Abstract
Background. Aeromonas necrotizing fasciitis (NF) causes high rates of amputation and mortality, even after aggressive surgical debridement and antibacterial therapy. This study investigated the effects of rational use of antibiotics and education by infectious disease (ID) physicians on Aeromonas NF treatment outcomes. Methods. Retrospective review for conducted for four years (period I, without an ID physician, December 2001 to December 2005) and 15 years (period II, with an ID physician, January 2006 to March 2021). In period II, the hospital-wide computerized antimicrobial approval system (HCAAS) was also implemented. A pretest-posttest time series analysis compared the two periods. Differences in clinical outcomes, demographics, comorbidities, signs and symptoms, laboratory findings, Aeromonas antibiotic susceptibility, and antibiotic regimens were compared between the two periods. Results. There were 19 patients in period I and 53 patients in period II. Patients had a lower rate of amputation or mortality in period II (35.8%) compared with period I (63.2%). Forty-four patients (61.1%) had polymicrobial infections. In the emergency room, the rate of misdiagnosis decreased from 47.4% in period I to 28.3% in period II, while effective empiric antibiotic usage increased from 21.1% in period I to 66.0% in period II. After the ID physician’s adjustment, 69.4% received monotherapy in period II compared to 33.3% in period I. Conclusions. Because Aeromonas NF had a high mortality rate and was often polymicrobial, choosing an antibiotic regimen was difficult. Using the HCAAS by an experienced ID physician can improve rational antibiotic usage and clinical outcomes in Aeromonas NF.
1. Introduction
In 1952, Wilson used necrotizing fasciitis (NF) to describe the infection’s most consistent characteristic, fascial necrosis [1]. When NF is present, the underlying muscle is intact while the skin appears normal. Upon histopathology, swelling, necrosis, and inflammation were evident in the skin, skin fat, and fascia. There is a prominent presence of thrombosis at all levels of vessels [2].
Aeromonas NF is a rare and life-threatening necrotizing skin and soft tissue infection (NSSTI) characterized by rapidly spreading necrosis in the subcutaneous layers, especially in the fascia [1,3]. Despite aggressive surgical debridement and antibacterial treatment, Aeromonas NF causes high rates of amputation and mortality, with 27.3–50% of patients losing limbs [4,5,6] and 26.7–100% dying [4,5,6,7,8].
The Chang Gung Memorial Hospital (CGMH)-Chiayi is a tertiary teaching hospital located on the western coast of southern Taiwan, and it has been in service since December 2001. Residents in this region are typically exposed to seawater, raw seafood, brackish water, and soil. As a result, there has been a relatively high incidence of Vibrio spp. and Aeromonas spp. infections in our hospital [4,5,6,7,8,9,10,11,12]. We set up a Vibrio NSSTIs Treatment and Research (VTR) Group comprising professional staff from various departments, including emergency medicine, orthopedic surgery, infectious disease (ID), plastic surgery, and the intensive care unit (ICU), and the hyperbaric oxygen treatment center [6,13,14]. The VTR Group also set up an NF diagnosis and treatment protocol (Figure 1).
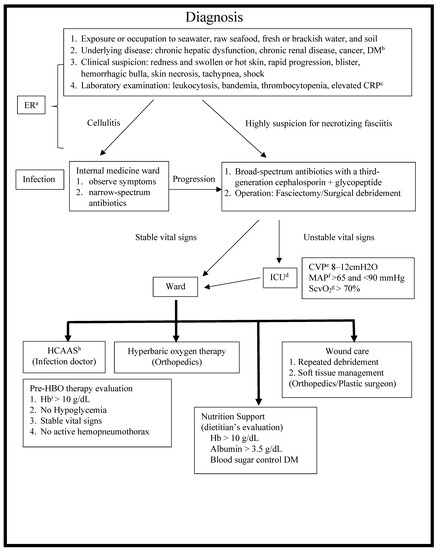
Figure 1.
Diagnosis and treatment protocol of NF. NF, necrotizing fasciitis; a ER, emergency room; b DM, diabetes mellitus; c CRP, C-reactive protein; d ICU, intensive care unit; e CVP, central venous pressure; f MAP, mean arterial pressure; g ScvO2, central venous oxygen saturation; h HCAAS, hospital-wide computerized antimicrobial approval system; i Hb, hemoglobin.
