicrob. Agents 2009, 34, 439–445. [Google Scholar] [CrossRef]
- Weiss, G.; Reimnitz, P.; Hampel, B.; Muehlhofer, E.; Lippert, H. Moxifloxacin for the Treatment of Patients with Complicated Intra-Abdominal Infections (the AIDA Study). J. Chemother. 2009, 21, 170–180. [Google Scholar] [CrossRef]
- De Waele, J.J.; Tellado, J.M.; Alder, J.; Reimnitz, P.; Jensen, M.; Hampel, B.; Arvis, P. Randomised Clinical Trial of Moxifloxacin versus Ertapenem in Complicated Intra-Abdominal Infections: Results of the PROMISE Study. Int. J. Antimicrob. Agents 2013, 41, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.; del Castillo, M.; Yi, A.; Gotuzzo, E. Clindamycin plus Amikacin versus Clindamycin plus Aztreonam in Established Intraabdominal Infections. Surgery 1994, 116, 28–35. [Google Scholar] [PubMed]
- de Groot, H.G.W.; Hustinx, P.A.; Lampe, A.S.; Oosterwijk, W.M. Comparison of Imipenem/Cilastatin with the Combination of Aztreonam and Clindamycin in the Treatment of Intra-Abdominal Infections. J. Antimicrob. Chemother. 1993, 32, 491–500. [Google Scholar] [CrossRef]
- Mosdell, D.M.; Twiest, M.W. Antibiotic Treatment for Surgical Peritonitis. Ann. Surg. 1991, 214, 543. [Google Scholar] [CrossRef]
- Baré, M.; Castells, X.; Garcia, A.; Riu, M.; Comas, M.; Egea, M.J.G. Importance of Appropriateness of Empiric Antibiotic Therapy on Clinical Outcomes in Intra-Abdominal Infections. Int. J. Technol. Assess Health Care 2006, 22, 242–248. [Google Scholar] [CrossRef]
- Krobot, K.; Yin, D.; Zhang, Q.; Sen, S.; Altendorf-Hofmann, A.; Scheele, J.; Sendt, W. Effect of Inappropriate Initial Empiric Antibiotic Therapy on Outcome of Patients with Community-Acquired Intra-Abdominal Infections Requiring Surgery. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 682–687. [Google Scholar] [CrossRef]
- Rüddel, H.; Thomas-Rüddel, D.O.; Reinhart, K.; Bach, F.; Gerlach, H.; Lindner, M.; Marshall, J.C.; Simon, P.; Weiss, M.; Bloos, F.; et al. Adverse Effects of Delayed Antimicrobial Treatment and Surgical Source Control in Adults with Sepsis: Results of a Planned Secondary Analysis of a Cluster-Randomized Controlled Trial. Crit. Care 2022, 26, 51. [Google Scholar] [CrossRef]
- Takesue, Y.; Kusachi, S.; Mikamo, H.; Sato, J.; Watanabe, A.; Kiyota, H.; Iwata, S.; Kaku, M.; Hanaki, H.; Sumiyama, Y.; et al. Antimicrobial Susceptibility of Common Pathogens Isolated from Postoperative Intra-Abdominal Infections in Japan. J. Infect. Chemother. 2018, 24, 330–340. [Google Scholar] [CrossRef]
- Edelsberg, J.; Berger, A.; Schell, S.; Mallick, R.; Kuznik, A.; Oster, G. Economic Consequences of Failure of Initial Antibiotic Therapy in Hospitalized Adults with Complicated Intra-Abdominal Infections. Surg. Infect. 2008, 9, 335–347. [Google Scholar] [CrossRef]
- Edmiston, C.E.; Krepel, C.J.; Seabrook, G.R.; Somberg, L.R.; Nakeeb, A.; Cambria, R.A.; Towne, J.B. In Vitro Activities of Moxifloxacin against 900 Aerobic and Anaerobic Surgical Isolates from Patients with Intra-Abdominal and Diabetic Foot Infections. Antimicrob. Agents Chemother. 2004, 48, 1012–1016. [Google Scholar] [CrossRef][Green Version]
