Abstract
Targeted protein degradation is a new aspect in the field of drug discovery. Traditionally, developing an antibiotic includes tedious and expensive processes, such as drug screening, lead optimization, and formulation. Proteolysis-targeting chimeras (PROTACs) are new-generation drugs that use the proteolytic mechanism to selectively degrade and eliminate proteins involved in human diseases. The application of PROTACs is explored immensely in the field of cancer, and various PROTACs are in clinical trials. Thus, researchers have a profound interest in pursuing PROTAC technology as a new weapon to fight pathogenic viruses and bacteria. This review highlights the importance of antimicrobial PROTACs and other similar “PROTAC-like” techniques to degrade pathogenic target proteins (i.e., viral/bacterial proteins). These techniques can perform specific protein degradation of the pathogenic protein to avoid resistance caused by mutations or abnormal expression of the pathogenic protein. PROTAC-based antimicrobial therapeutics have the advantage of high specificity and the ability to degrade “undruggable” proteins, such as nonenzymatic and structural proteins.
1. Introduction
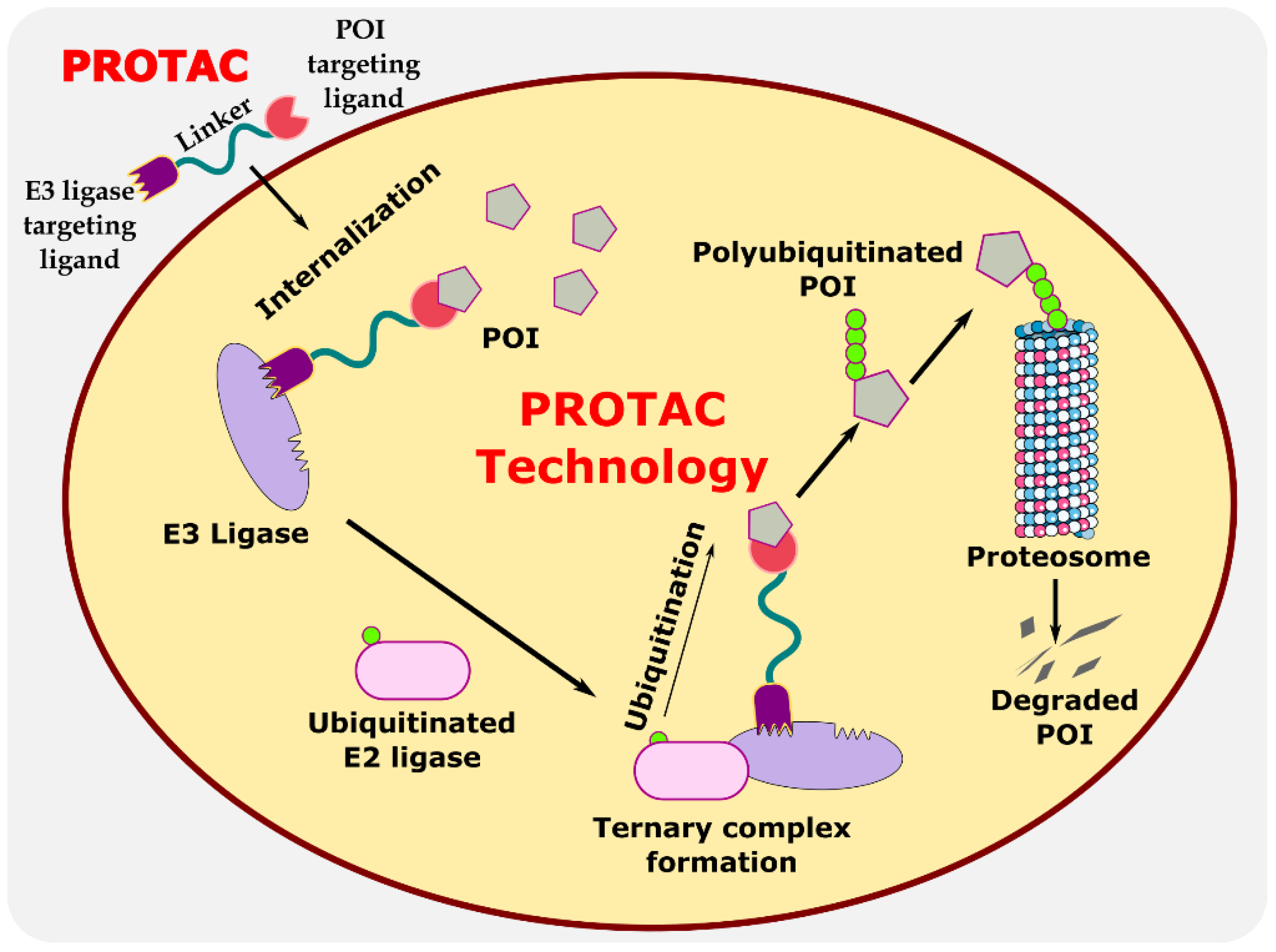
Proteolysis-targeting chimeras (PROTACs) are bifunctional protein degraders that use the E3 ubiquitin ligase pathway for the degradation of the protein of interest. A PROTAC molecule consists of three components: a ligand moiety that targets the protein of interest (POI), another ligand that binds to E3 ligase, and a linker, which bridges between these two ligands [1]. The main function of these ligands is to attract E3 ligase and POI and initiate polyubiquitination for degrading POI via the ubiquitin–proteasome system (UPS) (Figure 1) [2]. The ubiquitin–proteasome system is an essential pathway of every eukaryotic cell for