The hospital-wide computerized antimicrobial approval system (HCAAS) is an online antimicrobial control system implemented at the CGMH in Taiwan [15,16,17]. The HCAAS reduces antimicrobial consumption and expenditures without compromising or reducing healthcare quality [15,16,17]. By using HCAAS and education by ID physicians, we evaluated Aeromonas NF treatment outcomes and improved rational antibiotic use.
2. Methods
2.1. Study Design
A retrospective study was conducted by the VTR Group at CGMH-Chiayi from December 2001 to March 2021. The study enrolled patients admitted to the emergency room (ER) with Aeromonas NF of the limbs. A pretest-posttest time series analysis compared the two study periods: period I (without an ID physician, December 2001 to December 2005 [4 years]) and period II (with an ID physician, January 2006 to March 2021 [15 years]). Data such as clinical outcomes, demographics, comorbidities, presenting signs and symptoms, laboratory findings, Aeromonas antibiotic susceptibility, and antibiotic regimens were recorded and compared between the two periods. This study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (Number: 202001656B0, 201801530B1B0, 97-1073B, 99-1123C, and 102-5105B), and all patients provided informed consent.
2.2. HCAAS
A HCAAS application is an intranet-based system that integrates with electronic medical records [14,15,16]. The entire algorithm is displayed in Figure 2 of the system flow. In the ICU, all parenteral antimicrobial agents must be approved by ID physicians. ID physicians are notified when antimicrobial agents are prescribed in preassigned regions. The information above is automatically processed by HCAAS. The ID physician will review online patient clinical records, laboratory reports, cultures, and images submitted by the prescribing physician. The ID physician consults with the physician in charge before making a decision. When a disapproval decision is made, the unit-dose delivery system of the pharmacy will discontinue the antimicrobial after 48 h, and the prescriber will be notified immediately.
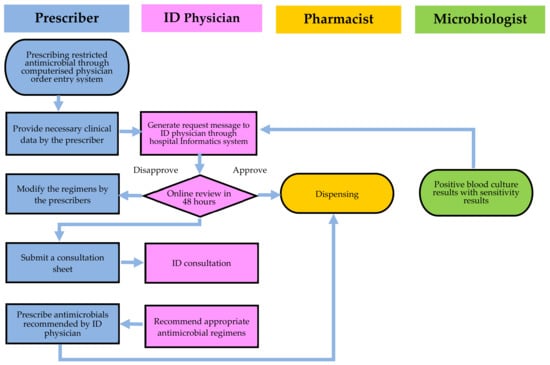
Figure 2.
An overview of the hospital-wide computerized antimicrobial approval system (HCAAS). Infectious diseases (ID) physicians have three options following online review of the antimicrobial prescription: approval, disapproval, or an on-site consultation.
2.3. Definitions
For inclusion in the Aeromonas NF patient group, the following criteria were used: (1) necrosis of the skin, subcutaneous fat, superficial fascia, or muscles beneath the skin; (2) Aeromonas sp. isolated from soft-tissue lesions and/or blood collected during surgery or after patients arrived at the ER [17,18].
The following antibiotics are not restricted: cefazolin, penicillin G, oxacillin, ampicillin, gentamicin, clindamycin, metronidazole, and trimethoprim-sulfamethoxazole. Many other antibiotics are restricted, such as cephalosporins (cefuroxime, ceftriaxone, ceftazidime, cefpirome, and cefepime), flomoxef, penicillins (amoxicillin, amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin, and piperacillin-tazobactam), carbapenems (imipenem, meropenem, doripenem, and ertapenem), aztreonam, amikacin, fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin), glycopeptides (vancomycin and teicoplanin), and other kinds of antibiotics (colistin, tigecycline, linezolid, and daptomycin).
Antimicrobial regimens prescribed before the results of the microbiologic susceptibility test became available were defined as empirical, while those subsequently adjusted according to the microbiologic susceptibility test results were defined as definitive [18]. Effective empirical antimicrobial usage was defined as the administration of an initial antimicrobial regimen that provided coverage for all infectious isolates based on antimicrobial susceptibility testing [11,19].
2.4. Laboratory Methods
Aeromonas species consists of oxidase-positive, polar flagellated, glucose-fermenting, facultatively anaerobic, motile bacteria that do not grow in gram-negative rods containing 6.5% NaCl. Conventional methods were used to identify all strains. For further verification, API-20E and ID32 GN Systems (bioMérieux Inc., Hazelwood, MO, USA) or Vitek 2 ID-GNB identification cards (bioMérieux Inc., Durham, NC, USA) were used. Clinical and Laboratory Standards Institute (CLSI) criteria for microorganisms were used to interpret the results of antimicrobial susceptibility tests.