- Dupont, H.; Friggeri, A.; Touzeau, J.; Airapetian, N.; Tinturier, F.; Lobjoie, E.; Lorne, E.; Hijazi, M.; Régimbeau, J.-M.; Mahjoub, Y. Enterococci Increase the Morbidity and Mortality Associated with Severe Intra-Abdominal Infections in Elderly Patients Hospitalized in the Intensive Care Unit. J. Antimicrob. Chemother. 2011, 66, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Morvan, A.-C.; Hengy, B.; Garrouste-Orgeas, M.; Ruckly, S.; Forel, J.-M.; Argaud, L.; Rimmelé, T.; Bedos, J.-P.; Azoulay, E.; Dupuis, C.; et al. Impact of Species and Antibiotic Therapy of Enterococcal Peritonitis on 30-Day Mortality in Critical Care—An Analysis of the OUTCOMEREA Database. Crit. Care 2019, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, C.; Cherifi, S.; Karmali, R. Management and Outcome of High-Risk Peritonitis: A Retrospective Survey 2005–2009. Int. J. Infect. Dis. 2011, 15, e769–e773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, W.-Q.; Chen, W.; Wei, T.; Wang, C.-W.; Zhang, J.-Y.; Zhang, Y.; Liang, T.-B. Systematic Review and Meta-Analysis of the Efficacy of Appropriate Empiric Anti-Enterococcal Therapy for Intra-Abdominal Infection. Surg. Infect. 2021, 22, 131–143. [Google Scholar] [CrossRef]
- MIC EUCAST. Available online: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=-1&search%5Bspecies%5D=254&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 (accessed on 1 October 2022).
- Pilmis, B.; Lécuyer, H.; Lortholary, O.; Charlier, C. Enterococcus Faecalis-Related Prostatitis Successfully Treated with Moxifloxacin. Antimicrob. Agents Chemother. 2015, 59, 7156–7157. [Google Scholar] [CrossRef][Green Version]
- Khan, F.; Ismail, M.; Khan, Q.; Ali, Z. Moxifloxacin-Induced QT Interval Prolongation and Torsades de Pointes: A Narrative Review. Expert. Opin. Drug Saf. 2018, 17, 1029–1039. [Google Scholar] [CrossRef]
- Sotto, A. Evaluation of Antimicrobial Therapy Management of 120 Consecutive Patients with Secondary Peritonitis. J. Antimicrob. Chemother. 2002, 50, 569–576. [Google Scholar] [CrossRef]
- Lavigne, J.-P.; Nicolas-Chanoine, M.-H.; Bourg, G.; Moreau, J.; Sotto, A. Virulent Synergistic Effect between Enterococcus Faecalis and Escherichia Coli Assayed by Using the Caenorhabditis Elegans Model. PLoS ONE 2008, 3, e3370. [Google Scholar] [CrossRef]
- Montravers, P.; Lepape, A.; Dubreuil, L.; Gauzit, R.; Pean, Y.; Benchimol, D.; Dupont, H. Clinical and Microbiological Profiles of Community-Acquired and Nosocomial Intra-Abdominal Infections: Results of the French Prospective, Observational EBIIA Study. J. Antimicrob. Chemother. 2009, 63, 785–794. [Google Scholar] [CrossRef]
- Augustin, P.; Kermarrec, N.; Muller-Serieys, C.; Lasocki, S.; Chosidow, D.; Marmuse, J.-P.; Valin, N.; Desmonts, J.-M.; Montravers, P. Risk Factors for Multidrug Resistant Bacteria and Optimization of Empirical Antibiotic Therapy in Postoperative Peritonitis. Crit Care 2010, 14, R20. [Google Scholar] [CrossRef]
- ONERBA 2018 Anual Report [Internet]. Available online: http://onerba-doc.onerba.org/Rapports/Rapport-ONERBA-2018/Rap18_onerba_synthese.pdf (accessed on 27 October 2022).
