Antifungal and Anti-Inflammatory Activities of PS1-2 Peptide against Fluconazole-Resistant Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Antifungal Activity of PS1-2, PS1-5 and PS1-6 against Drug-Resistant C. albicans
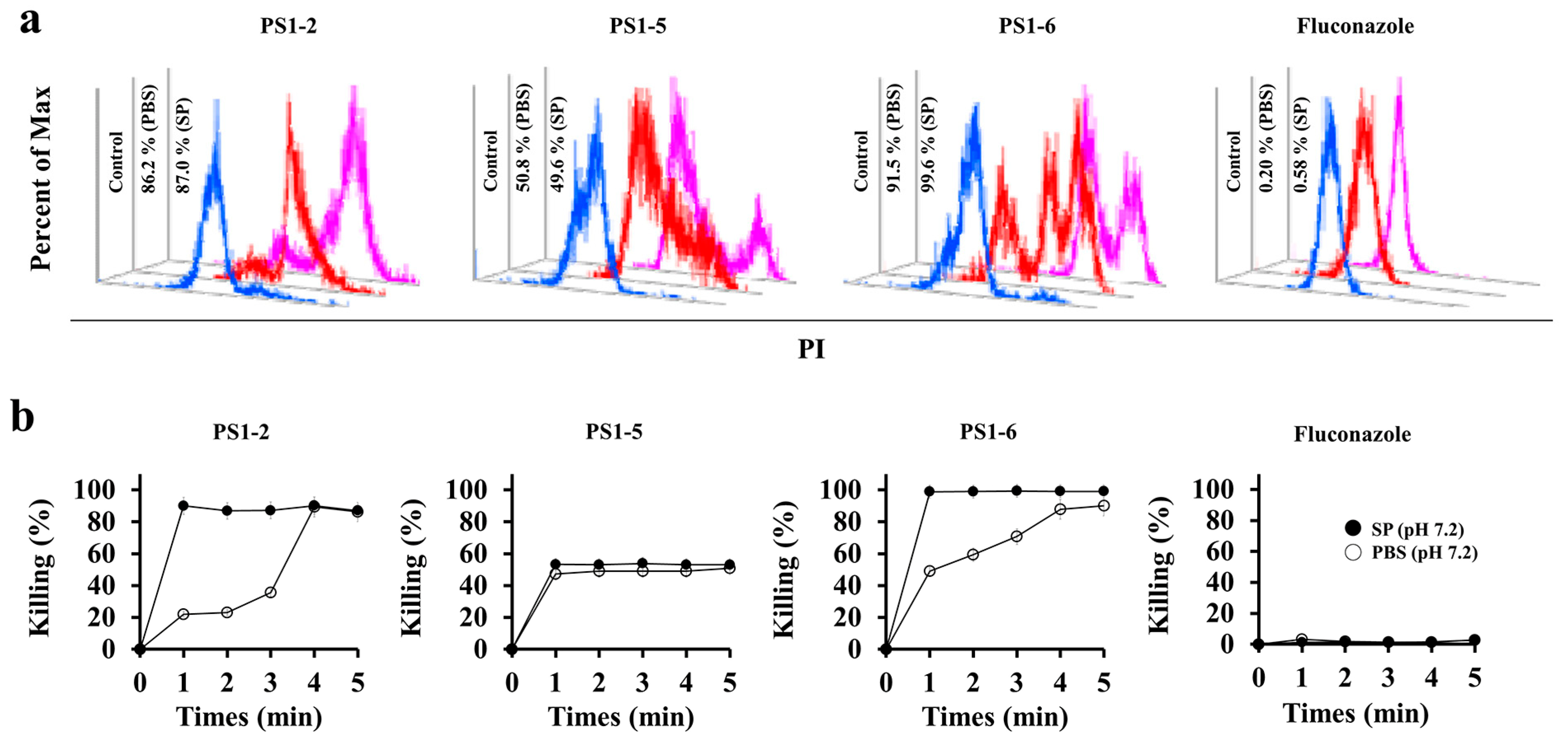
2.2. Fungicidal Kinetics of PS1-2, PS1-5 and PS1-6
2.3. Binding Affinity of FAM-Labeled PS1-2 Peptide to C. albicans Cells
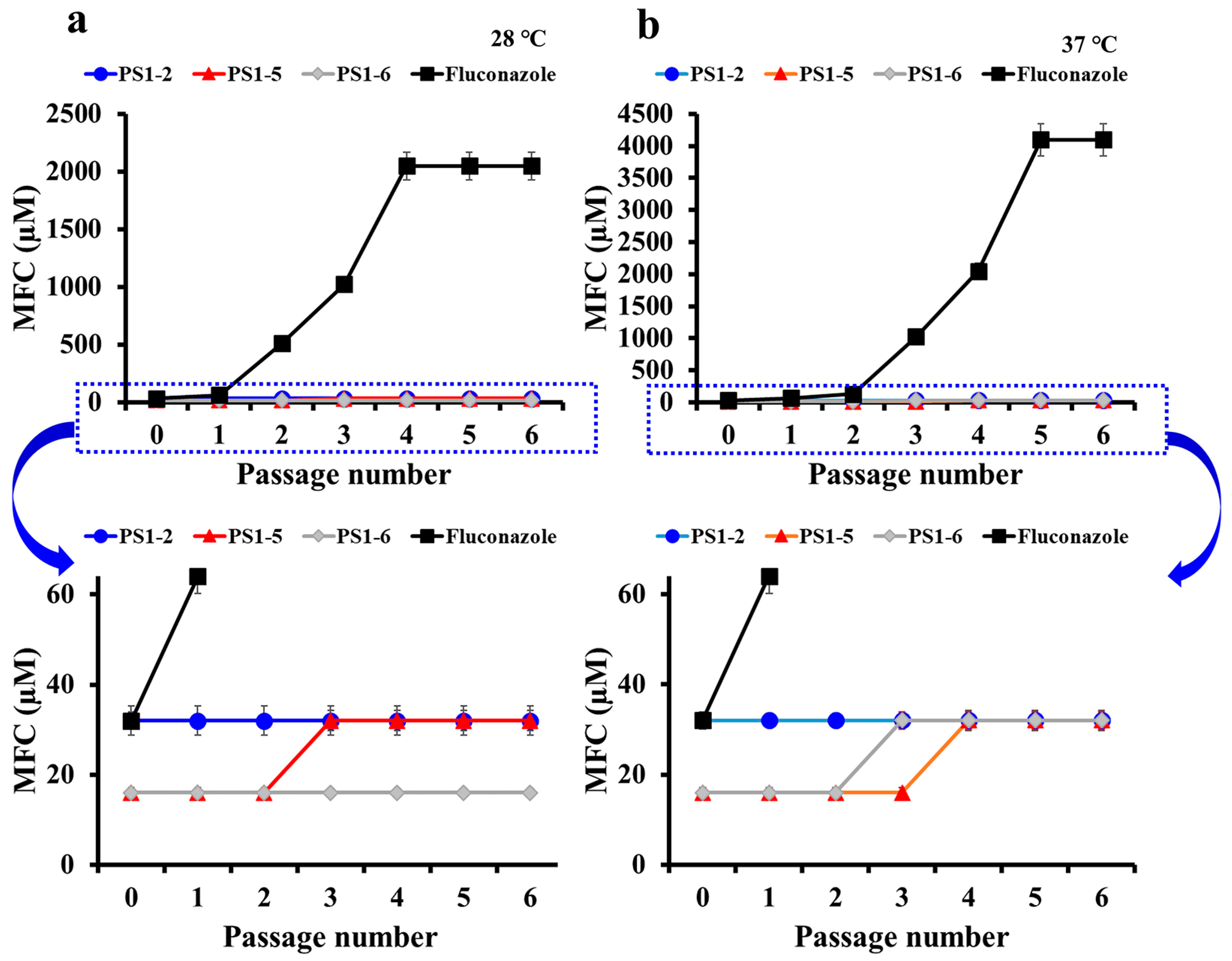
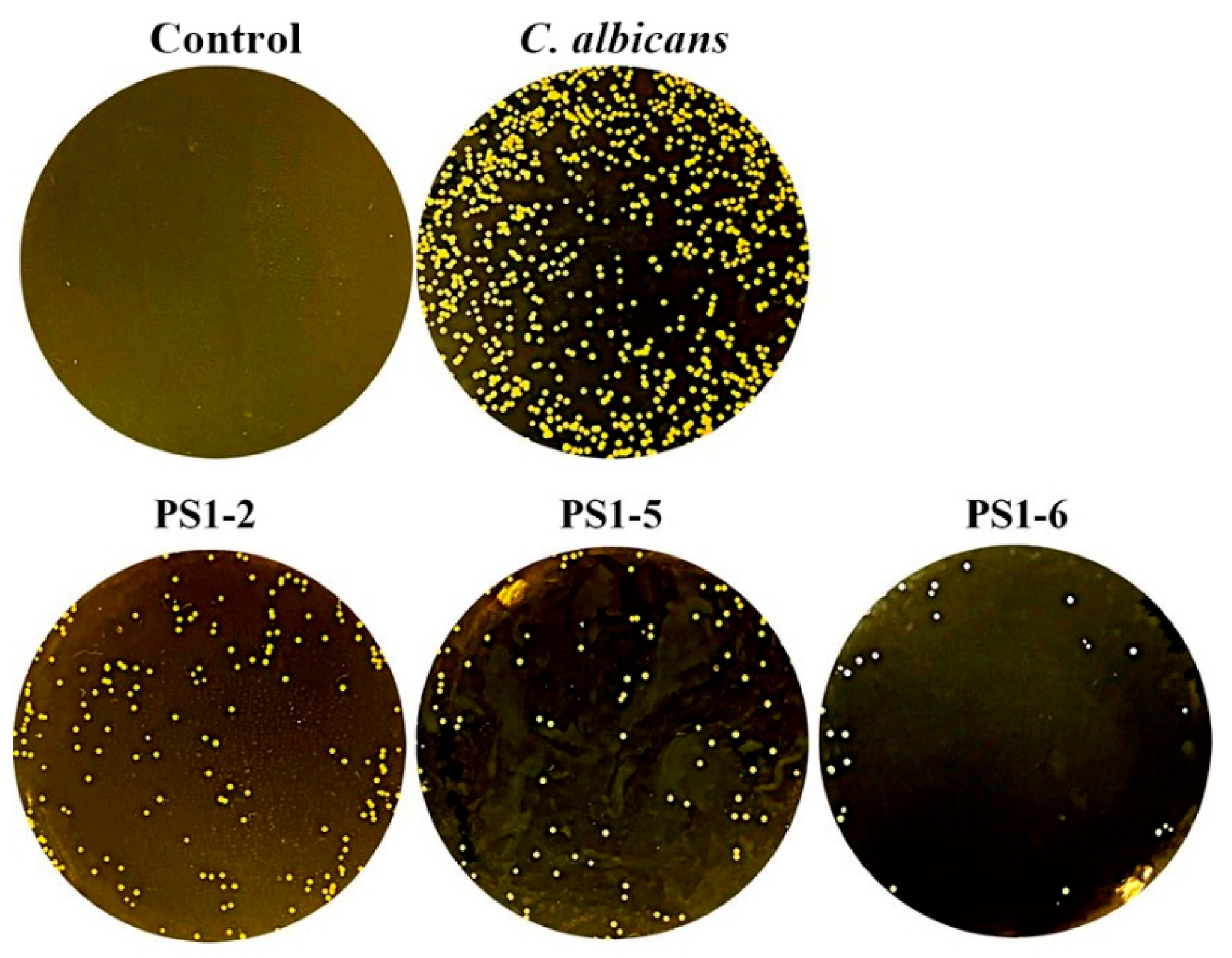
2.4. Peptides Did Not Induce the Drug-Resistance
2.5. Protection for Protease Degradation
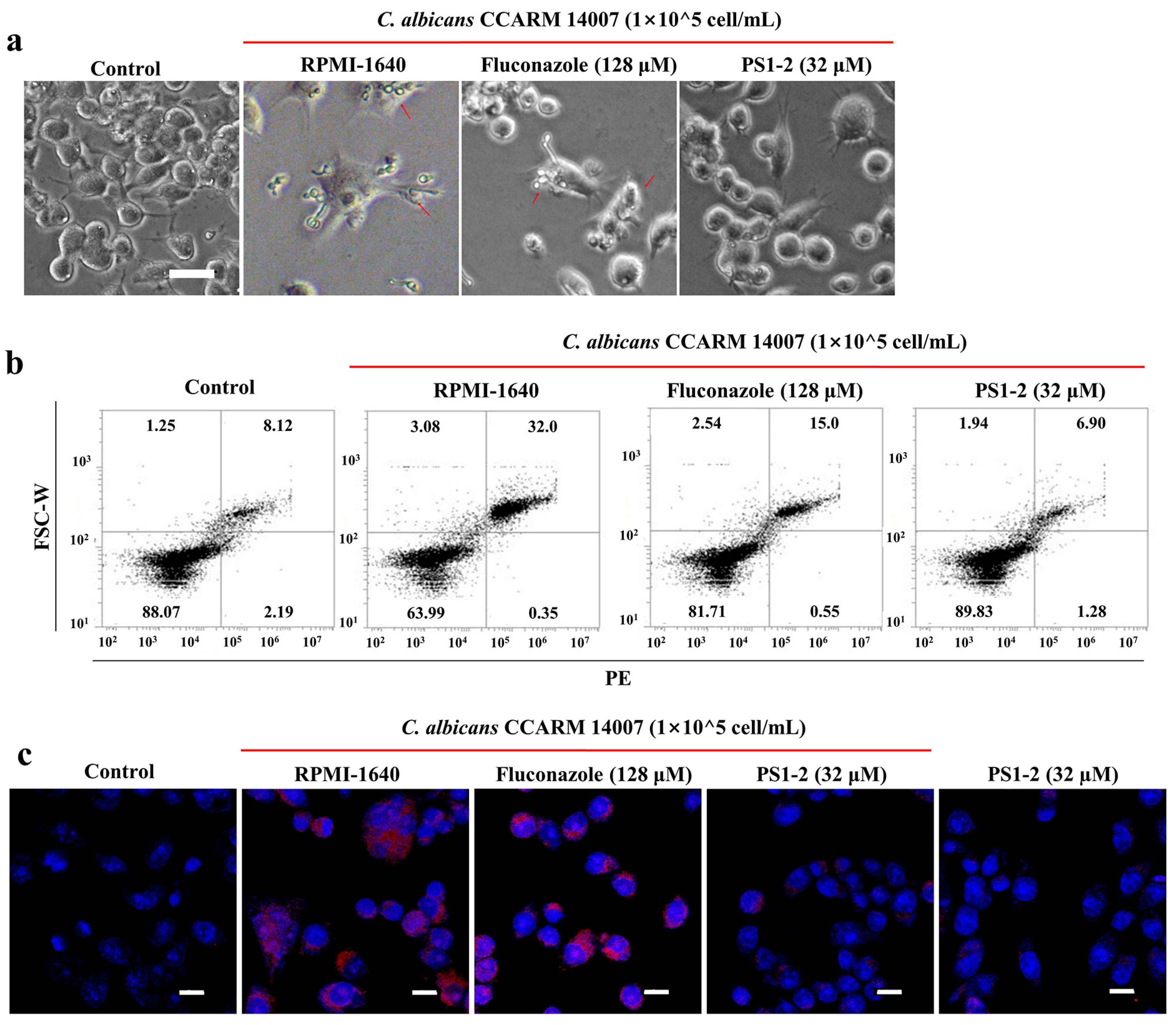
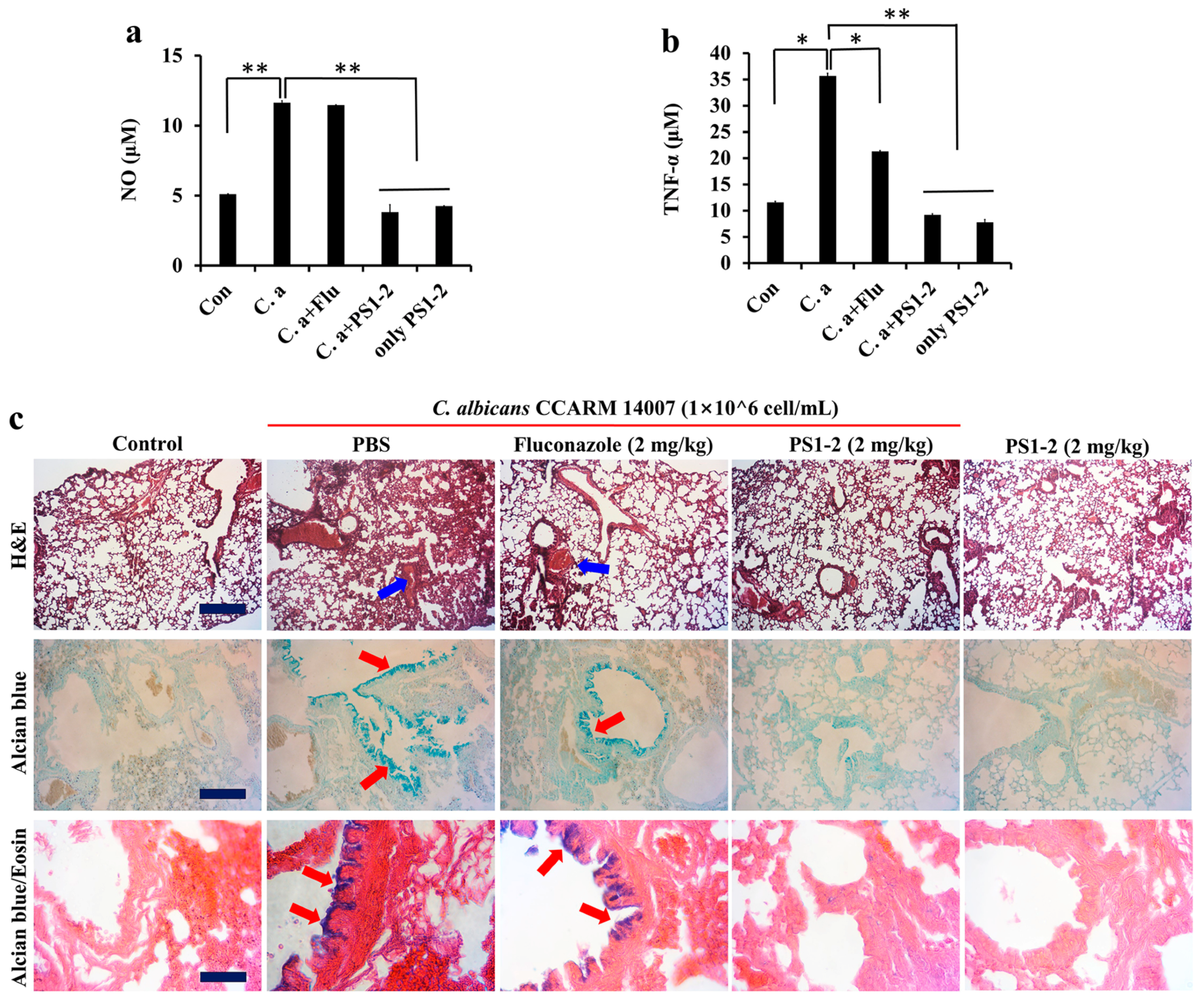
2.6. In Vitro Anti-Inflammatory Activity of PS1-2 Peptide in Co-Culture of RAW 264.7 and C. albicans
2.7. In Vivo Anti-Inflammatory Effects of PS1-2 in Acute Lung Injury
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the PS1P by Fmoc Solid-Phase Method
3.3. Anti-Fungicidal Activity
3.4. Binding Affinity of FAM-Labeled Peptide
3.5. Time-Killing Kinetic Assay
3.6. Drug-Resistance Assay
