Awareness of Antibiotics and Antibiotic Resistance in a Rural District of Ha Nam Province, Vietnam: A Cross-Sectional Survey
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
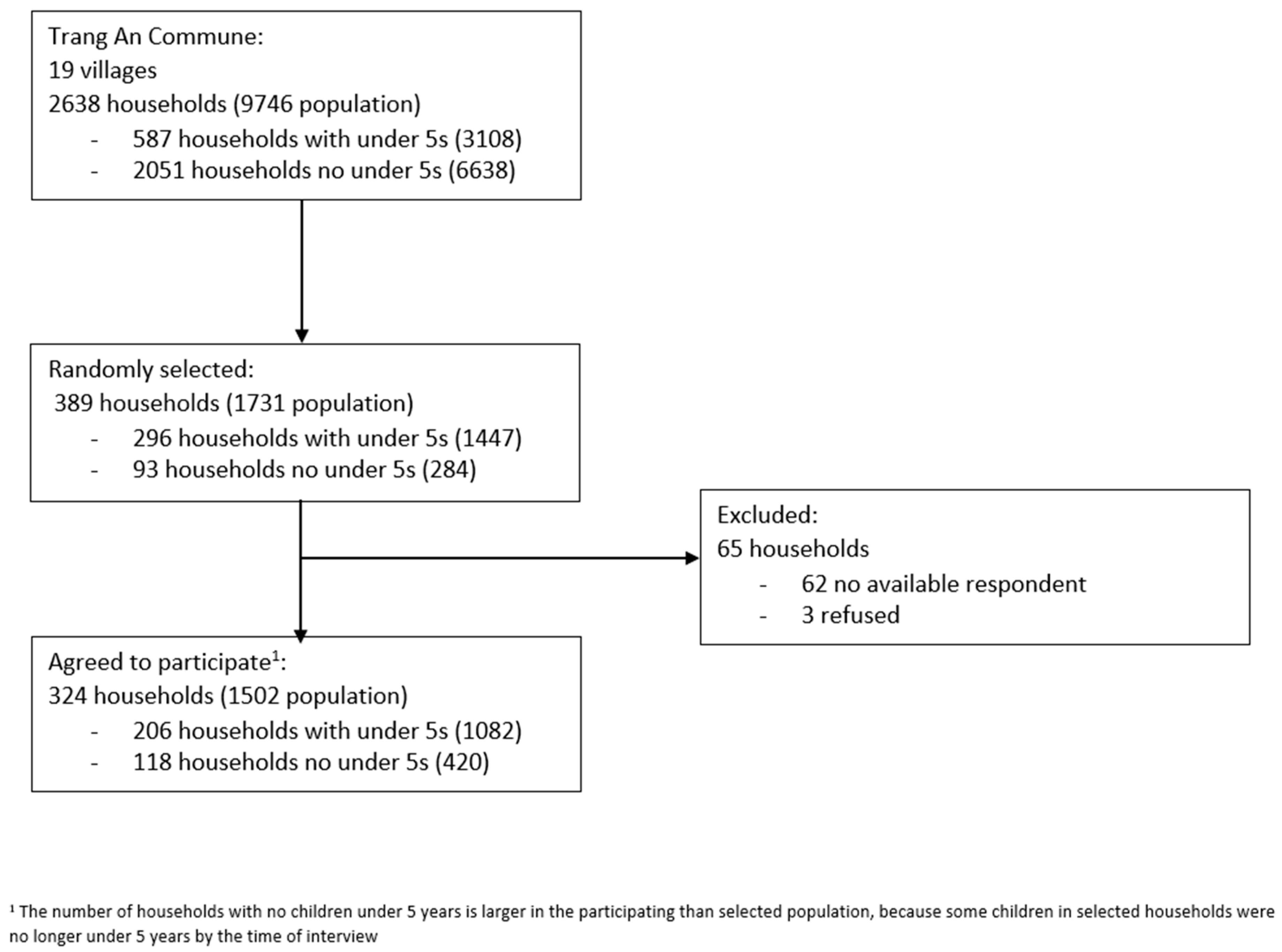
4.2. Sample Size
4.3. Definitions of Variables
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cars, O.; Nordberg, P. Antibiotic Resistance—The Faceless Threat. Int. J. Risk Saf. Med. 2005, 17, 103–110. [Google Scholar]
- Bush, K. The Coming of Age of Antibiotics: Discovery and Therapeutic Value. Ann. N. Y. Acad. Sci. 2010, 1213, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Smith, R.; Coast, J. The True Cost of Antimicrobial Resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The Negative Impact of Antibiotic Resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Heymann, D.L. Challenges of Drug Resistance in the Developing World. BMJ 2012, 344, e1567. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- and Middle-Income Countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Nga, D.T.T.; Chuc, N.T.K.; Hoa, N.P.; Hoa, N.Q.; Nguyen, N.T.T.; Loan, H.T.; Toan, T.K.; Phuc, H.D.; Horby, P.; Van Yen, N.; et al. Antibiotic Sales in Rural and Urban Pharmacies in Northern Vietnam: An Observational Study. BMC Pharmacol. Toxicol. 2014, 15, 6. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Resistance: Multi-Country Public Awareness Survey; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-150981-7.
- Vanden Eng, J.; Marcus, R.; Hadler, J.L.; Imhoff, B.; Vugia, D.J.; Cieslak, P.R.; Zell, E.; Deneen, V.; McCombs, K.G.; Zansky, S.M.; et al. Consumer Attitudes and Use of Antibiotics. Emerg. Infect. Dis. 2003, 9, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Trepka, M.J.; Belongia, E.A.; Chyou, P.-H.; Davis, J.P.; Schwartz, B. The Effect of a Community Intervention Trial on Parental Knowledge and Awareness of Antibiotic Resistance and Appropriate Antibiotic Use in Children. Pediatrics 2001, 107, e6. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.L.A.; Tolfree, R.; Kipping, R. Systematic Review of Public-Targeted Communication Interventions to Improve Antibiotic Use. J. Antimicrob. Chemother. 2017, 72, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.V.; Nguyen, A.M.T.; Nguyen, H.S.T. Public Awareness about Antibiotic Use and Resistance among Residents in Highland Areas of Vietnam. Available online: https://www.hindawi.com/journals/bmri/2019/9398536/ (accessed on 13 May 2020).