maintaining homeostasis and regulating gene transcription and translation, cell cycle, and apoptosis [3]. In the ubiquitin proteolysis pathway, the ubiquitin molecule binds to the ubiquitin-activating enzyme E1. The E1-bound ubiquitin transfers the ubiquitin to the E2-conjugating enzyme, which is later transferred to E3 ligase, and, finally, ubiquitin binds to POI. These are ATP-driven cascades of reaction where the ubiquitin molecule is transferred from one molecule to another and, finally, to POI. Similarly, several ubiquitin molecules bind to the POI, which signals the proteasome to initiate the degradation of the polyubiquitinated POI. This innate protein degradation pathway is utilized for degrading POI.
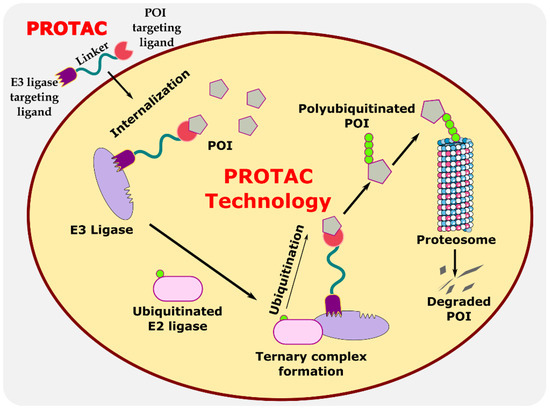
Figure 1.
Illustration explaining the mechanism of PROTAC in targeted protein degradation.
The targeted degradation of the proteins has been explored using various techniques like ligand-induced degradation [4] (LID), hydrophobic tagging (HyT) [5], etc. These techniques later lead to the development of PROTACs [6]. PROTACs are more efficient than conventional small molecule inhibitors [7]. For instance, traditional small molecule inhibitors could only inhibit the activity of certain enzymes or could block the partial function of the protein, while PROTACs can completely eliminate the disease-related proteins [8]. A significantly lower concentration of the drug is required in case of targeted protein degradation using PROTACs as compared to small molecule inhibitors. Many proteins which remain undruggable over the decades, like scaffold proteins, transcriptional factors, or proteins without active binding sites, could be easily targeted by PROTACs and other similar targeted technologies [9]. Such molecules have the great advantages of high selectivity, catalytic, and drugging the undruggable targets.