- Thelwall, S.; Harrington, P.; Sheridan, E.; Lamagni, T. Impact of Obesity on the Risk of Wound Infection Following Surgery: Results from a Nationwide Prospective Multicentre Cohort Study in England. Clin. Microbiol. Infect. 2015, 21, 1008.e1–1008.e8. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Mui, E.; Holubar, M.K.; Deresinski, S.C. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy 2017, 37, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.; Kinzig, M.; Boivin, G.; Sörgel, F.; LeBel, M. Intravenous Ciprofloxacin Disposition in Obesity. Clin. Pharm. 1993, 54, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, P.G.; Bhavnani, S.M.; Owens, R.C. Clinical Pharmacodynamics of Quinolones. Infect. Dis. Clin. 2003, 17, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.; Guerci, P.; Pape, E.; Thilly, N.; Luc, A.; Germain, A.; Butin-Druoton, A.-L.; Losser, M.-R.; Birckener, J.; Scala-Bertola, J.; et al. Serum and Peritoneal Exudate Concentrations after High Doses of β-Lactams in Critically Ill Patients with Severe Intra-Abdominal Infections: An Observational Prospective Study. J. Antimicrob. Chemother. 2020, 75, 156–161. [Google Scholar] [CrossRef]
- Ramsey, C.; MacGowan, A.P. A Review of the Pharmacokinetics and Pharmacodynamics of Aztreonam. J. Antimicrob. Chemother. 2016, 71, 2704–2712. [Google Scholar] [CrossRef]
- Abaziou, T.; Vardon-Bounes, F.; Conil, J.-M.; Rouget, A.; Ruiz, S.; Grare, M.; Fourcade, O.; Suc, B.; Leone, M.; Minville, V.; et al. Outcome of Community- versus Hospital-Acquired Intra-Abdominal Infections in Intensive Care Unit: A Retrospective Study. BMC Anesth. 2020, 20, 295. [Google Scholar] [CrossRef]
- Lepape, A.; Machut, A.; Savey, A. Comité de Réa-Raisin Réseau national Réa-Raisin de surveillance des infections acquises en réanimation adulte: Méthodes et principaux résultats. Méd. Intensive Réa. 2018, 27, 197–203. [Google Scholar] [CrossRef]
- Enquête Nationale de Prévalence des Infections Nosocomiales et des Traitements Anti-Infectieux en Etablissements de Santé, France, Mai-Juin 2017. [Internet]. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/infections-associees-aux-soins/documents/enquetes-etudes/enquete-nationale-de-prevalence-des-infections-nosocomiales-et-des-traitements-anti-infectieux-en-etablissements-de-sante-mai-juin-2017 (accessed on 27 October 2022).
- Headley, J.; Theriault, R.; Smith, T.L. Independent Validation of Apache Ii Severity of Illness Score for Predicting Mortality in Patients with Breast Cancer Admitted to the Intensive Care Unit. Cancer 1992, 70, 497–503. [Google Scholar] [CrossRef]
- Patriarca, G.; Schiavino, D.; Lombardo, C.; Altomonte, G.; Decinti, M.; Buonomo, A.; Nucera, E. Tolerability of Aztreonam in Patients with IgE-Mediated Hypersensitivity to Beta-Lactams. Int. J. Immunopathol. Pharm. 2008, 21, 375–379. [Google Scholar] [CrossRef]
- Buonomo, A.; Nucera, E.; Pasquale, T.D.; Pecora, V.; Lombardo, C.; Sabato, V.; Colagiovanni, A.; Rizzi, A.; Aruanno, A.; Pascolini, L.; et al. Tolerability of Aztreonam in Patients with Cell-Mediated Allergy to β-Lactams. IAA 2011, 155, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vaccinations Des Personnes Immunodéprimées Ou Aspléniques. Recommandations. Available online: https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=322 (accessed on 1 October 2022).