2.5. The Judgment of Antimicrobial Use and Education by ID Physicians
Through the HCAAS and consultation formally, ID physicians find all NF patients and follow up on these patients’ conditions. ID physicians reviewed the initial empirical antimicrobial prescriptions, categories of judgment for antibiotic regimen at ER, and definitive antibiotic regimen (≥72 h after admission). ID physicians recorded all inappropriate prescriptions during admission and recommendations on medical charts and further classified them by escalation, de-escalation, and other reasons [17,19]. ID physicians have been conducting combined meetings with emergency medicine doctors and educating the diagnosis of NF, rational antimicrobial usage, microbial resistance, and infection control every 2 months since March 2008 till now.
2.6. End-Point Measurements
This study was conducted to compare the outcome of treatment of Aeromonas NF between a new, large hospital in the two different periods with and without ID physicians, which had not been previously studied. The endpoints were mortality, amputation rate, effective rates of empirical antimicrobial usage at ER, and definitive antibiotic regimen after admission.
2.7. Statistical Analysis
Fisher’s exact test was used to test continuous variables and Student’s t-tests were used to test categorical variables. When the p-value was less than 0.05 with a two-tailed test, it was considered statistically significant. To compare gradients between periods I and II, segmented linear regression techniques were used. Statistics were calculated using SPSS 25.0 (SPSS Corporation, Chicago, IL, USA).
3. Results
3.1. Uptake
From December 2001 to March 2021, 72 patients admitted via the ER were surgically confirmed to have Aeromonas NF of the limbs. Period I had 19 admissions and period II had 53. Table 1 lists the demographic data, characteristics, and clinical outcomes.

Table 1.
Demographic data, characteristics, and clinical outcomes of 72 patients with Aeromonas NF a between periods I and II.
3.2. Inpatient Outcomes
Period II had a lower rate of amputation (17.0%) than period I (52.6%), in addition to a lower rate of amputations or mortality (35.8%) than period I (63.2%). The trend lines are listed in Figure 3. The 95% confidence interval was listed in Table 2. The rate of misdiagnosis in the ER decreased from 47.4% in period I to 28.3% in period II, and a delay of >24 h in taking the patient from the ER to surgery decreased from 42.1% in period I to 26.4% in period II, without statistical difference.
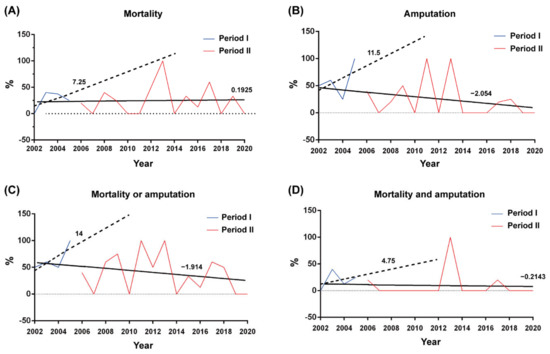
Figure 3.
The hospitalized patients, the rates of (A) mortality, (B) amputation, (C) mortality or amputation, and (D) mortality and amputation per year before (blue lines) and after (red lines) the deployment of the HCAAS in January 2006. The trend lines established by linear regression before and after deployment of HCAAS are depicted as dotted and solid lines, respectively.

Table 2.
A 95% confidence interval for the rates of mortality, amputation, mortality or amputation, and mortality and amputation between periods I and II.
3.3. Demographic Data
Period II was characterized by a statistically higher Charlson score (6.1 ± 3.1) than period I (4.5 ± 2.7), in addition to a higher incidence of chronic liver dysfunction but a lower rate of peripheral vascular disease (Table 1).
3.4. Antimicrobial Resistance and the Empiric Antimicrobial Prescription
The proportion of bloodstream infection was higher in period II than in period I, without a statistical difference (Table 3). We found a higher susceptibility rate of Aeromonas to penicillin in period II (81.1%) than period I (26.3%), with a statistically significant difference.

Table 3.
Microbiological results and judgment for antimicrobial agents for 72 patients with Aeromonas NF between periods I and II.