The first PROTAC molecule was successfully developed in 2001, and, to date, more than 3270 PROTACs have been developed [10]. Some of the PROTAC molecules are currently in different phases of clinical trials, and their initial results have provided a great modality for PROTAC-based degraders (Table 1) [11]. Thus, PROTACs have grabbed the attention of various pharmaceutical companies. Companies such as Arvinas [12], Pfizer [13], Accutar Biotech [14], Bristol Myers Squibb [15], Dialectic Therapeutics [16], Foghorn Therapeutics [17], Kymera Therapeutics [18], Nurix Therapeutics [19], C4 Therapeutics [20], and Cullgen [21] have already entered in the race of clinical trials for their respective PROTAC molecules. It has been predicted that within a few years, approximately 15 PROTAC molecules will be in clinics [22]. Due to the inarguable potential of PROTACs in the current era, researchers are exploring the possibilities of developing new protein degraders for various diseases, such as immunological disorders [23], inflammatory disorders [24,25,26], cancer [27,28], auto-immune diseases [29], neurological diseases [30], bacterial infections [31], and viral infections [32]. It is undeniable to state that PROTAC-based degraders are highly investigated in the field of cancer research, and many protein degraders are in the pipeline for clinical trials. However, the exploration of PROTACs in the field of anti-microbial remains marginal. This review is an attempt to highlight the state-of-the-art protein-based degraders targeting microorganisms. It also emphasizes PROTACs as an alternative to antibiotics.

Table 1.
Comprehensive representation of clinical cases of PROTACs.
In the current scenario, due to the inappropriate/overuse of antibiotics, the use of antibiotics in the agricultural field and feeding livestock has led to the emergence of resistant strains of pathogens, which is a major threat to humankind. Consequently, pharmaceutical industries consider the development of new antibiotic as potentially effective for a shorter duration and also requires hefty investment [45]. Thus, there is a need to develop new strategies for targeting multidrug-resistant pathogens. Thus, PROTAC could be a glimmer of hope for destroying resistant pathogens [31]. Due to the characteristic nature of direct degrading of the disease-related protein or POI instead of inhibiting them could provide enhanced sensitivity towards multidrug resistant pathogens [46]. Since PROTACs utilize a chemical knockdown approach, an innate cellular mechanism, there are likely fewer chances of the generation of spontaneous mutations in the target protein [8]. There are several approaches for degrading the POI, and they are classified based on the type of degradation system used, namely, the eukaryotic system and prokaryotic system.