- Di, K.N.; Tay, S.T.; Ponnampalavanar, S.S.L.S.; Pham, D.T.; Wong, L.P. Socio-Demographic Factors Associated with Antibiotics and Antibiotic Resistance Knowledge and Practices in Vietnam: A Cross-Sectional Survey. Antibiotics 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Huy Hoang, N.; Notter, J.; Hall, J. The Application of a Conceptual Framework and Model for Information, Education and Communication (IEC) to Reduce Antibiotic Misuse in Vu Ban District, Nam Dinh Province. AJPHR 2019, 7, 58–72. [Google Scholar] [CrossRef]
- Lim, K.K.; Teh, C.C. A Cross Sectional Study of Public Knowledge and Attitude towards Antibiotics in Putrajaya, Malaysia. South Med. Rev. 2012, 5, 26–33. [Google Scholar]
- Do, N.T.T.; Vu, H.T.L.; Nguyen, C.T.K.; Punpuing, S.; Khan, W.A.; Gyapong, M.; Asante, K.P.; Munguambe, K.; Gómez-Olivé, F.X.; John-Langba, J.; et al. Community-Based Antibiotic Access and Use in Six Low-Income and Middle-Income Countries: A Mixed-Method Approach. Lancet Glob. Health 2021, 9, e610–e619. [Google Scholar] [CrossRef]
- Haenssgen, M.J.; Charoenboon, N.; Zanello, G.; Mayxay, M.; Reed-Tsochas, F.; Lubell, Y.; Wertheim, H.; Lienert, J.; Xayavong, T.; Zaw, Y.K.; et al. Antibiotic Knowledge, Attitudes and Practices: New Insights from Cross-Sectional Rural Health Behaviour Surveys in Low-Income and Middle-Income South-East Asia. BMJ Open 2019, 9, e028224. [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Rattanaumpawan, P.; Boonyasiri, A.; Pumsuwan, V.; Judaeng, T.; Tiengrim, S.; Paveenkittiporn, W.; Rojanasthien, S.; Jaroenpoj, S.; Issaracharnvanich, S. Thailand Antimicrobial Resistance Containment and Prevention Program. J. Glob. Antimicrob. Resist. 2015, 3, 290–294. [Google Scholar] [CrossRef]
- Mohrs, S. Factors Influencing the Use of Antibiotics and Knowledge about Antibiotic Resistance in Jakarta: A Qualitative Study on the Perceptions of Stakeholders Involved in Yayasan Orangtua Peduli’s Smart Use of Antibiotics Campaign in Indonesia; DiVA: Laval, QC, Australia, 2015; p. 71. [Google Scholar]
- Tangcharoensathien, V.; Chanvatik, S.; Kosiyaporn, H.; Kirivan, S.; Kaewkhankhaeng, W.; Thunyahan, A.; Lekagul, A. Population Knowledge and Awareness of Antibiotic Use and Antimicrobial Resistance: Results from National Household Survey 2019 and Changes from 2017. BMC Public Health 2021, 21, 2188. [Google Scholar] [CrossRef]
- Ferdiana, A.; Liverani, M.; Khan, M.; Wulandari, L.P.L.; Mashuri, Y.A.; Batura, N.; Wibawa, T.; Yeung, S.; Day, R.; Jan, S.; et al. Community Pharmacies, Drug Stores, and Antibiotic Dispensing in Indonesia: A Qualitative Study. BMC Public Health 2021, 21, 1800. [Google Scholar] [CrossRef] [PubMed]
- Mazińska, B.; Strużycka, I.; Hryniewicz, W. Surveys of Public Knowledge and Attitudes with Regard to Antibiotics in Poland: Did the European Antibiotic Awareness Day Campaigns Change Attitudes? PLoS ONE 2017, 12, e0172146. [Google Scholar] [CrossRef] [PubMed]
- Magis-Weinberg, L.; Ballonoff Suleiman, A.; Dahl, R.E. Context, Development, and Digital Media: Implications for Very Young Adolescents in LMICs. Front. Psychol. 2021, 12, 632713. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yuan, J.; Dong, M.; Paterson, P.; Lam, W.W.T. Drivers of Global Media Attention and Representations for Antimicrobial Resistance Risk: An Analysis of Online English and Chinese News Media Data, 2015–2018. Antimicrob. Resist. Infect. Control 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Cutilli, C.C. Seeking Health Information: What Sources Do Your Patients Use? Orthop. Nurs. 2010, 29, 214–219. [Google Scholar] [CrossRef]