The effective empirical and appropriate antimicrobial usage in the ER increased from 21.1% in period I to 66.0% in period II and from 15.8% in period I to 32.1% in period II, respectively. Empirical antimicrobial regimen escalation decreased from 78.9% in period I to 30.2% in period II, but de-escalation increased from 5.3% in period I to 30.2% in period II. After admission >72 h later, 15 patients (78.9%) in period I and 45 patients (84.9%) in period II were changed to different antimicrobial regimens. In period II, more antimicrobial regimens were re-adjusted to monotherapy (69.4%) than in period I (33.3%).
3.5. Microbiological Analysis
A. hydrophila was the most common infectious bacterium, accounting for 49 of 72 patients (68.1%), followed by 10 patients with A. sobria (13.9%), 10 with Aeromonas sp. (13.9%), and 3 with A. caviae (4.2%). Of the 72 patients, 44 (61.1%) were diagnosed with polymicrobial infections. The most common isolates obtained from patients with co-infective microorganisms were Clostridium sp. (21, 47.7%), followed by Enterobacter sp. (14, 31.8%) (Table 4).

Table 4.
Summary of identified infectious microorganisms in 44 patients of Aeromonas polymicrobial NF of the limbs.
3.6. Clinical Presentation
There were no significant differences in the presentation of fever (>38 ℃), tachycardia (heartbeat > 100/min), tachypnea (respiratory rate > 20/min), or shock (systolic blood pressure < 90 mmHg) and in the proportion of patients presenting with erythematous, swollen, painful lesions; bullae formation; and skin necrosis between the two periods (Table 5).

Table 5.
Comparison of clinical presentations and important laboratory findings of 72 patients with Aeromonas NF between periods I and II.
3.7. Laboratory Findings
More than 10% of banded leukocyte cells were observed more frequently in period II than in period I (Table 5). In addition, the serum albumin level was lower (p = 0.005) in period II than in period I.
3.8. Surgical Treatment
When the first operation was performed in period I, three patients (15.8%) underwent amputation, five (26.3%) underwent debridement, and eleven (57.9%) underwent fasciotomy. In period II, the first surgery performed was amputation for two patients (3.8%), debridement for 12 (22.6%), and fasciotomy for 39 (73.6%).
4. Discussion
Aeromonads are flagellated, rod-shaped, nonsporulating facultative anaerobic gram-negative bacteria of the family Aeromonadaceae with typing of the aquatic environment [20]. Currently, there are more than 20 Aeromonas species identified, of which three, namely A. hydrophila, A. caviae, and A. veronii biovar sobria, are the major clinical important [20]. These bacteria can cause human infections, including hepatobiliary infections, blood-borne infections, traumatic events, and burns and scalds related to skin and soft-tissue infections, in previously healthy subjects [6,18,21,22,23].
This 19-year study is the first report on an online antimicrobial control system used by ID specialists for Aeromonas NF treatment. A delay of >24 h in the first surgical intervention from symptom onset to surgery adversely affects survival outcomes [3,24]. This delay of >24 h decreased from 42.1% in period I to 26.4% in period II. Mortality is associated with a delay in the diagnosis of NF [25]. In the ER, the mean rate of misdiagnosis is 71.4% (range from 41% to 96%) [26], while in this study, the rate of misdiagnosis was only 27.8% in period II. Due to prompt NF diagnosis, surgery can be arranged as quickly as possible. The trend lines indicate that mortality or amputations decreased between periods II and I (Figure 3). We consider persistent education of emergency medicine doctors by an ID physician should help in improving this delay [27]. In period I, we aggressively amputated patients’ limbs to save their lives, but we still found higher mortality rates than in period II. In the first operation, we change the patient’s surgical treatment from amputation to fasciotomy. In this manner, period II has lower amputation rates and rates of mortality or amputations.
With more than 19 years of experience in NF treatment, our VTR Group found a lot of medical information, clinical phenomena, and important laboratory findings for the early diagnosis of NF, especially that caused by Vibrio and Aeromonas spp. Because Aeromonas spp. are often located in fresh or brackish water, sewage, or nonfecal organic materials [7,22]. First, we need to take the occupational history of farmers, recent trauma, and exposure to soil, wood, or dirty ditches. Second, a significantly higher mortality rate was observed in patients with NF who also had chronic kidney disease, chronic liver dysfunction, diabetes mellitus, or cancer [5,6,24,28,29]. About 55–57% of patients with Aeromonas NF had chronic liver dysfunction [5,14]. Hepatic cirrhosis was found in 36–54% of patients with Aeromonas bacteremia [18,22] and in 27–32% of patients with Aeromonas NF [5,6]. So, we aggressively checked viral hepatitis and liver cirrhosis in period II.