2. Eukaryotic System
2.1. Anti-Viral PROTACs
2.1.1. Degradation of Viral Protein
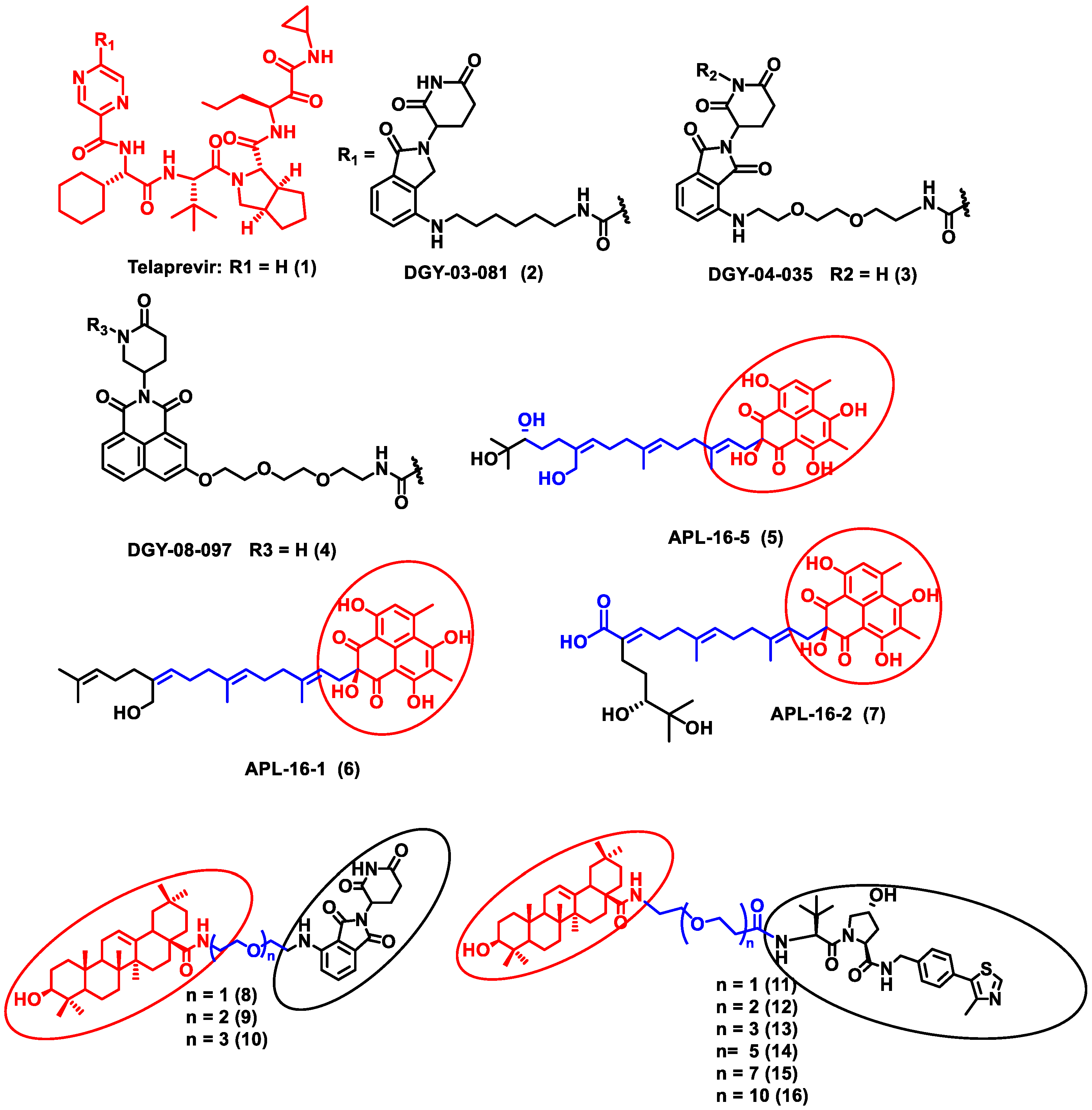
In this section, PROTACs that target viral proteins have been enlisted. PROTAC-based protein degraders are highly explored in this section as compared to other strategies of protein degradation. Many viral proteins have been targeted for protein degradation (Table 2). For instance, Montrose et al. developed a peptide-based PROTAC molecule that targets X-protein, which is an essential protein required for the replication of the hepatitis B virus (HBV). It was also found that the presence of X-protein could also induce hepatocellular Carcinoma (HCC). This PROTAC molecule consists of an ODD degrons (oxygen-dependent degradation) domain, an oligomerization domain, and a cell-penetrating peptide. The ODD degrons domain binds to Von Hippel–Lindau (VHL) E3 ligase, the oligomerization domain interacts with the X-protein, and octa-arginine is used as a CPP for to ease cellular entry. In vitro studies verified the ability of peptide-based PROTACs to efficiently degrade X-protein [47]. In another study, instead of the peptide as a ligand for POI, the authors used telaprevir, an anti-viral peptidomimetic protease inhibitor. They developed three different molecules (DGY-03-081 (2), DGY-04-035 (3), and DGY-08-097 (4)) that target the NS3/4A protease of the hepatitis C virus (Figure 2). Lenalidomide, pomalidomide and novel tricyclic imide moiety were used as the ligands for CRBN E3 ligase. The function of NS3/4A serine protease is to cleave viral polyprotein, which acts as an essential step in viral replication [48]. Thus, degradation of NS3/4A protease via PROTAC will inhibit virion formation and multiplication. These compounds were evaluated in Hep C virus-infected HEK293T cells. Interestingly, all three degraders exhibited anti-viral activity and did not show cytotoxicity to the uninfected cells. Compound DGY-08-097 (4) had the highest degradation ability and the least DC50 value (50 nM at 4 h). One of the reasons for the increased affinity might be due to the tricyclic imide moiety in the DGY-08-097 (4) that showed increased affinity towards CRBN E3 ligase [49].

Table 2.
Comprehensive information on the targeted degradation of viral protein in the eukaryotic system using PROTAC molecules.