- Tran, H.H.; Nguyen, H.A.T.; Tran, H.B.; Vu, B.N.T.; Nguyen, T.C.T.; Tacoli, C.; Tran, T.P.; Trinh, T.S.; Cai, T.H.N.; Nadjm, B.; et al. Feasibility, Acceptability, and Bacterial Recovery for Community-Based Sample Collection to Estimate Antibiotic Resistance in Commensal Gut and Upper Respiratory Tract Bacteria: A Cross-Sectional Mixed-Methods Study. Sci. Rep. 2022; in press. [Google Scholar]

| Characteristic | Total Study Population | ||
|---|---|---|---|
| n | % | ||
| Age (years) | 18–29 years | 53 | 16.4 |
| 30–49 years | 129 | 39.8 | |
| 50 years and above | 142 | 43.8 | |
| Sex | Female | 274 | 84.6 |
| Male | 50 | 15.4 | |
| Highest level of education | Never attended school/unknown | 59 | 18.7 |
| Attended any school level | 257 | 81.3 | |
| Occupation | Employed | 91 | 28.5 |
| Farmer | 210 | 65.8 | |
| Not working | 18 | 5.6 | |
| Household wealth tertile | Poor | 115 | 35.5 |
| Middle | 104 | 32.1 | |
| Rich | 105 | 32.4 | |
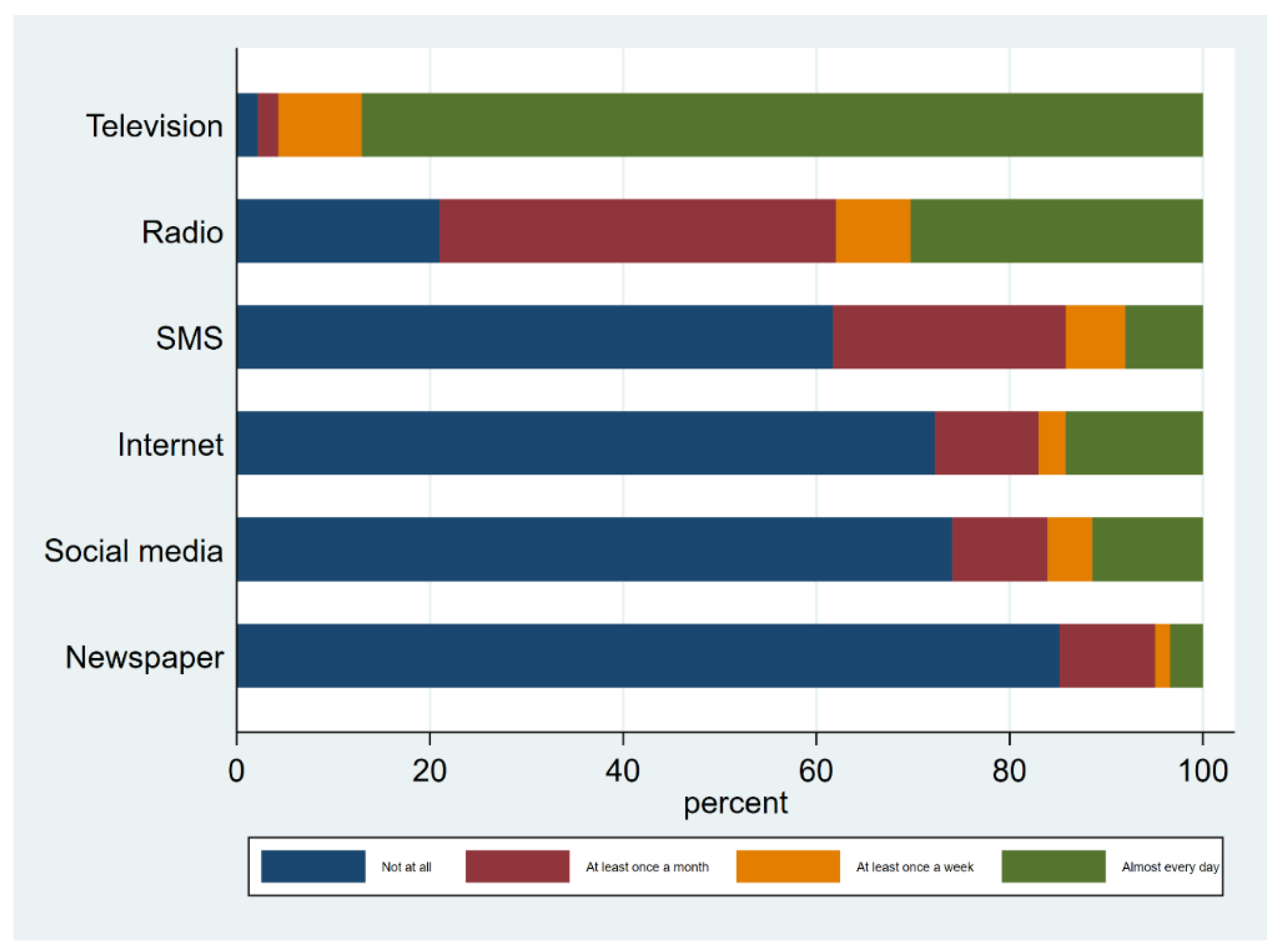
| Used print media in last month (newspapers and magazines) | Not used | 276 | 85.2 |
| Used | 48 | 14.8 | |
| Listened to radio in last month | Not used | 68 | 21 |
| Used | 256 | 79 | |
| Watched television in last month | Not used | 7 | 2.2 |
| Used | 317 | 97.8 | |
| Used Short Message Service (SMS) in last month | Not used | 200 | 61.7 |
| Used | 124 | 38.3 | |
| Used internet in last month | Not used | 234 | 72.2 |
| Used | 90 | 27.8 | |
| Used social media in last month | Not used | 240 | 74.1 |
| Used | 84 | 25.9 | |
| Television | Never | 15 | 4.6 |
| Sometimes, often, very often | 308 | 95.4 | |
| Radio | Never | 193 | 59.8 |
| Sometimes, often, very often | 130 | 40.2 | |
| Newspaper | Never | 280 | 86.7 |
| Sometimes, often, very often | 43 | 13.3 | |
| Magazine | Never | 285 | 88.2 |
| Sometimes, often, very often | 38 | 11.8 | |
| Book | Never | 274 | 84.8 |
| Sometimes, often, very often | 49 | 15.2 | |
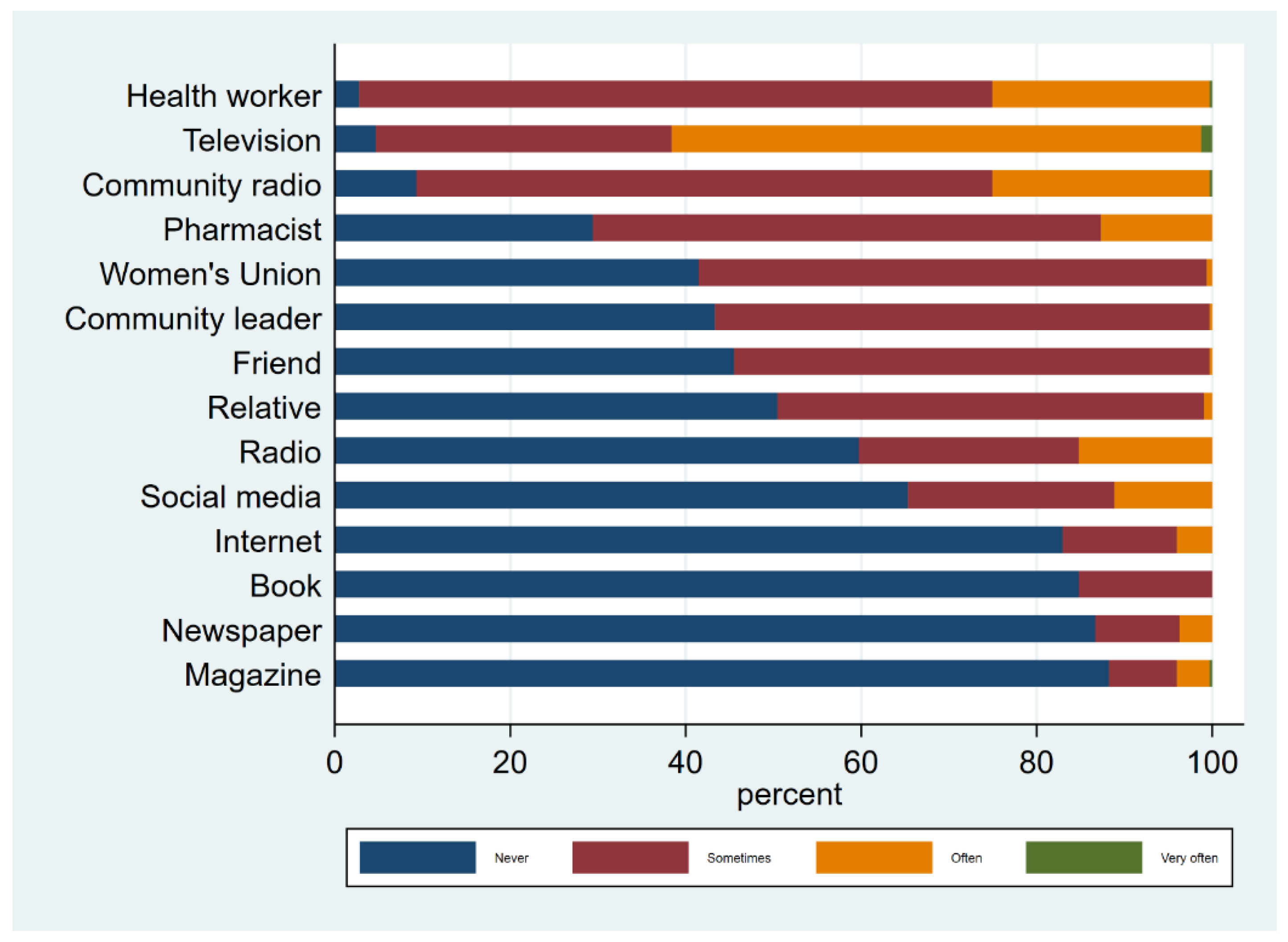
| Community radio | Never | 30 | 9.3 |
| Sometimes, often, very often | 293 | 90.7 | |
| Health worker | Never | 9 | 2.8 |
| Sometimes, often, very often | 314 | 97.2 | |
| Pharmacist | Never | 95 | 29.4 |
| Sometimes, often, very often | 228 | 70.6 | |
| Community leader | Never | 140 | 43.3 |
| Sometimes, often, very often | 183 | 56.7 | |
| Women’s Union | Never | 134 | 41.5 |
| Sometimes, often, very often | 189 | 58.5 | |
| Relative | Never | 163 | 50.5 |
| Sometimes, often, very often | 160 | 49.5 | |
| Friend | Never | 147 | 45.5 |
| Sometimes, often, very often | 176 | 54.5 | |
| Internet | Never | 268 | 83 |
| Sometimes, often, very often | 55 | 17 | |
| Social media | Never | 211 | 65.3 |
| Sometimes, often, very often | 112 | 34.7 | |
| Total | Heard of Antibiotics | Not Heard of Antibiotics | Heard of Antibiotics | Antibiotic Knowledge Score (Based on Naming Antibiotics) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Odds Ratio | Adjusted Odds Ratio | Crude Regression Coefficient | Adjusted Regression Coefficient | |||||||||||||||
| Characteristic | N | n | % | n | % | OR | 95% CI | p-Value | aOR | 95% CI | p-Value | B | 95% CI | p-Value | aB | 95% CI | p-Value | |
| Total | 323 | 232 | 71.8 | 91 | 28.2 | |||||||||||||
| Age (years) | 18–29 years | 52 | 40 | 76.9 | 12 | 23.1 | 1 | |||||||||||
| 30–49 years | 127 | 98 | 77.2 | 29 | 22.8 | 1.42 | (0.44–4.56) | 0.556 | 1.69 | (−1.12–4.50) | 0.237 | |||||||
| 50 years and above | 136 | 90 | 66.2 | 46 | 33.8 | 0.42 | (0.14–1.26) | 0.123 | −1.50 | (−4.41–1.41) | 0.312 | |||||||
| Sex | Female | 267 | 204 | 76.4 | 63 | 23.6 | 1 | |||||||||||
| Male | 48 | 24 | 50 | 24 | 50 | 0.30 | (0.13–0.73) | 0.008 | −3.16 | (−5.66–−0.66) | 0.013 | |||||||
| Highest level of education | Never attended school/unknown | 59 | 15 | 25.4 | 44 | 74.6 | 1 | |||||||||||
| Attended any school level | 256 | 213 | 83.2 | 43 | 16.8 | 9.99 | (3.90–25.60) | <0.001 | 2.26 | (0.13–4.39) | 0.037 | |||||||
| Occupation | Employed | 91 | 71 | 78 | 20 | 22 | 1 | |||||||||||