Third, Aeromonas NF was often initially present with tachypnea, shock, hemorrhagic bullae, and skin necrosis [4,5,6,7,14]. These important early clinical signs and symptoms are easily observed when patients arrive at the ER. Patients with Aeromonas NF have a statistical tendency to have tachypnea and initially present with septic shock tending to mortality [6]. The emergence of hemorrhagic bullae is considered a feature of Vibrio infection, but they are also found in 38–40% of patients with Aeromonas NF [6,14]. Patients with NF presenting with hemorrhagic bullae have a higher rate of amputation and mortality than patients with serous-filled bullae or without bullae [14]. In one study, 27.9% of patients with Aeromonas NF had skin necrosis, and this phenomenon is a poor predictor of mortality [6]. In period II, more patients had tachypnea, shock, and skin necrosis, but there was no statistically significant difference between period I and period II.
Fourth, we also found some important laboratory results to predict the poor outcome of NF. Lower counts of total and segmented leukocytes, higher counts of banded leukocytes, thrombocytopenia, and lower serum albumin levels are significantly associated with mortality [6,8,30,31]. More than 10% of these forms of leukocytes indicate gram-negative NF, especially that caused by Vibrio spp. and Aeromonas spp. [6,8,30]. In this study, we also found more patients with >10% banded leukocytes and lower serum albumin levels in period II.
The fatality rate associated with Aeromonas bacteremia is reported to be 30–36% [18,22]. In our previous study, 38.2% of patients with Aeromonas NF had bacteremia [6], and bloodstream infection significantly increased the mortality rate in NF [6,28]. A total of 17–61% of patients with NF receive postoperative intubation [14,30], and 36–100% of patients require ICU care [6,14,25,30]. Although patients exhibited high disease severity in period II, including a higher Acute Physiology and Chronic Health Evaluation II score, a higher bacteremia rate, postoperative intubation, and ICU care, lower rates of mortalities or amputations were found. These better outcomes must rely on the long-term cooperation of VTR Group.
At ER, empiric antibiotic therapy with oxacillin plus gentamicin was prescribed for suspicion of NF in period I, and third-generation cephalosporins were empirically prescribed when a Vibrio infection was suspected in period II [30]. Antibiotics ordered with evidence of culture and susceptibility results are found to be more appropriate than those ordered empirically [32]. Aeromonas SSTIs are often polymicrobial [6,21] and have high drug resistance [6], so the selection of an antimicrobial regimen is challenging. The initial ineffective empirical antimicrobial usage is related to poor outcomes for patients with Aeromonas NF [6].
However, culture-directed antimicrobial therapy and an antibiotic order with consultation with an ID physician for critically ill patients are more likely to be appropriate [7,17,32]. The HCAAS and persisted on-the-spot education by ID physicians in a trauma ICU can decrease 54% sepsis-related and 41% overall infection-related mortality rates, respectively, and increase the appropriate ratio of antibiotic usage from 60.5% to 73.7% [17]. Early prompt fasciotomy, combined with appropriate empiric antimicrobial therapy supported by ID physicians and aggressive ICU care, should be initially administered to critically ill patients suffering from fulminant NF to save lives and limbs [7,17,24,33].
Most Aeromonas strains are uniformly resistant to penicillin but invariably susceptible to aminoglycosides, sulfa drugs, second–fourth-generation cephalosporins, carbapenems, fluoroquinolones, and tetracyclines [22,34,35]. Third-generation cephalosporins combined with tetracycline are commonly the empiric prescription in highly suspected Vibrio infection cases before the pathogen is identified [36,37]. But the high resistance of cephalosporins was related to Aeromonas NF mortality [6]. Fluoroquinolones are seemed higher active against Aeromonas spp. [6,35], but a mutation in the gyrA gene induces fluoroquinolone resistance [35]. However, in highly suspected fulminate Aeromonas NF cases, we sometimes add quinolones before the final pathogen is identified [6]. Most Aeromonas spp. infections are treatable with monotherapy and studies with combination therapy do not show better outcomes [38]. In period II, 84.9% of patients adjusted the definitive antibiotic regimen based on the ID physician’s suggestion using the HCAAS, monotherapy was more common, simpler, and rational than combination therapy. At the same time, we should limit antibiotic prescriptions to patients, and we should reduce the length of time these treatments are required [39].