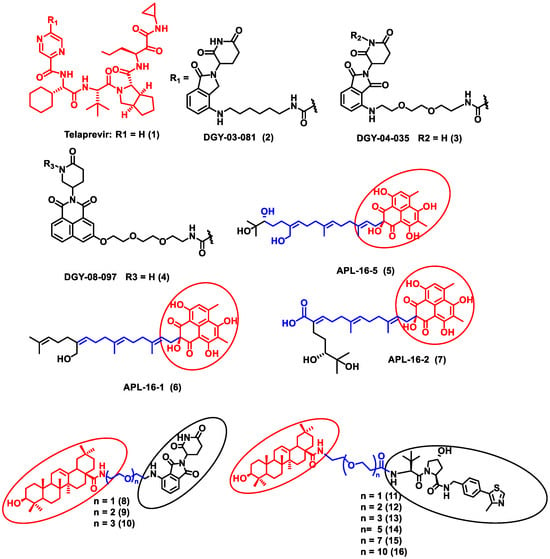
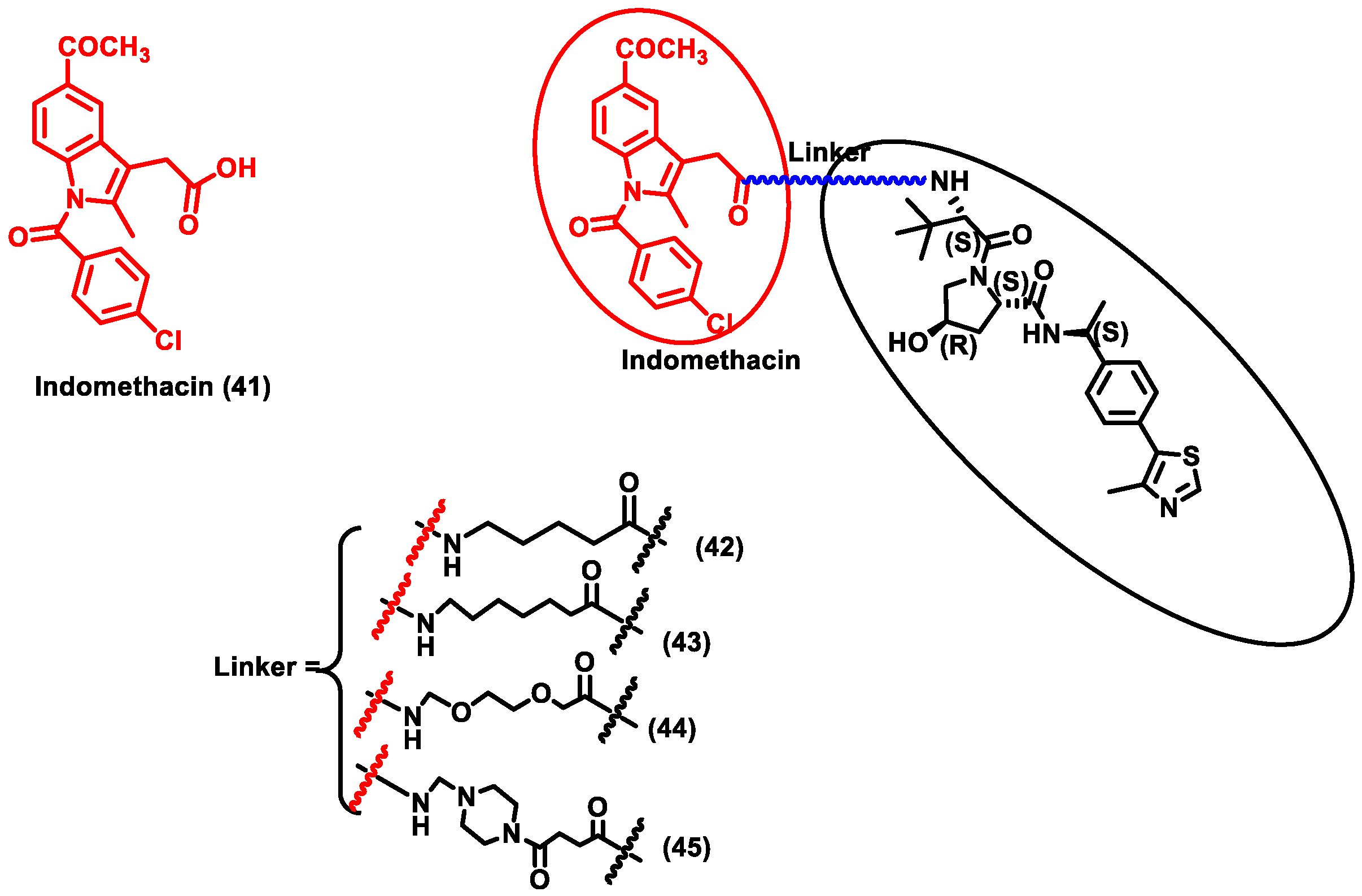
Figure 2.