| Farmer | 203 | 150 | 73.9 | 53 | 26.1 | 0.59 | (0.26–1.38) | 0.225 | −1.56 | (−3.63–0.50) | 0.137 | |||||||
| Not working | 17 | 5 | 29.4 | 12 | 70.6 | 0.14 | (0.03–0.60) | 0.008 | −5.78 | (−9.69–−1.86) | 0.004 | |||||||
| Household wealth tertile | Poor | 109 | 75 | 68.8 | 34 | 31.2 | 1 | |||||||||||
| Middle | 102 | 79 | 77.5 | 23 | 22.5 | 1.22 | (0.54–2.78) | 0.626 | 1.72 | (−0.61–4.04) | 0.148 | |||||||
| Rich | 104 | 74 | 71.2 | 30 | 28.8 | 1.75 | (0.78–3.95) | 0.177 | 2.43 | (0.11–4.75) | 0.040 | |||||||
| Usual health facility | Government facility | 218 | 165 | 75.7 | 53 | 24.3 | 1 | |||||||||||
| Private/pharmacy/drugstore | 50 | 40 | 80 | 10 | 20 | 1.70 | (0.58–4.97) | 0.331 | 1.54 | (0.48–4.96) | 0.468 | 3.51 | (0.97–6.05) | 0.007 | 4.07 | (1.70–6.43) | 0.001 | |
| Traditional practitioner | 47 | 23 | 48.9 | 24 | 51.1 | 0.23 | (0.10–0.57) | 0.001 | 0.40 | (0.13–1.20) | 0.102 | −2.70 | (−5.28–−0.12) | 0.040 | −1.29 | (−3.80–1.23) | 0.315 | |
| Distance to nearest health facility | Less than 10 min | 151 | 136 | 90.1 | 15 | 9.9 | 1 | |||||||||||
| 10 min or more | 160 | 89 | 55.6 | 71 | 44.4 | 0.06 | (0.02–0.15) | <0.001 | 0.08 | (0.04–0.19) | <0.001 | −2.77 | (−4.56–−0.98) | 0.003 | −1.43 | (−3.24–0.38) | 0.121 | |
| Medical insurance card | No | 63 | 41 | 65.1 | 22 | 34.9 | 1 | |||||||||||
| Yes | 238 | 181 | 76.1 | 57 | 23.9 | 1.11 | (0.48–2.57) | 0.814 | 1.77 | (0.61–5.13) | 0.289 | −1.07 | (−3.36–1.22) | 0.360 | −0.57 | (−2.66–1.52) | 0.592 | |
| Media use frequency | Low | 120 | 92 | 76.7 | 28 | 23.3 | 1 | |||||||||||
| Medium | 89 | 53 | 59.6 | 36 | 40.4 | 0.34 | (0.15–0.76) | 0.009 | 0.41 | (0.14–1.24) | 0.114 | −2.13 | (−4.63–0.36) | 0.093 | −2.01 | (−4.82–0.79) | 0.159 | |
| High | 106 | 83 | 78.3 | 23 | 21.7 | 1.42 | (0.59–3.40) | 0.435 | 1.67 | (0.43–6.52) | 0.459 | 0.32 | (−1.71–2.35) | 0.756 | −0.30 | (−2.61–2.01) | 0.798 | |
| Health information source | ||||||||||||||||||
| Television | Never | 14 | 4 | 28.6 | 10 | 71.4 | 1 | |||||||||||
| Sometimes, often, very often | 301 | 224 | 74.4 | 77 | 25.6 | 14.62 | (2.81–75.93) | 0.001 | 7.32 | (0.44–122.44) | 0.166 | 7.21 | (3.73–10.70) | <0.001 | 5.15 | (1.10–9.20) | 0.013 | |
| Radio | Never | 190 | 154 | 81.1 | 36 | 18.9 | ||||||||||||
| Sometimes, often, very often | 125 | 74 | 59.2 | 51 | 40.8 | 0.48 | (0.24–0.96) | 0.039 | 0.81 | (0.33–1.99) | 0.641 | −0.22 | (−2.15–1.70) | 0.820 | 0.54 | (−1.39–2.48) | 0.582 | |
| Newspaper | Never | 273 | 206 | 75.5 | 67 | 24.5 | ||||||||||||
| Sometimes, often, very often | 42 | 22 | 52.4 | 20 | 47.6 | 0.50 | (0.19–1.32) | 0.159 | 1.91 | (0.51–7.15) | 0.333 | 0.92 | (−1.05–2.89) | 0.359 | 2.36 | (0.12–4.60) | 0.039 | |
| Magazine | Never | 278 | 212 | 76.3 | 66 | 23.7 | ||||||||||||
| Sometimes, often, very often | 37 | 16 | 43.2 | 21 | 56.8 | 0.31 | (0.11–0.88) | 0.028 | 1.17 | (0.30–4.53) | 0.820 | 0.46 | (−1.74–2.65) | 0.683 | 2.32 | (−0.45–5.08) | 0.100 | |
| Book | Never | 268 | 205 | 76.5 | 63 | 23.5 | ||||||||||||
| Sometimes, often, very often | 47 | 23 | 48.9 | 24 | 51.1 | 0.30 | (0.12–0.74) | 0.010 | 0.57 | (0.21–1.54) | 0.266 | −0.30 | (−2.13–1.53) | 0.748 | 0.38 | (−1.69–2.45) | 0.721 | |
| Community radio | Never | 28 | 17 | 60.7 | 11 | 39.3 | ||||||||||||
| Sometimes, often, very often | 287 | 211 | 73.5 | 76 | 26.5 | 3.07 | (1.06–8.90) | 0.039 | 2.95 | (0.65–13.37) | 0.159 | 4.20 | (0.44–7.95) | 0.029 | 2.80 | (−0.62–6.21) | 0.108 | |
| Health worker | Never | 8 | 1 | 12.5 | 7 | 87.5 | ||||||||||||
| Sometimes, often, very often | 307 | 227 | 73.9 | 80 | 26.1 | 104.80 | (12.14–904.50) | <0.001 | 172.78 | (13.49–2213.05) | <0.001 | 12.00 | (8.60–15.40) | <0.001 | 11.31 | (8.27–14.35) | <0.001 | |
| Pharmacist | Never | 92 | 64 | 69.6 | 28 | 30.4 | ||||||||||||