The HCAAS provided a comprehensive evaluation of a novel, ID specialist-led, and sustainable system to provide antibiotic stewardship in a busy medical ecology [17]. HCAAS was not used in this study to reduce antimicrobial consumption, expenditure, and de-escalation, thus helping ID physicians detect such major diseases earlier. The average duration of hospitalization for survivors is usually more than 30 days [6,14,24,30]. ID physicians can monitor the following antibiotic regimen through the HCAAS during the working day and suggest an appropriate antibiotic regimen to improve rational antibiotic usage.
The major limitation of this study was that the HCAAS is only used at admission but not in ER. Second, there was no report of the minimum inhibitory concentration of Aeromonas spp. Third, we did not analyze antibiotic consumption, the occurrence of new Aeromonas antibiotic-resistant strains, and the rates of hospital-associated infection.
5. Conclusions
As soon as possible, long-term, close cooperation and experienced teamwork can help diagnose and aggressively treat Aeromonas NF. An effective antimicrobial regimen against Aeromonas spp. confirmed by an experienced ID physician using the HCAAS can improve clinical outcomes and rational antibiotic usage. However, antibiotic resistance is also an important consideration.
Author Contributions
T.-Y.H. participated in the design of the study, collected data, performed the statistical analysis, and drafted the manuscript. Y.-K.H., C.-H.H. and K.-T.P. participated in the design of the study and the drafting of the manuscript. Y.-H.T., C.-Y.L. and W.-H.H. conceived the study, carried out surgeries, and coordinated the research groups. Y.-Y.L., J.-L.C. and J.-C.H. participated in the design of the study and assisted in the surgery. C.-T.H. and H.-J.L. participated in the design of the study and the revision of the manuscript. S.-F.K. and J.-C.H. participated in the design of the study and statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chang Gung Medical Research Program Foundation [grant numbers CMRPG6L0071, CMRPG6H0641, CMRPG680051~52, and CORPG6E0051~53], and the Ministry of Science and Technology (R.O.C.) (grants NMRPG6K6011-6013) [MOST 109-2314-B-182A-024-MY3].
Institutional Review Board Statement
This study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (Numbers: 202001656B0, 201801530B1B0, 97-1073B, 99-1123C, and 102-5105B), and all patients provided informed consent.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Chun-Yen Huang and Chun-Wei Huang for their assistance in English modification.
Conflicts of Interest
The authors declare that they have no competing interest.
Abbreviations
NF, necrotizing fasciitis; HCAAS, hospital-wide computerized antimicrobial approval system; ID, infectious disease; ER, emergency room; ICU, intensive care unit; NSSTI, necrotizing skin and soft-tissue infection; OR, odds ratio; CI, confidence interval.
References
- Wilson, B. Necrotizing fasciitis. Am. Surg. 1952, 18, 416–431. [Google Scholar] [PubMed]
- Umbert, I.J.; Winkelmann, R.; Oliver, G.F.; Peters, M.S. Necrotizing fasciitis: A clinical, microbiologic, and histopathologic study of 14 patients. J. Am. Acad. Dermatol. 1989, 20, 774–781. [Google Scholar] [CrossRef]
- Green, R.J.; Dafoe, D.C.; Raffin, T.A. Necrotizing fasciitis. Chest 1996, 110, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Huang, K.-C.; Huang, T.-J.; Hsu, R.W.-W. Case Reports: Fatal Necrotizing Fasciitis Caused by Aeromonas sobria in Two Diabetic Patients. Clin. Orthop. Relat. Res. 2009, 467, 846–849. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Shen, S.-H.; Yang, T.-Y.; Chen, P.-H.; Huang, K.-C.; Lee, M.S. Monomicrobial Necrotizing Fasciitis Caused by Aeromonas hydrophila and Klebsiella pneumoniae. Med. Princ. Pract. 2015, 24, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Peng, K.