Structures of the PROTAC molecules used for the degradation of viral proteins. The red circle indicates the POI ligand. The blue wavy line indicates the linker, and the black circles indicate the E3 ligand moiety.
Similarly, the endonuclease polymerase subunit (PA) of influenza virus A was the POI for developing a novel PROTAC molecule. Asperphenalenone E (APL-16-5) (5) was derived from an endophytic fungus Aspergillus, which was used as a ligand for PA. APL-16-5 (5) induces degradation of the viral polymerase subunit (PA) by ubiquitin–proteasome machinery, as it can bind to both the E3 ligase enzyme (TRIM25) and PA. The endonuclease polymerase enzyme is essential in the polymerization of DNA during DNA replication. Derivatives of asperphenalenone, APL-16-1 (6), and APL-16-2 (7) were also synthesized, and the results were compared with the known anti-viral drug ribavirin. HEK293T, A549, and MDCK cells were cultured and infected with influenza virus A WSN/33 for in vitro analysis. The cytotoxicity of APL-16-5 (5) and APL-16-1 (6) against the Influenza virus was in micromolar concentration (EC50) 0.28 to 0.36 μM. Proteosome-mediated degradation of PA with APL-16-5 (5) exhibited a marked decrease in viral RNA components. Later, APL-16-5 (5) was evaluated against influenza virus B, hepatitis C, and Zika viruses. The results from the study confirmed that APL-16-5 is a selective inhibitor for influenza viruses. Dose-dependent studies were conducted to determine the interaction of PA with TRIM25 and concluded that compound 5 induces the destabilization of PA by ubiquitination, and thereby it degrades the PA [50].
Li et al. designed a pentacyclic triterpenoid group (PTG) containing PROTAC molecule for targeting hemagglutinin (HA) of the influenza virus. Pentacyclic triterpenoids are secondary metabolites present in various medicinal plants, and they possess significant anti-viral activity. Oleanolic acid (OA) and its derivatives are compounds that were selected as the warhead for the PROTAC molecule. OA exhibited anti-viral action against the influenza A/WSN/33 virus, and it had a moderate binding affinity with HA; thus, it became an ideal molecule for PROTAC technology. Two sets of PROTAC molecules (8–10) and (11–16) were designed and studied employing different E3 ligases, such as CRBN and VHL ligands, respectively. HEK293T cells were transfected using HA plasmids, and the level of HA degradation was studied using the synthesized PROTAC molecules. A cell viability assay, immunofluorescence microscopy assay, immunoprecipitation assay, hemagglutination inhibition assay, etc., were performed to evaluate the molecules. Compound 13 (DC50 = 1.44 μM) exhibited the maximum HA depletion as compared to other compounds. This was also validated by molecular docking analysis by Schrodinger Suite. Furthermore, it was concluded from these assays that the VHL ligand containing PROTACs showed better HA degradation [51].