| Sometimes, often, very often | 223 | 164 | 73.5 | 59 | 26.5 | 2.23 | (1.09–4.59) | 0.029 | 2.45 | (0.88–6.87) | 0.087 | 3.38 | (1.20–5.56) | 0.003 | 2.67 | (0.57–4.78) | 0.013 | |
| Community leader | Never | 137 | 94 | 68.6 | 43 | 31.4 | ||||||||||||
| Sometimes, often, very often | 178 | 134 | 75.3 | 44 | 24.7 | 1.77 | (0.90–3.50) | 0.099 | 2.53 | (0.99–6.44) | 0.052 | 0.24 | (−1.87–2.35) | 0.823 | −0.33 | (−2.39–1.73) | 0.754 | |
| Woman’s Union | Never | 131 | 84 | 64.1 | 47 | 35.9 | ||||||||||||
| Sometimes, often, very often | 184 | 144 | 78.3 | 40 | 21.7 | 2.46 | (1.24–4.90) | 0.011 | 5.08 | (1.71–15.09) | 0.004 | 2.14 | (0.14–4.14) | 0.037 | 1.51 | (−0.52–3.55) | 0.145 | |
| Relative | Never | 160 | 112 | 70 | 48 | 30 | ||||||||||||
| Sometimes, often, very often | 155 | 116 | 74.8 | 39 | 25.2 | 1.68 | (0.85–3.33) | 0.136 | 4.08 | (1.42–11.74) | 0.009 | −0.59 | (−2.44–1.26) | 0.529 | −0.95 | (−2.73–0.84) | 0.297 | |
| Friend | Never | 143 | 96 | 67.1 | 47 | 32.9 | ||||||||||||
| Sometimes, often, very often | 172 | 132 | 76.7 | 40 | 23.3 | 1.56 | (0.79–3.09) | 0.199 | 1.73 | (0.69–4.34) | 0.243 | −0.24 | (−2.12–1.64) | 0.801 | −1.51 | (−3.30–0.28) | 0.098 | |
| Internet | Never | 262 | 186 | 71 | 76 | 29 | ||||||||||||
| Sometimes, often, very often | 53 | 42 | 79.2 | 11 | 20.8 | 1.91 | (0.65–5.59) | 0.240 | 2.95 | (1.10–7.94) | 0.032 | 0.77 | (−0.72–2.27) | 0.310 | 0.19 | (−1.83–2.21) | 0.855 | |
| Social media | Never | 207 | 147 | 71 | 60 | 29 | ||||||||||||
| Sometimes, often, very often | 108 | 81 | 75 | 27 | 25 | 1.63 | (0.76–3.48) | 0.206 | 2.62 | (0.69–9.99) | 0.157 | 0.47 | (−1.47–2.42) | 0.631 | 0.48 | (−1.83–2.80) | 0.683 | |
| Health information seeking type | Low information seeking | 124 | 94 | 75.8 | 30 | 24.2 | ||||||||||||
| Official sources | 34 | 16 | 47.1 | 18 | 52.9 | 0.32 | (0.11–0.97) | 0.044 | 0.48 | (0.13–1.81) | 0.277 | −3.90 | (−6.75–−1.04) | 0.008 | −2.56 | (−5.56–0.43) | 0.093 | |
| Interpersonal sources | 123 | 105 | 85.4 | 18 | 14.6 | 2.05 | (0.88–4.80) | 0.096 | 4.06 | (1.32–12.46) | 0.015 | −1.50 | (−3.64–0.64) | 0.169 | −1.63 | (−3.67–0.40) | 0.116 | |
| High information seeking | 34 | 13 | 38.2 | 21 | 61.8 | 0.38 | (0.12–1.17) | 0.092 | 2.12 | (0.37–12.14) | 0.398 | −0.61 | (−3.32–2.10) | 0.659 | 0.44 | (−2.56–3.44) | 0.772 | |
| Total | Heard of AMR | Not Heard of AMR | Heard of Antibiotic Resistance (AMR) | Antibiotic Resistance Knowledge Score (Based on Questions about Antibiotic Resistance) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Odds Ratio | Adjusted Odds Ratio | Crude Regression Coefficient | Adjusted Regression Coefficient | |||||||||||||||
| Characteristic | N | n | % | n | % | OR | 95% CI | p-Value | aOR | 95% CI | p-Value | B | 95% CI | p-Value | aB | 95% CI | p-Value | |
| Total | 322 | 57 | 17.7 | 265 | 82.3 | |||||||||||||
| Age (years) | 18–29 years | 52 | 21 | 40.4 | 31 | 59.6 | 1 | |||||||||||
| 30–49 years | 127 | 26 | 20.5 | 101 | 79.5 | 0.40 | (0.14–1.15) | 0.089 | −3.11 | (−6.84–0.63) | 0.103 | |||||||
| 50 years and above | 135 | 10 | 7.4 | 125 | 92.6 | 0.09 | (0.03–0.33) | <0.001 | −6.44 | (−9.81–−3.06) | <0.001 | |||||||
| Sex | Female | 266 | 49 | 18.4 | 217 | 81.6 | 1 | |||||||||||
| Male | 48 | 8 | 16.7 | 40 | 83.3 | 0.49 | (0.14–1.68) | 0.253 | −2.68 | (−4.11–−1.25) | <0.001 | |||||||
| Highest level of education | Never attended school/unknown | 59 | 6 | 10.2 | 53 | 89.8 | 1 | |||||||||||
| Attended any school level | 255 | 51 | 20 | 204 | 80 | 1.20 | (0.37–3.89) | 0.764 | 1.44 | (−1.05–3.92) | 0.256 | |||||||
| Occupation | Employed | 90 | 25 | 27.8 | 65 | 72.2 | 1 | |||||||||||
| Farmer | 203 | 29 | 14.3 | 174 | 85.7 | 0.33 | (0.14–0.78) | 0.012 | −3.54 | (−6.11–−0.97) | 0.007 | |||||||
| Not working | 17 | 3 | 17.6 | 14 | 82.4 | 0.10 | (0.02–0.43) | 0.002 | −5.40 | (−7.94–−2.87) | <0.001 | |||||||
