1. Introduction
A disinfectant is an agent that destroys disease-causing microorganisms. Typically, disinfectants are substances applied to inanimate objects which distinguish them from antiseptics which are applied to living tissues. Some common examples of disinfectants are phenolic compounds, alcohols, quaternary ammonium compounds, chlorine compounds, aldehydes, and gases such as ethylene oxide. Disinfectants are used extensively in hospitals and other health care facilities for killing microorganisms on a variety of hard surfaces [
1]. These substances are essential components of infection control practices and aid in the prevention of nosocomial infections. Mounting concerns over the potential for microbial contamination and infection risks in the food and general consumer markets have also led to increased use of antiseptics and disinfectants by the general public. Disinfectants comprise a wide variety of active chemical agents (or “biocides”). Many of these compounds have been used for hundreds of years for antisepsis, disinfection, and preservation. Despite this, the modes of action of active agents remain largely unknown. In general, biocides have a broader spectrum of activity than antibiotics, and, while antibiotics tend to have specific intracellular targets, biocides may have multiple targets. One example of such biocides includes aerosols, such as fog and mineral oils.
Aerosols generated through volatilization and subsequent recondensation of oil vapors have been used as obscurant (smoke) screens during military operations since the early twentieth century [
2,
3]. The battlefield obscurant used for impairing view in the visible region produces a dense white cloud which attenuates light transmission in the visible region of the electromagnetic spectrum thereby confounding enemy sensors and smart munitions [
2,
3]. For the past four decades, fog oil (de-aromatized middle distillate petroleum (MIL-F-12070E)) has been used by the US military as obscurant in the battlefield and during training exercises. Recently, use of biogenic (vegetable) oils has been investigated as a substitute for the fog oil [
4,
5,
6]. Biogenic oils are non-petroleum-based oils with physical properties similar to fog oil. Compared to other disinfectants, fog and biogenic oils have properties that make them superior to other commonly used disinfectants [
7,
8,
9,
10,
11,
12,
13]. First, fog oils are free of potentially carcinogenic poly aromatic hydrocarbons (PAHs). Second, the individual components of these oils (e.g., esters, aldehydes, terpenes, alcohols, and hydrocarbons) are more effective antimicrobial agents when used in the vapor phase than in the solution phase [
6]. Lastly, the diversity of compounds allows for a broad range and potency of antimicrobial activities against different bacteria [
6]. The antimicrobial activity of oils is associated with their physico-chemical properties, which affect the depth of penetration into the bilayer. For example, lipophilic oils can lower the plasma membrane integrity in mammalian and bacterial cells.
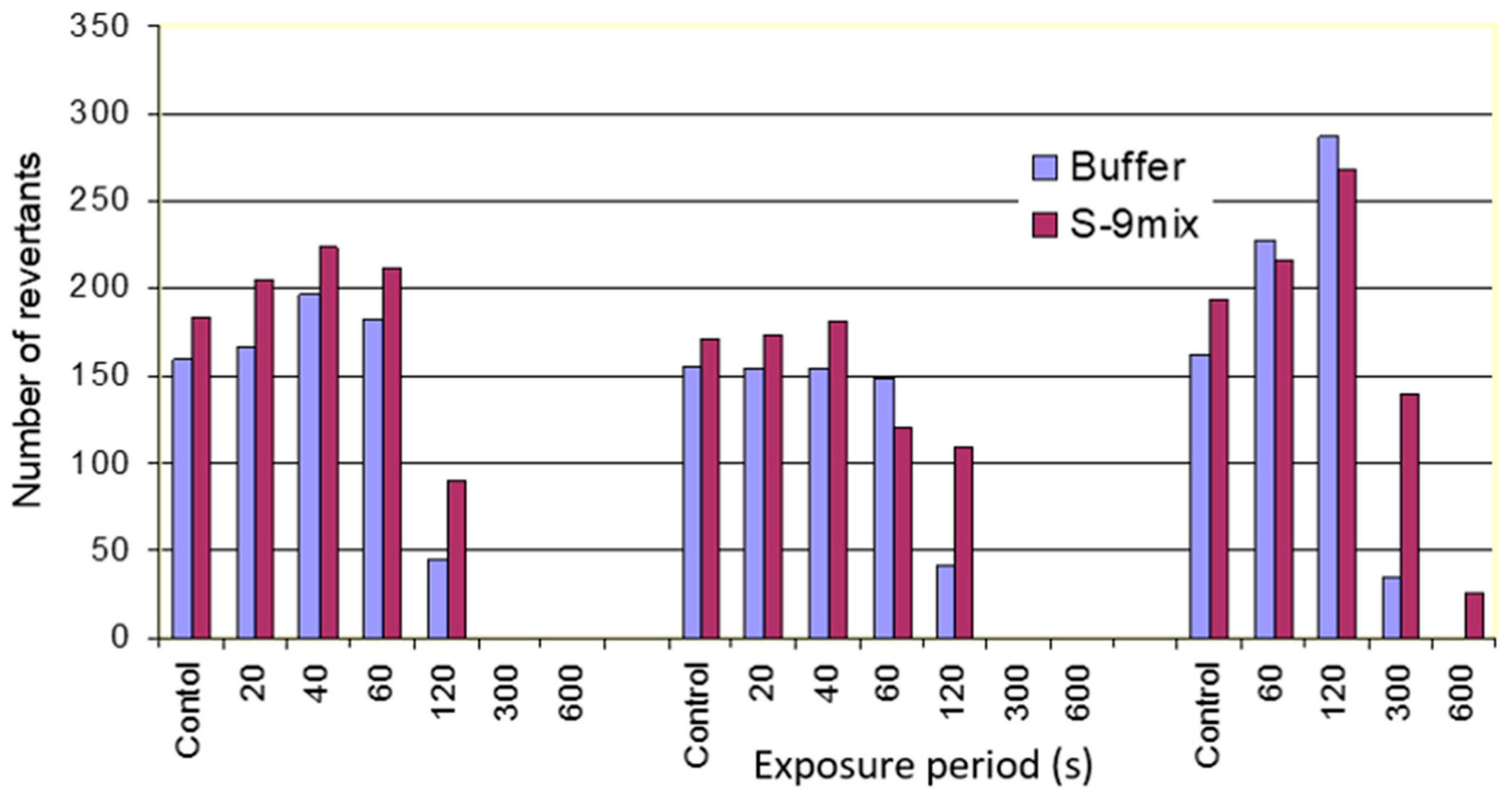
During these evaluations, the fog oil and vegetable oil esters and their vapors were subjected to the Ames test [
14]. The Ames test is one of the most widely used assay techniques to test the mutagenicity of chemicals [
14]. The Ames test involves exposure of auxotrophic strains of
Salmonella typhimurium (histidine requiring) to potential mutagenic compounds and measuring the relative increase in reversion of histidine auxotrophy [