3.7. Trypsin Hydrolysis Assay
3.8. Anti-Inflammatory Assay
3.9. In Vivo Study
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackman, L.D.; Sutherland, T.D.; De-Barro, P.J.; Thissen, H.; Locock, K.E.S. Addressing a future pandemic: How can non-biological complex drugs prepare us for antimicrobial resistance threats? Mater. Horiz. 2022, 9, 2076–2096. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef] [PubMed]
- Ksiezopolska, E.; Gabaldón, T. Evolutionary Emergence of Drug Resistance in Candida Opportunistic Pathogens. Genes 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, N.; Cowen, L.E. Antifungal discovery. Curr. Opin. Microbiol. 2022, 69, 102198. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Langfeldt, A.; Gold, J.A.W.; Chiller, T. Emerging Fungal Infections: From the Fields to the Clinic, Resistant Aspergillus fumigatus and Dermatophyte Species: A One Health Perspective on an Urgent Public Health Problem. Curr. Clin. Microbiol. Rep. 2022, 27, 1–6. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Perrine-Walker, F. Caspofungin resistance in Candida albicans: Genetic factors and synergistic compounds for combination therapies. Braz. J. Microbiol. 2022, 53, 1101–1113. [Google Scholar] [CrossRef]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e0114621. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Yu, W.L. Antifungal susceptibility testing. New technology and clinical applications. Infect. Dis. Clin. North. Am. 2001, 15, 1227–1261. [Google Scholar] [CrossRef]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Erwing, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Romani, L.; Mencacci, A.; Cenci, E.; Del Sero, G.; Bistoni, F.; Puccetti, P. An immunoregulatory role for neutrophils in CD4+ T helper subset sselection in mice with candidiasis. J. Immunol. 1997, 58, 1256–2362. [Google Scholar]
- Cannon, G.J.; Swanson, J.A. The macrophage capacity for phagocytosis. J. Cell Sci. 1992, 101, 907–913. [Google Scholar] [CrossRef]
- Perez-Rodriguez, A.; Eraso, E.; Quindós, G.; Mateo, E. Antimicrobial Peptides with Anti-Candida Activity. Int. J. Mol. Sci. 2022, 23, 9264. [Google Scholar] [CrossRef]