| Household wealth tertile | Poor | 109 | 15 | 13.8 | 94 | 86.2 | 1 | |||||||||||
| Middle | 103 | 14 | 13.6 | 89 | 86.4 | 0.97 | (0.31–3.02) | 0.957 | −0.03 | (−1.99–1.93) | 0.974 | |||||||
| Rich | 102 | 28 | 27.5 | 74 | 72.5 | 2.60 | (0.97–6.96) | 0.057 | 2.66 | (0.26–5.06) | 0.030 | |||||||
| Usual health facility | Government facility | 219 | 43 | 19.6 | 176 | 80.4 | 1 | |||||||||||
| Private/pharmacy/drugstore | 49 | 5 | 10.2 | 44 | 89.8 | 0.14 | (0.05–0.39) | <0.001 | 0.14 | (0.05–0.44) | 0.001 | −2.18 | (−3.76–−0.61) | 0.007 | −1.96 | (−3.41–−0.51) | 0.008 | |
| Traditional practitioner | 46 | 9 | 19.6 | 37 | 80.4 | 0.91 | (0.30–2.78) | 0.869 | 1.55 | (0.41–5.82) | 0.512 | −1.95 | (−4.19–0.30) | 0.089 | −0.56 | (−2.85–1.74) | 0.635 | |
| Distance to nearest health facility | Less than 10 min | 151 | 38 | 25.2 | 113 | 74.8 | 1 | |||||||||||
| 10 min or more | 160 | 19 | 11.9 | 141 | 88.1 | 0.31 | (0.13–0.74) | 0.009 | 0.43 | (0.18–1.07) | 0.068 | −4.29 | (−6.27–−2.31) | <0.001 | −3.26 | (−5.20–−1.33) | 0.001 | |
| Medical insurance card | No | 62 | 8 | 12.9 | 54 | 87.1 | 1 | |||||||||||
| Yes | 238 | 48 | 20.2 | 190 | 79.8 | 2.94 | (0.86–10.03) | 0.085 | 3.70 | (1.06–12.96) | 0.041 | 2.42 | (0.97–3.86) | 0.001 | 2.85 | (1.35–4.36) | <0.001 | |
| Media use frequency | Low | 121 | 8 | 6.6 | 113 | 93.4 | 1 | |||||||||||
| Medium | 89 | 7 | 7.9 | 82 | 92.1 | 1.36 | (0.31–5.98) | 0.686 | 1.38 | (0.30–6.31) | 0.680 | 0.09 | (−1.68–1.87) | 0.919 | 0.69 | (−1.35–2.73) | 0.506 | |
| High | 104 | 42 | 40.4 | 62 | 59.6 | 11.60 | (3.73–36.09) | <0.001 | 9.54 | (2.39–38.07) | 0.001 | 5.86 | (3.44–8.28) | <0.001 | 5.46 | (2.49–8.42) | <0.001 | |
| Health information source | ||||||||||||||||||
| Television | Never | 14 | 0 | 0 | 14 | 100 | 1 | |||||||||||
| Sometimes, often, very often | 299 | 57 | 19.1 | 242 | 80.9 | 1.00 | 1.00 | 3.98 | (2.97–4.98) | <0.001 | 2.34 | (0.65–4.03) | 0.007 | |||||
| Radio | Never | 189 | 26 | 13.8 | 163 | 86.2 | ||||||||||||
| Sometimes, often, very often | 124 | 31 | 25 | 93 | 75 | 1.89 | (0.82–4.34) | 0.135 | 3.10 | (1.09–8.81) | 0.034 | 1.12 | (−0.89–3.12) | 0.273 | 2.25 | (0.20–4.30) | 0.032 | |
| Newspaper | Never | 271 | 41 | 15.1 | 230 | 84.9 | ||||||||||||
| Sometimes, often, very often | 42 | 16 | 38.1 | 26 | 61.9 | 4.53 | (1.60–12.83) | 0.005 | 9.37 | (1.82–48.15) | 0.008 | 4.34 | (0.16–8.52) | 0.042 | 5.82 | (1.78–9.86) | 0.005 | |
| Magazine | Never | 276 | 45 | 16.3 | 231 | 83.7 | ||||||||||||
| Sometimes, often, very often | 37 | 12 | 32.4 | 25 | 67.6 | 3.61 | (1.17–11.10) | 0.025 | 5.88 | (0.85–40.71) | 0.072 | 2.78 | (−1.32–6.87) | 0.183 | 3.53 | (−0.37–7.43) | 0.076 | |
| Book | Never | 266 | 42 | 15.8 | 224 | 84.2 | ||||||||||||
| Sometimes, often, very often | 47 | 15 | 31.9 | 32 | 68.1 | 2.15 | (0.77–6.01) | 0.142 | 1.85 | (0.40–8.66) | 0.432 | 1.92 | (−1.50–5.34) | 0.271 | 2.06 | (−2.11–6.24) | 0.332 | |
| Community radio | Never | 28 | 5 | 17.9 | 23 | 82.1 | ||||||||||||
| Sometimes, often, very often | 285 | 52 | 18.2 | 233 | 81.8 | 3.83 | (1.28–11.46) | 0.016 | 3.82 | (1.15–12.67) | 0.029 | 3.35 | (2.09–4.61) | <0.001 | 3.23 | (1.63–4.84) | <0.001 | |
| Health worker | Never | 8 | 1 | 12.5 | 7 | 87.5 | ||||||||||||
| Sometimes, often, very often | 305 | 56 | 18.4 | 249 | 81.6 | 8.28 | (0.95–72.26) | 0.056 | 5.05 | (0.60–42.30) | 0.135 | 3.43 | (2.00–4.87) | <0.001 | 2.53 | (0.60–4.47) | 0.010 | |
| Pharmacist | Never | 91 | 22 | 24.2 | 69 | 75.8 | ||||||||||||
| Sometimes, often, very often | 222 | 35 | 15.8 | 187 | 84.2 | 1.10 | (0.46–2.66) | 0.826 | 0.91 | (0.33–2.56) | 0.861 | 1.22 | (−0.60–3.04) | 0.189 | 0.87 | (−0.84–2.58) | 0.319 | |
| Community leader | Never | 135 | 22 | 16.3 | 113 | 83.7 | ||||||||||||