14]. The antibacterial activity of the fog oil and vegetable esters was observed through the elimination of the background growth in the Ames test. The results of the Ames test revealed that the vapors obtained from oils under certain conditions were not mutagenic but lethal to bacterial strains used in the Ames test. These results opened the potential use of oil-derived vapors as antimicrobial agents. The overall objective of this study was to evaluate antimicrobial properties of volatiles produced during rapid volatilization of biogenic oil esters and mineral oils through modifications in the lethality of the biogenic oil esters through different times, temperatures, and exposures.
4. Methods
Oils: Methyl soyate was purchased from AG Environmental Products LLC (Lenexa, Kansas). Fog oil was obtained from US Army Chemical School (Fort Leonard Wood, MO, USA). Fog oil is a middle distillate of petroleum similar to the commercially available mineral oil. It is used for generation of smoke screens through vaporization and condensation processes. Soy oil propyl ester (SOPE), olive oil methyl ester (OOME), and linseed oil methyl esters were prepared in the laboratory through transesterification reactions of the oils with the appropriate alcohol in the presence of a basic catalyst. The biogenic oil esters used had different carbon chain lengths and unsaturation. Diesel is a specific fractional distillate of fuel oil (mostly petroleum) and made up of 75% saturated hydrocarbons and 25% aromatic hydrocarbons. It was obtained from a local gas station (Rolla, MO, USA). Jet Propellant 8 (JP-8) is a kerosene-like fuel for jet aircrafts and Department of Defense (DOD) tactical vehicles. It was obtained from US Army Chemical School at Fort Leonard Wood, MO, USA.
Ames strains: TA97, TA98, and TA100 were obtained from Dr. Bruce Ames Laboratory, University of California, Berkeley [
14]. These stains are histidine-dependent
Salmonella strains each carrying different mutations in various genes in the histidine operon. Stock cultures were stored at −80 °C and fresh sub-cultures from frozen stock were prepared on a regular basis [
14]. Active cultures were transferred to fresh media every week.
Bacterial cultures: K. pneumoniae, P. aeruginosa, E. cloacae, E. coli, S. marcescens, S. flexneri, B. megaterium, S. aureus, and S. typhimuirum were obtained from American Type Collection Center (ATCC) and stock cultures were stored at −80 °C. Fresh sub-cultures from frozen stock were prepared on a regular basis. Active cultures were transferred to fresh media every week.
Bacterial culture media: The following culture media were used for basic bacterial culturing, mutagenicity testing, and exposure experiments. Tryptic soy agar is a nutrient medium suitable for sub-culturing, serial dilutions, and exposure experiments. It was prepared with 1.5% agar and 3.5% tryptic soy broth. Glucose minimal media is the bottom agar used for mutagenicity assay. It was prepared with 1.5% agar, 1X Vogel Bonner (VB) salt solution, and 0.2% glucose solution. Agar was added to water in a flask and autoclaved 10 X VB stock solutions and 10% glucose solution were added, mixed, and poured into Petri dishes. Top agar supplemented with histidine/biotin was used to deliver the bacteria, chemical, and buffer or S9 mix to the bottom agar. The main ingredients include 0.7% agar and sodium chloride. Filter-sterilized histidine/biotine was added after autoclaving.