- Zolin, G.V.S.; Fonseca, F.H.D.; Zambom, C.R.; Garrido, S.S. Histatin 5 Metallopeptides and Their Potential against Candida albicans Pathogenicity and Drug Resistance. Biomolecules 2021, 11, 1209. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides-Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Abdi, M.; Mirkalantari, S.; Amirmozafari, N. Bacterial resistance to antimicrobial peptides. J. Pept. Sci. 2019, 25, e3210. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Park, S.C.; Jung, M.; Shin, M.K.; Kang, H.L.; Baik, S.C.; Cheong, G.W.; Jang, M.K.; Lee, W.K. Cell-selectivity of tryptophan and tyrosine in amphiphilic α-helical antimicrobial peptides against drug-resistant bacteria. Biochem. Biophys. Res. Commun. 2018, 505, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Lee, M.Y.; Kim, J.Y.; Kim, H.; Jung, M.; Shin, M.K.; Lee, W.K.; Cheong, G.W.; Lee, J.R.; Jang, M.K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Park, S.; Kim, Y.M.; Guk, T.; Lee, M.Y.; Park, S.C.; Lee, J.R.; Jang, M.K. Candidacidal and Antibiofilm Activity of PS1-3 Peptide against Drug-Resistant Candida albicans on Contact Lenses. Pharmaceutics 2022, 14, 1602. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Makovitzki, A.; Shai, Y. pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry 2005, 44, 9775–9784. [Google Scholar] [CrossRef]
- Boris, S.; Barb, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes. Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- van Houte, J.; Lopman, J.; Kent, R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J. Dent. Res. 1996, 75, 1008–1014. [Google Scholar] [CrossRef]
- Hunt, J.F.; Fang, K.; Malik, R.; Snyder, A.; Malhotra, N.; Platts-Mills, T.A.; Gaston, B. Endogenous airway acidification. Implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2000, 161, 694–699. [Google Scholar] [CrossRef]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Rose, M.C.; Nickola, T.J.; Voynow, J.A. Airway mucus obstruction: Mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am. J. Respir. Cell Mol. Biol. 2001, 25, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef]