| Sometimes, often, very often | 178 | 35 | 19.7 | 143 | 80.3 | 1.29 | (0.55–3.02) | 0.550 | 1.29 | (0.52–3.19) | 0.580 | 1.36 | (−0.50–3.23) | 0.151 | 1.43 | (−0.40–3.26) | 0.125 | |
| Woman’s Union | Never | 129 | 20 | 15.5 | 109 | 84.5 | ||||||||||||
| Sometimes, often, very often | 184 | 37 | 20.1 | 147 | 79.9 | 1.70 | (0.72–4.05) | 0.227 | 1.43 | (0.58–3.56) | 0.439 | 2.95 | (1.22–4.68) | 0.001 | 2.77 | (1.10–4.44) | 0.001 | |
| Relative | Never | 158 | 27 | 17.1 | 131 | 82.9 | ||||||||||||
| Sometimes, often, very often | 155 | 30 | 19.4 | 125 | 80.6 | 1.54 | (0.67–3.52) | 0.306 | 1.46 | (0.63–3.40) | 0.379 | 2.06 | (0.15–3.96) | 0.035 | 2.09 | (0.27–3.91) | 0.024 | |
| Friend | Never | 141 | 18 | 12.8 | 123 | 87.2 | ||||||||||||
| Sometimes, often, very often | 172 | 39 | 22.7 | 133 | 77.3 | 1.93 | (0.82–4.53) | 0.130 | 1.20 | (0.52–2.77) | 0.675 | 2.40 | (0.51–4.29) | 0.013 | 1.37 | (−0.41–3.15) | 0.132 | |
| Internet | Never | 260 | 35 | 13.5 | 225 | 86.5 | ||||||||||||
| Sometimes, often, very often | 53 | 22 | 41.5 | 31 | 58.5 | 3.29 | (1.26–8.64) | 0.016 | 2.00 | (0.74–5.40) | 0.173 | 3.17 | (−0.27–6.62) | 0.071 | 1.81 | (−1.43–5.05) | 0.272 | |
| Social media | Never | 207 | 20 | 9.7 | 187 | 90.3 | ||||||||||||
| Sometimes, often, very often | 106 | 37 | 34.9 | 69 | 65.1 | 6.16 | (2.58–14.74) | <0.001 | 4.43 | (1.65–11.86) | 0.003 | 4.30 | (1.88–6.72) | 0.001 | 3.23 | (0.64–5.82) | 0.015 | |
| Health information seeking type | Low information seeking | 124 | 20 | 16.1 | 104 | 83.9 | ||||||||||||
| Official sources | 32 | 6 | 18.8 | 26 | 81.3 | 1.92 | (0.46–8.06) | 0.369 | 3.88 | (1.01–14.86) | 0.048 | 0.28 | (−2.60–3.15) | 0.849 | 1.50 | (−0.94–3.95) | 0.227 | |
| Interpersonal sources | 123 | 21 | 17.1 | 102 | 82.9 | 1.25 | (0.47–3.34) | 0.654 | 1.33 | (0.48–3.66) | 0.583 | 1.53 | (−0.40–3.46) | 0.119 | 1.74 | (−0.13–3.60) | 0.067 | |
| High information seeking | 35 | 10 | 28.6 | 25 | 71.4 | 5.08 | (1.48–17.51) | 0.010 | 12.85 | (1.63–101.10) | 0.015 | 4.77 | (0.03–9.51) | 0.048 | 6.75 | (1.88–11.62) | 0.007 | |
| Variable | Definition | Categories |
|---|---|---|
| Antibiotic awareness | Have you ever heard of a type of medicine called an antibiotic? | Yes No/don’t know |
| Antibiotic knowledge | Which antibiotics have you heard of? (Mentioned spontaneously or after probing—penicillin, doxycycline, tetracycline, erythromycin, ampicillin/amoxicillin, Augmentin, streptomycin, cotrimoxazole, cephalexin, ciprofloxacin, colistin) | Score (sum of scores for each antibiotic, where 3 for each antibiotic mentioned spontaneously, 2 for each antibiotic mentioned after probing, and 1 for each antibiotic not known) |
| Antibiotic resistance awareness | Some antibiotic medicines that used to work in fighting infections no longer work. This problem is called antibiotic resistance. Have you heard of this problem before? | Yes No/don’t know |
| Antibiotic resistance knowledge | What could the consequences of getting an antibiotic resistant infection be? (Multiple choice answers: Be sick for longer; May have to visit doctor more or be treated in hospital; May need more expensive medicine that may cause side-effects; Other) Can you think of any ways of reducing the problem of antibiotic resistance? (Don’t take antibiotics when they are not needed (e.g., for colds and sore throats); Don’t demand antibiotics from health-workers or drug suppliers; Make sure antibiotics are good quality and within expiry date; Complete the course as recommended by a health-worker; Don’t use antibiotics prescribed for someone else; Make sure you use the right antibiotic for the right infection; Make sure you take antibiotics as soon as you feel sick; Make sure you take a very strong antibiotic to kill the infection; Take several different antibiotics to make sure the infection is killed; Don’t use antibiotics in animal feed as a growth promoter; Washing hands after contact with a live animal, slaughtering animals, or preparing meat; Washing hands after contact with someone or something that has been touched by a person who has an antibiotic-resistant infection) | Score summing each correct answer |