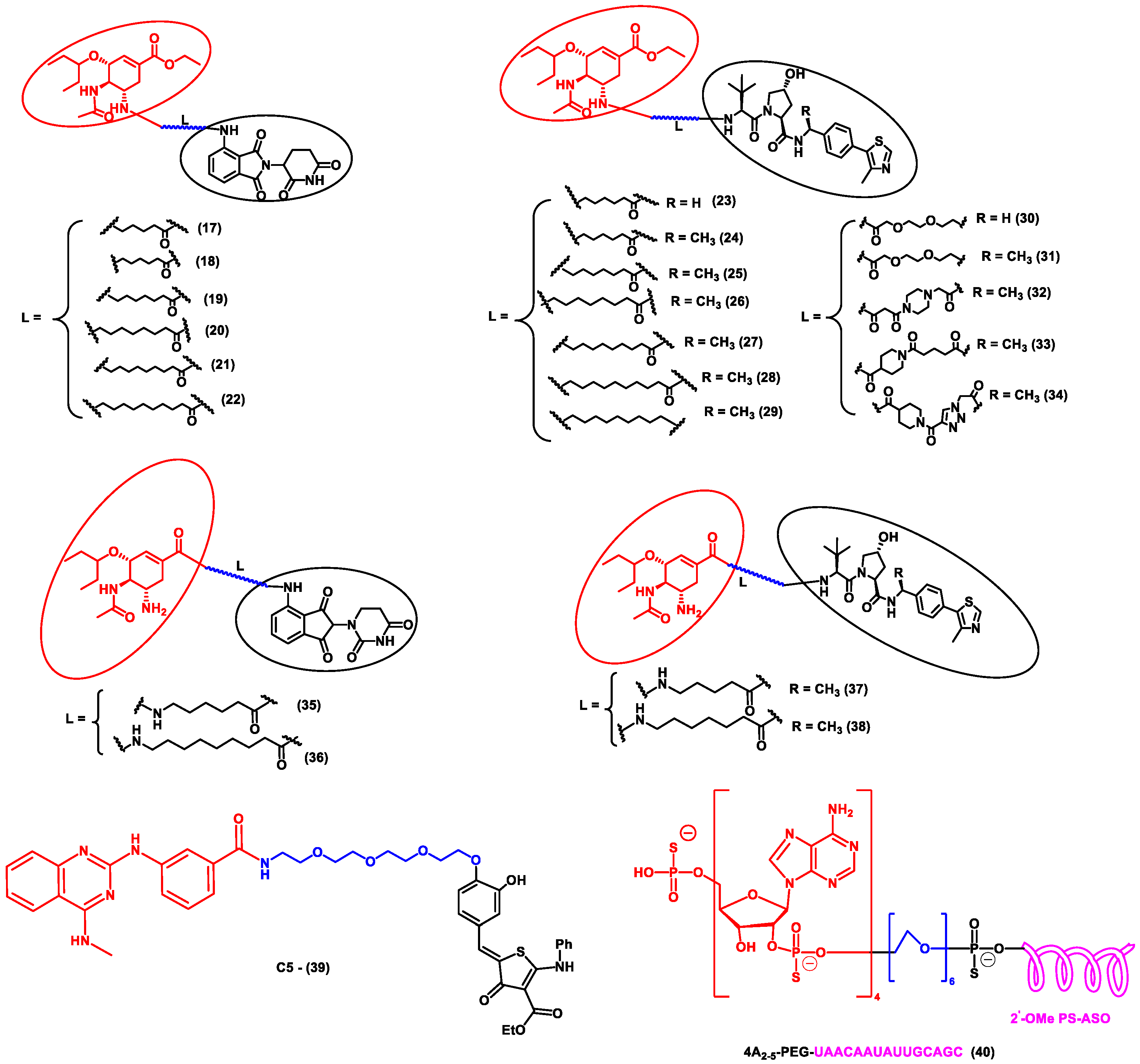
In another independent study conducted by Xu et al., oseltamivir is an approved drug for influenza that targets influenza neuraminidase (NA). Neuraminidase is an essential enzyme for viral replication. They have used oseltamivir-based compounds for targeting neuraminidase and linked them with a discrete variety of E3 ligase ligands such as VHL or CRBN. The amino or carboxylate group of oseltamivir was modified to improve its anti-viral activity. A wide variety of linker combinations like rigid as well as flexible groups like PEG, pyridyl, triazole, and piperazinyl were also involved. A set of PROTAC combinations (17–38) were designed, and from these, N-substituted oseltamivir showed increased potency than the carboxylate-substituted compound. According to the in vitro studies, compound 27 showed the best anti-viral activity having an EC50 of 0.33 µM, which was almost similar to the reference drug oseltamivir phosphate (EC50 = 0.36 µM). Furthermore, interestingly, all the synthesized compounds do not show cytotoxicity towards the normal cells with a concentration up to CC50 > 50 µM. Docking studies indicated that these ternary complexes showed great hydrogen bonding and hydrophobic interactions between neuraminidase and E3 ligase [52]. From these above studies, it could be concluded that there are various strategies being evolved to target viral proteins and inhibit their replication.