S9 mix creation: Some carcinogenic chemicals are biologically inactive unless they are metabolized in the liver to active forms. Since bacteria do not have appropriate metabolic capability, an exogenous metabolic mammalian organ activation system can be added to the Petri dish together with the test chemical and the bacteria. Tests were performed with and without S9 to determine if liver metabolism significantly impacts mutagenicity. S9 was generated through a rodent metabolic activation system which mainly consists of 90008g supernatant fraction of a rat liver homogenate and was delivered to the test system in the presence of NADP and cofactor NAPDH-supported oxidation. Sodium phosphate buffer: This was added to the control sample (without S9) and used to test chemicals in the absence of metabolic activation. Monobasic sodium phosphates (0.1 M) and dibasic sodium phosphates (0.1 M) were combined to achieve a pH of 7.4. The buffer was autoclaved and stored in screw-cap bottles.
Exposure of Ames strains to aerosols: All smoke generation and exposure experiments were carried out in a fume hood. The exposure of Ames strains and other bacteria was conducted in the gas-tight stainless steel–borosilicate chamber described earlier. The chamber was placed inside the fume hood. The test specimens were placed inside or retrieved from the chamber through a hinged door. In the following experiments, the air flow was maintained at 10 L min−1 while oil flow was maintained at 0.5 mL min−1 unless otherwise mentioned. Temperature inside the chamber was 24 °C.
Mutagenicity testing of oil aerosols and vapor produced from linseed oil methyl ester, fog oil, and methyl soyate: Top agar was mixed with Ames strain TA97 and S9 mix or buffer. The contents of the tube were poured on the surface of minimal glucose agar medium. The plates were then exposed to oil aerosols and vapor obtained through rapid volatilization of linseed oil methyl ester, fog oil, and methyl soyate for specific exposure periods. The plates were incubated at 37 °C for 72 h. The plates were examined for revertant colonies.
Direct exposure to oil aerosols and vapor: Exposure of Salmonella strains TA97, TA98, and TA100 to oil aerosols and vapors of fog oil, methyl soyate, soybean oil isopropyl ester, and olive oil methyl ester was performed. A 5 mL culture (100 μL) was added to the Petri dish and spread with glass beads to produce a lawn of bacteria. The plates were exposed to oil aerosols and vapors for specific exposure periods, removed from the chamber, and incubated at 37 °C for 24 h.
Oil vs. vapor: Two sets of nutrient agar plates were used in this experiment. In one set, the plates were covered completely with a Kimwipe® and stationery paper. In the second set, the plates were covered with papers with a hole in the center. After the exposure, the plates were transferred to a sterile hood. A 24 h culture was added as described above and the plates were incubated for 24 h at 37 °C.
Residual activity: Nutrient agar plates were exposed to fog oil and methyl soyate aerosols for 5 min. The plates were kept open in a sterile hood for 5 min, 10 min, and 30 min durations. A 24 h culture (100 μL) was used and the plates were incubated for 24 h at 37 °C. As a control, unexposed plates were incubated for 24 h at 37 °C.
Standard test for disinfectant properties: A test to examine the disinfectant efficacy on solid surfaces was also carried out. Sterile metal pins were inoculated with 24 h broth culture of TA97, 98, and 100, and dried in a sterile hood. Pins treated with each organism were exposed to oil aerosols and neat oils. Baby oil and pins not exposed to smoke were used as negative controls. The pins were exposed for 2 min and inoculated into 5 mL sterile nutrient broth and incubated for 24 h at 37 °C with shaking (200 rpm).
Exposure of bacterial endospores to aerosols: Duo-spore sterility test kits (obtained from Fisher Scientific Waltham, MA, USA), generally used to test the efficiency of autoclaves, were used to test the effectiveness of oil aerosols against bacterial endospores. The strips contain endospores of Bacillus subtilis and Bacillus stearothermophilus enclosed in a sterile envelope. The strips were exposed to fog oil and methyl soyate aerosols generated at 550 °C and 650 °C for exposure periods of 60 and 120 min. After exposure, the strips were inoculated in tryptic soy broths. The broths were incubated at 37 °C for 24 h with shaking (200 rpm).
Exposure of bacteria: A 24 h culture (100 μL) of Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus cloacae, Escherichia coli, and Serratia marcescens was added to Petri dishes with nutrient agar and spread using sterile glass beads. The plates were kept inside the glass chamber for a 30 min time period.
Methyl soyate vs. other oils: A 24 h culture (100 μL) of Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus cloacae, Escherichia coli, Serratia marcescens, Staphylococcus aureus, Bacillus megaterium, Shigella flexneri, and Salmonella typhimurium was cultured on nutrient agar plates. The plates were exposed to methyl soyate, JP-8, and diesel aerosols and vapors generated at 650 °C for 15 and 30 min exposure periods.