| C. albicans CCARM 14007 | MFCa (μM) | ||||
|---|---|---|---|---|---|
| PS1-2 | PS1-5 | PS1-6 | Fluconazole | ||
| Temperature | |||||
| 28 °C | pH | ||||
| pH 5.5 a | 32 | 16 | 16 | >128 | |
| pH 6.0 a | 32 | 16 | 16 | >128 | |
| pH 7.2 a | 32 | 32 | 16 | >128 | |
| Ion (pH 5.5) | |||||
| 150 mM NaCl | 32 | 64 | 32 | >128 | |
| 24 mM KCl | 32 | 64 | 32 | >128 | |
| 6 mM CaCl2 | 64 | 32 | 64 | >128 | |
| 6 mM MgCl2 | 32 | 32 | 32 | >128 | |
| 37 °C | pH | ||||
| pH 5.5 a | 32 | 16 | 16 | >128 | |
| pH 6.0 a | 32 | 16 | 16 | >128 | |
| pH 7.2 a | 32 | 32 | 16 | >128 | |
| Ion (pH 5.5) | |||||
| 150 mM NaCl | 32 | 64 | 32 | >128 | |
| 24 mM KCl | 32 | 64 | 32 | >128 | |
| 6 mM CaCl2 | 64 | 32 | 64 | >128 | |
| 6 mM MgCl2 | 32 | 32 | 32 | >128 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-K.; Park, S.; Kim, Y.-M.; Guk, T.; Choi, J.K.; Kim, J.-Y.; Lee, M.-Y.; Jang, M.-K.; Park, S.-C. Antifungal and Anti-Inflammatory Activities of PS1-2 Peptide against Fluconazole-Resistant Candida albicans. Antibiotics 2022, 11, 1779. https://doi.org/10.3390/antibiotics11121779