-T.; Hsu, W.-H.; Hung, C.-H.; Chuang, F.-Y.; Tsai, Y.-H. Independent Predictors of Mortality for Aeromonas Necrotizing Fasciitis of Limbs: An 18-year Retrospective Study. Sci. Rep. 2020, 10, 7716–7719. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Hsu, R.W.W.; Huang, T.J.; Hsu, W.H.; Huang, K.C.; Li, Y.Y.; Peng, K.T. Necrotizing soft-tissue infections and sepsis caused by Vibrio vulnificus compared with those caused by Aeromonas species. J. Bone Jt. Surg. Am. 2007, 89, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Huang, K.-C.; Shen, S.-H.; Hsu, W.-H.; Peng, K.-T.; Huang, T.-J. Microbiology and surgical indicators of necrotizing fasciitis in a tertiary hospital of southwest Taiwan. Int. J. Infect. Dis. 2012, 16, e159–e165. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Huang, T.-Y.; Chen, J.-L.; Hsiao, C.-T.; Kuo, L.-T.; Huang, K.-C. Bacteriology and mortality of necrotizing fasciitis in a tertiary coastal hospital with comparing risk indicators of methicillin-resistant Staphylococcus aureus and Vibrio vulnificus infections: A prospective study. BMC Infect. Dis. 2021, 21, 771. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-D.; Lai, L.-J.; Hsu, W.-H.; Huang, T.-Y. Vibrio cholerae non-O1-the first reported case of keratitis in a healthy patient. BMC Infect. Dis. 2019, 19, 916. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Hsu, R.W.-W.; Huang, K.-C.; Chen, C.-H.; Cheng, C.-C.; Peng, K.-T.; Huang, T.-J. Systemic Vibrio Infection Presenting as Necrotizing Fasciitis and Sepsis: A Series of Thirteen Cases. J. Bone Jt. Surg. 2004, 86, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Huang, T.Y.; Kuo, L.T.; Chuang, P.Y.; Hsiao, C.T.; Huang, K.C. Comparison of Surgical Outcomes and Predictors in Patients with Monomicrobial Necrotizing Fasciitis and Sepsis Caused by Vibrio vulnificus, Aeromonas hydrophila, and Aeromonas sobria. Surg. Infect. 2022, 23, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Peng, K.-T.; Hsiao, C.-T.; Fann, W.-C.; Tsai, Y.-H.; Li, Y.-Y.; Hung, C.-H.; Chuang, F.-Y.; Hsu, W.-H. Predictors for Gram-negative monomicrobial necrotizing fasciitis in southern Taiwan. BMC Infect. Dis. 2020, 20, 60. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Tsai, Y.-H.; Kuo, L.-T.; Hsu, W.-H.; Hsiao, C.-T.; Hung, C.-H.; Huang, W.-Y.; Wu, H.-R.; Chuang, H.-J.; Li, Y.-Y.; et al. Different types of bullae of limbs with necrotizing fasciitis predict different outcome: A prospective study. Infection 2021, 49, 135–144. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Lin, T.-Y.; Huang, C.-T.; Deng, S.-T.; Wu, T.-L.; Leu, H.-S.; Chiu, C.-H. Implementation and outcomes of a hospital-wide computerised antimicrobial stewardship programme in a large medical centre in Taiwan. Int. J. Antimicrob. Agents 2011, 38, 486–492. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chiu, C.-H.; Huang, C.-T.; Cheng, C.-W.; Lin, Y.-J.; Hsu, Y.-J.; Chen, C.-H.; Deng, S.-T.; Leu, H.-S. Blood culture-guided de-escalation of empirical antimicrobial regimen for critical patients in an online antimicrobial stewardship programme. Int. J. Antimicrob. Agents 2014, 44, 520–527. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Hung, C.-H.; Lai, L.-J.; Chuang, H.-J.; Wang, C.-C.; Lin, P.-T.; Hsu, W.-H. Implementation and outcomes of hospital-wide computerized antimicrobial approval system and on-the-spot education in a traumatic intensive care unit in Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Lee, H.-C.; Chuang, Y.-C.; Liu, C.-C.; Wu, J.-J. Clinical Features and Therapeutic Implications of 104 Episodes of Monomicrobial Aeromonas Bacteraemia. J. Infect. 2000, 40, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M.; Tupasi, T.; Craig, W.A. Use of antibiotics. A brief exposition of the problem and some tentative solutions. Ann. Intern. Med. 1973, 79, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Gold, W.L.; Salit, I.E. Aeromonas hydrophila Infections of Skin and Soft Tissue: Report of 11 Cases and Review. Clin. Infect. Dis. 1993, 16, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Chuang, Y.