| Education | Whether one had attended any school beyond nursery level | Any school level—primary to tertiary Never attended school—less than primary school level—or unknown |
| Occupation | Main work of the respondent | Farmers Employed (factories, labourers, office, shops and others) Not working |
| Household wealth | Tertiles based on principal component analysis of household assets (telephone, mobile phone, computer, tablet, radio, TV, bed, table and chairs, sofa, fan, air conditioner, gas cooker, electric cooker, washing machine, refrigerator, bicycle, motorcycle car, tractor, motorboat), electricity, crowding, type of flooring, type of roofing, type of walls | Poor Middle Rich |
| Usual health facility | The facility that respondents considered as their primary facility where their children or themselves go when they get sick | Government Private/pharmacy/drug stores Others (traditional healers, shops, and those that do not seek care) |
| Distance to health facility | Time it took from their house to the commune primary healthcare centre | Less than 10 min More than 10 min |
| Medical insurance card | Whether they had a government provided medical insurance card | Yes—when they had the card No—when they did not have |
| Access to different media | Respondents were asked whether they had access to different media platforms | Access to Print media, Radio, Television, SMS, social media and Internet No access to the above. |
| Media use frequency | Tertiles based on principal component analysis of frequency of access to different media platforms | Low Medium High |
| Health information seeking type | Groups based on latent class analysis of frequency of access to different sources of health information | Low information seeking across all sources Official sources-mainly newspaper, television, radio, community radio, health worker Interpersonal sources-mainly pharmacist, friends, relatives, community leader, women’s union High information seeking across all sources |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulaya, G.; Nguyen, T.C.T.; Vu, B.N.T.; Dang, D.A.; Nguyen, H.A.T.; Tran, H.H.; Tran, H.K.T.; Reeve, M.; Pham, Q.D.; Trinh, T.S.; et al. Awareness of Antibiotics and Antibiotic Resistance in a Rural District of Ha Nam Province, Vietnam: A Cross-Sectional Survey. Antibiotics 2022, 11, 1751. https://doi.org/10.3390/antibiotics11121751
Ulaya G, Nguyen TCT, Vu BNT, Dang DA, Nguyen HAT, Tran HH, Tran HKT, Reeve M, Pham QD, Trinh TS, et al. Awareness of Antibiotics and Antibiotic Resistance in a Rural District of Ha Nam Province, Vietnam: A Cross-Sectional Survey. Antibiotics. 2022; 11(12):1751. https://doi.org/10.3390/antibiotics11121751
Chicago/Turabian StyleUlaya, Godwin, Tu Cam Thi Nguyen, Bich Ngoc Thi Vu, Duc Anh Dang, Hien Anh Thi Nguyen, Hoang Huy Tran, Huong Kieu Thi Tran, Matthew Reeve, Quynh Dieu Pham, Tung Son Trinh, and et al. 2022. "Awareness of Antibiotics and Antibiotic Resistance in a Rural District of Ha Nam Province, Vietnam: A Cross-Sectional Survey" Antibiotics 11, no. 12: 1751. https://doi.org/10.3390/antibiotics11121751
APA StyleUlaya, G., Nguyen, T. C. T., Vu, B. N. T., Dang, D. A., Nguyen, H. A. T., Tran, H. H., Tran, H. K. T., Reeve, M., Pham, Q. D., Trinh, T. S., van Doorn, H. R., & Lewycka, S. (2022). Awareness of Antibiotics and Antibiotic Resistance in a Rural District of Ha Nam Province, Vietnam: A Cross-Sectional Survey. Antibiotics, 11(12), 1751. https://doi.org/10.3390/antibiotics11121751