Lee J-K, Park S, Kim Y-M, Guk T, Choi JK, Kim J-Y, Lee M-Y, Jang M-K, Park S-C. Antifungal and Anti-Inflammatory Activities of PS1-2 Peptide against Fluconazole-Resistant Candida albicans. Antibiotics. 2022; 11(12):1779. https://doi.org/10.3390/antibiotics11121779
Chicago/Turabian StyleLee, Jong-Kook, Soyoung Park, Young-Min Kim, Taeuk Guk, Jong Kwon Choi, Jin-Young Kim, Min-Young Lee, Mi-Kyeong Jang, and Seong-Cheol Park. 2022. "Antifungal and Anti-Inflammatory Activities of PS1-2 Peptide against Fluconazole-Resistant Candida albicans" Antibiotics 11, no. 12: 1779. https://doi.org/10.3390/antibiotics11121779
APA StyleLee, J.-K., Park, S., Kim, Y.-M., Guk, T., Choi, J. K., Kim, J.-Y., Lee, M.-Y., Jang, M.-K., & Park, S.-C. (2022). Antifungal and Anti-Inflammatory Activities of PS1-2 Peptide against Fluconazole-Resistant Candida albicans. Antibiotics, 11(12), 1779. https://doi.org/10.3390/antibiotics11121779