- Healthcare-Associated Infections. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 1 October 2022).
- Complicated Intra-Abdominal Infections: Developing Drugs for Treatment Guidance for Industry. 2018, 20. [Internet]. Available online: https://www.fda.gov/media/84691/download (accessed on 27 October 2022).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
| Characteristics | BLA (N = 43) N (%) or Median (IQR) | NBLA (N = 43) N (%) or Median (IQR) |
|---|---|---|
| Women | 32 (74%) | 32 (74%) |
| Median age, years | 65 (47–76) | 66 (47–76) |
| Alcohol > 20 g/day | 2 (5%) | 1 (2%) |
| Tobacco use > 4 unit/day | 8 (19%) | 3 (7%) |
| Charlson index | 2 (1–7) | 3 (1–6) |
| History of abdominal surgery | 25 (58%) | 29 (67%) |
| Corticosteroid treatment | 2 (5%) | 0 (0%) |
| Other immunodeficiency | 1 (2%) | 4 (9%) |
| BMI (kg/m2) | 25.56 (23.52–29.59) | 25.36 (22.75–29.27) |
| Type of BL allergy | ||
| - Unspecified rash | 3 (7%) | - |
| - Urticaria | 6 (14%) | - |
| - Angio-oedema | 13 (30%) | - |
| - Grade III anaphylaxis | 1 (2%) | - |
| - Toxidermy | 4 (9%) | - |
| - Unknown | 16 (37%) | - |
| Type of infection | ||
| - Upper digestive tract | 21 (49%) | 21 (49%) |
| - Cholangitis | 11 (26%) | 11 (26%) |
| - Cholecystitis | 9 (21%) | 12 (28%) |
| - Appendicitis | 10 (23%) | 10 (23%) |
| - Diverticulitis | 7 (16%) | 5 (12%) |
| Complicated IAI: | 19 (44%) | 19 (44%) |
| - Peritonitis | 10 (23%) | 15 (35%) |
| - Intra-abdominal abscess | 9 (21%) | 4 (9%) |
| HLOS (days) | 5 (3–9.5) | 7 (4–11) |
| Maximal temperature (°C) | 37.3 (37–38.3) | 37.5 (31–38.6) |
| Maximal CRP (mg/L) | 174.5 (130.2–241.1) | 192 (97.1–333.2) |
| Maximal leukocytosis (G/L) | 14.22 (11.21–19.11) | 16.73 (12.88–21.82) |
| Apache II score | 9 (6–13) | 9 (6–14) |
| Healthcare-associated IAI | 6 (14%) | 6 (14%) |
| Co-infection at admission | 1 (2%) | 2 (5%) |
| Diagnostic delay (days) | 1 (1–4) | 2 (1–3) |
| Source control delay (days) | 2 (1–5) | 3 (2–6) |
| Source control procedure | 26 (60%) | 31 (72%) |
| - Laparotomy | 6 (14%) | 7 (16%) |
| - Laparoscopy | 15 (35%) | 15 (35%) |
| - Percutaneous drainage | 2 (5%) | 4 (9%) |
| - ERCP | 3 (7%) | 5 (12%) |
| Antimicrobial therapy | 43 (100%) | 43 (100%) |
| - fluoroquinolones | 42 (98%) | 0 (0%) |
| - aztreonam | 6 (14%) | 0 (0%) |
| - ceftriaxone | 0 (0%) | 21 (49%) |
| - cefotaxime | 0 (0%) | 20 (47%) |
| - piperacillin–tazobactam | 0 (0%) | 9 (21%) |
| - amoxicillin–clavulanic acid | 0 (0%) | 18 (42%) |
| - metronidazole | 43 (100%) | 36 (84%) |
| - aminoglycoside | 12 (28%) | 10 (23%) |
| - glycopeptide | 5 (12%) | 6 (14%) |
| - antifungal agent | 0 (0%) | 3 (7%) 1 |