-C. Aeromonas Bacteremia: Review of 59 Episodes. Clin. Infect. Dis. 1995, 20, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Murray, G.F.; Maddrey, W.C. Aeromonas septicemia from hepatobiliary disease. Am. J. Dig. Dis. 1973, 18, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Chang, H.-C.; Pasupathy, S.; Khin, L.-W.; Tan, J.-L.; Low, C.-O. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J. Bone Jt. Surg. 2003, 85, 1454–1460. [Google Scholar] [CrossRef]
- Ward, R.G.; Walsh, M.S. Necrotizing fasciitis: 10 years’ experience in a district general hospital. Br. J. Surg. 1991, 78, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.; Goh, L.G.; Ang, C.H.; Wong, C.H. Early diagnosis of necrotizing fasciitis. Br. J. Surg. 2013, 101, e119–e125. [Google Scholar] [CrossRef]
- Dambrino, K.L.; Green, M. Antimicrobial Stewardship in College and University Health Settings: A Public Health Opportunity. Antibiotics 2022, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Li, W.-C.; Hong, Y.-C.; Shie, S.-S.; Fann, W.-C.; Hsiao, C.-T. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit. Care 2011, 15, R152. [Google Scholar] [CrossRef] [PubMed]
- Sproll, C.; Lommen, J.; Balasiu, A.; Schorn, L.; Kübler, N.R.; Henrich, B.; Kram, R.; Petersdorf, S. Lethal Aeromonas veronii Sepsis in the Course of Medicinal Leech Therapy. Antibiotics 2022, 11, 1180. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Kuo, L.-T.; Peng, K.-T.; Hsu, W.-H.; Huang, T.-W.; Chou, Y.-C. Prognostic factors and monomicrobial necrotizing fasciitis: Gram-positive versus Gram-negative pathogens. BMC Infect. Dis. 2011, 11, 5. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Hsu, R.W.-W.; Huang, K.-C.; Huang, T.-J. Laboratory Indicators for Early Detection and Surgical Treatment of Vibrio Necrotizing Fasciitis. Clin. Orthop. Relat. Res. 2010, 468, 2230–2237. [Google Scholar] [CrossRef]
- Erbay, A.; Bodur, H.; Akıncı, E.; Çolpan, A. Evaluation of antibiotic use in intensive care units of a tertiary care hospital in Turkey. J. Hosp. Infect. 2005, 59, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-T.; Weng, H.-H.; Yuan, Y.-D.; Chen, C.-T.; Chen, I.-C. Predictors of mortality in patients with necrotizing fasciitis. Am. J. Emerg. Med. 2008, 26, 170–175. [Google Scholar] [CrossRef]
- Aravena-Román, M.; Inglis, T.J.J.; Henderson, B.; Riley, T.V.; Chang, B.J. Antimicrobial Susceptibilities of Aeromonas Strains Isolated from Clinical and Environmental Sources to 26 Antimicrobial Agents. Antimicrob. Agents Chemother. 2012, 56, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Marco, F.; Soler, L.; Chacon, M.; Figueras, M.J. In vitro antimicrobial susceptibility of clinical isolates of Aeromonas caviae, Aeromonas hydrophila and Aeromonas veronii biotype sobria. J. Antimicrob. Chemother. 2002, 49, 701–702. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Yuan, C.-Y.; Liu, C.-Y.; Lan, C.-K.; Huang, A.H.-M. Vibrio vulnificus Infection in Taiwan: Report of 28 Cases and Review of Clinical Manifestations and Treatment. Clin. Infect. Dis. 1992, 15, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-W.; Lee, I.-K.; Tang, H.-J.; Ko, W.-C.; Lee, H.-C.; Liu, Y.-C.; Hsueh, P.-R.; Chuang, Y.-C. Prognostic Factors and Antibiotics in Vibrio vulnificus Septicemia. Arch. Intern. Med. 2006, 166, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Saliba, R.; Mizrahi, A.; Gauthier, P.d.P.; Alban, L.M.; Zahar, J.-R.; Pilmis, B. Antimicrobial Stewardship Program: Reducing Antibiotic’s Spectrum of Activity Is not the Solution to Limit the Emergence of Multidrug-Resistant Bacteria. Antibiotics 2022, 11, 70. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).