| - duration (days) | 10 (7–14) | 10 (7–13) |
| Characteristics | BLA (N = 43) N (%) or Median (IQR) | NBLA (N = 43) N (%) or Median (IQR) | p-Value |
|---|---|---|---|
| Surgical samples collected | 17 (40%) | 26 (60%) | 0.054 |
| Bloodstream infection | 2 (5%) | 6 (14%) | 0.156 |
| Patients with positive cultures | 14 (33%) | 22 (51%) | 0.082 |
| Gram-positive cocci | 9 (21%) | 11 (26%) | 0.610 |
| - Enterococcus spp. | 6 (14%) | 5 (12%) | 0.747 |
| - Streptococcus spp. | 4 (9%) | 7 (16%) | 0.338 |
| Gram-negative bacilli | 13 (30%) | 18 (42%) | 0.263 |
| - Escherichia coli | 10 (23%) | 14 (33%) | 0.338 |
| - Proteus spp. | 2 (5%) | 1 (2%) | 0.564 |
| - Klebsiella pneumoniae and K. oxytoca | 3 (7%) | 4 (9%) | 0.694 |
| - Enterobacter spp. | 1 (2%) | 1 (2%) | >0.999 |
| - Citrobacter spp. | 1 (2%) | 1 (2%) | >0.999 |
| - Pseudomonas aeruginosa | 2 (5%) | 0 (0%) | 0.992 |
| Anaerobes | 8 (19%) | 10 (23%) | 0.597 |
| - Bacteroides spp. | 6 (14%) | 3 (7%) | 0.299 |
| Candida spp. | 0 (0%) | 3 (7%) | 0.990 |
| Antimicrobial resistance in Enterobacterales | |||
| 3GC-resistant | 0/17 (0%) | 3/23 (13%) | |
| FQ-resistant | 2/17 (12%) | 3/23 (13%) | |
| Total | 2/17 (12%) | 6/23 (26%) | |
| Antimicrobial therapy duration (days) | 10 (7–14) | 10 (7–13) | 0.847 |
| Fluoroquinolones | 42 (98%) | 0 (0%) | |
| - ofloxacin | 33 (77%) | 0 (0%) | |
| - ciprofloxacin | 8 (19%) | 0 (0%) | |
| - levofloxacin | 7 (16%) | 0 (0%) | |
| Metronidazole | 43 (100%) | 36 (84%) | 0.991 |
| Aztreonam | 6 (14%) | 0 (0%) | 0.991 |
| Ceftriaxone | 0 (0%) | 21 (49%) | 0.989 |
| Cefotaxime | 0 (0%) | 20 (47%) | 0.990 |
| Piperacillin–Tazobactam | 0 (0%) | 9 (21%) | 0.990 |
| Amoxicillin–Clavulanic Acid | 0 (0%) | 18 (42%) | 0.990 |
| Amoxicillin | 0 (0%) | 1 (2%) | 0.991 |
| Aminoglycoside | 12 (28%) | 10 (23%) | 0.621 |
| Glycopeptide | 5 (12%) | 6 (14%) | 0.747 |
| Clindamycin | 4 (9%) | 0 (0%) | 0.989 |
| Antifungal agent | 0 (0%) | 3 (7%) 1 | 0.990 |
| Characteristics | BLA (N = 43) N (%) | NBLA (N = 43) N (%) | p-Value |
|---|---|---|---|
| Inadequate antibiotic therapy | |||
| Empiric antimicrobial therapy | 8 (19%) | 8 (19%) | >0.999 |
| Directed antimicrobial therapy | 5 (12%) | 1 (2%) | 0.2 |
| Details of inadequate antimicrobial therapy: empiric → directed | |||
| Enterococcus sp. or Streptococcus sp. | 7 → 4 | 5 → 0 | |
| Pseudomonas aeruginosa | 1 → 0 | 0 → 0 | |
| Fluoroquinolone-resistant Enterobacterales | 1 → 1 | 0 → 0 | |
| 3rd generation Cephalosporin resistant Enterobacterales | 0 → 0 | 1 → 0 | |
| Piperacillin–tazobactam-resistant Enterobacterales | 0 → 0 | 2 → 1 | |
| Methicillin-resistant Staphylococcus epidermidis | 0 → 0 | 1 → 0 | |
| Metronidazole-resistant Bacteroides thetaiotaomicron | 1 → 1 | 0 → 0 | |
| Therapeutic failure | |||
| 6 (14%) | 6 (14%) | >0.999 | |
| Death | - | 1 | |
| Surgical site infection | - | 1 | |
| Unplanned surgery due to complication or recurrence of IAI | 5 | 4 | |
| Initiation of another antibiotic for worsening symptoms of IAI | 2 | 4 | |
| Healthcare-associated infection | |||
| 2 (5%) | 6(14%) | 0.3 | |
| Candidemia | 1 1 | - | |
| Recurrent cholangitis | 1 | - | |
| Cystitis | 1 1 | ||
| Surgical site infection | - | 1 | |
| Pulmonary infection | - | 2 2 | |
| Bloodstream infection | - | 2 | |
| Clostridioides difficile infection | 1 | ||
| Adverse event due to antibiotics | |||
| 1 (2%) | 1 (2%) | >0.999 | |
| Thrombocytopenia | 1 3 | - | |
| Clostridioides difficile infection | - | 1 | |
| Characteristics | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Inadequate empiric antimicrobial therapy | 9.86 (2.27–44.76) | 0.002 | 11.71 (1.43–132.46) | 0.025 |
| Aminoglycosides | 5.51 (1.55–21.04) | 0.009 | 2.89 (0.39–23.96) | 0.293 |
| BMI (per kg/m2) | 1.12 (1.01–1.26) | 0.028 | 1.16 (1.00–1.36) | 0.041 |
| HLOS (per day) | 1.20 (1.10–1.35) | <0.001 | 1.20 (1.08–1.41) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naciri, T.; Monnin, B.; Pantel, A.; Roger, C.; Kinowski, J.-M.; Loubet, P.; Lavigne, J.-P.; Sotto, A.; Larcher, R. Outcomes of Beta-Lactam Allergic and Non-Beta-Lactam Allergic Patients with Intra-Abdominal Infection: A Case–Control Study. Antibiotics 2022, 11, 1786. https://doi.org/10.3390/antibiotics11121786
Naciri T, Monnin B, Pantel A, Roger C, Kinowski J-M, Loubet P, Lavigne J-P, Sotto A, Larcher R. Outcomes of Beta-Lactam Allergic and Non-Beta-Lactam Allergic Patients with Intra-Abdominal Infection: A Case–Control Study. Antibiotics. 2022; 11(12):1786. https://doi.org/10.3390/antibiotics11121786
Chicago/Turabian StyleNaciri, Tayma, Boris Monnin, Alix Pantel, Claire Roger, Jean-Marie Kinowski, Paul Loubet, Jean-Philippe Lavigne, Albert Sotto, and Romaric Larcher. 2022. "Outcomes of Beta-Lactam Allergic and Non-Beta-Lactam Allergic Patients with Intra-Abdominal Infection: A Case–Control Study" Antibiotics 11, no. 12: 1786. https://doi.org/10.3390/antibiotics11121786
APA StyleNaciri, T., Monnin, B., Pantel, A., Roger, C., Kinowski, J.-M., Loubet, P., Lavigne, J.-P., Sotto, A., & Larcher, R. (2022). Outcomes of Beta-Lactam Allergic and Non-Beta-Lactam Allergic Patients with Intra-Abdominal Infection: A Case–Control Study. Antibiotics, 11(12), 1786. https://doi.org/10.3390/antibiotics11121786