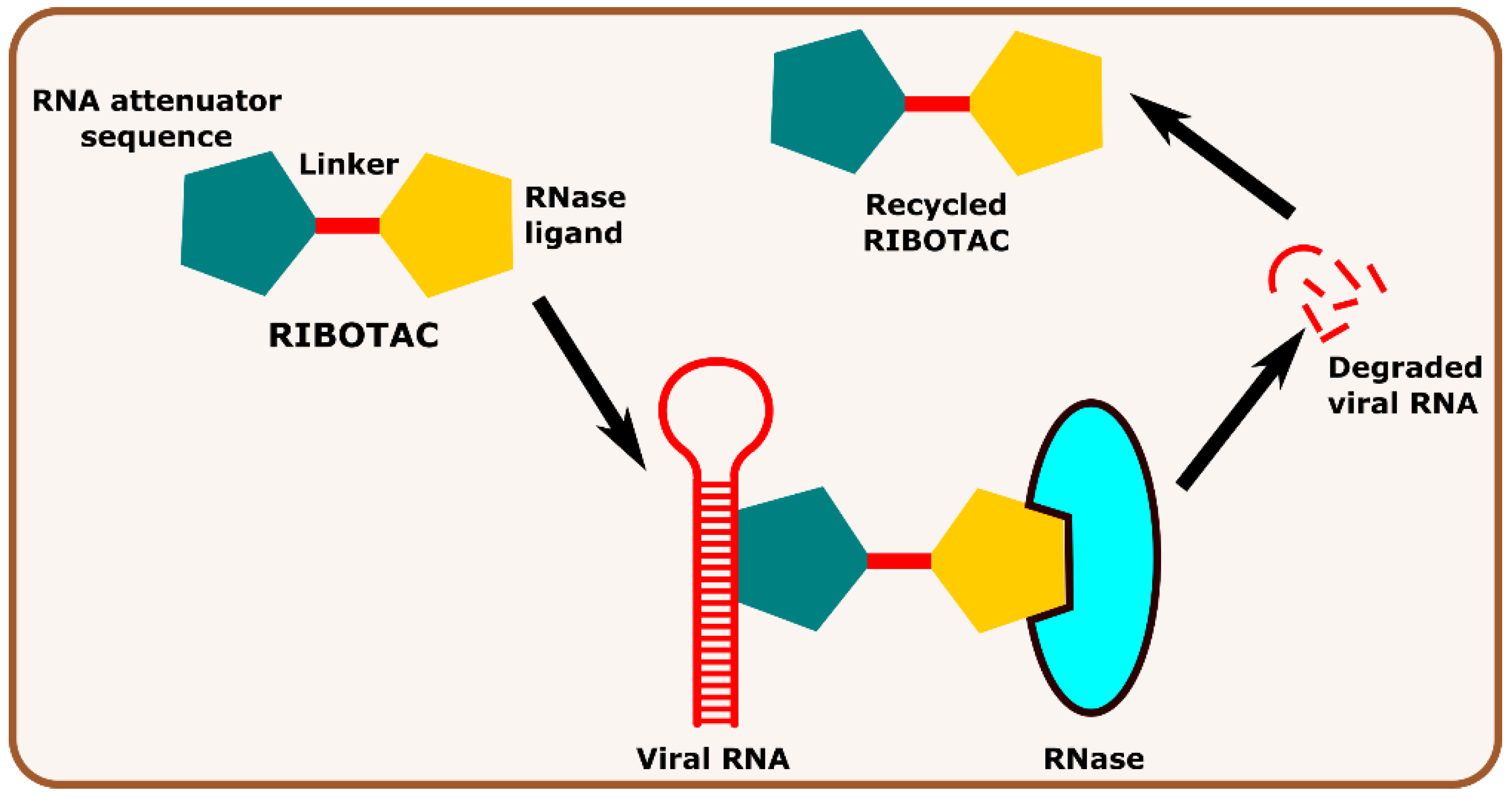
Other interesting subcategories of PROTAC are ribonuclease-targeting chimera (RIBOTAC) and nucleic acid-hydrolysis-targeting chimera (NATAC). Both strategies were used to develop novel degraders. In RIBOTAC, RNase is the degrader system, and it degrades viral RNA, while NATAC uses oligonucleotide sequences to identify the POI, and further, they could be degraded by RNase L (specific for ss-RNA). Haniff et al. developed a RIBOTAC degrader that targets the RNA genome of the SARS-CoV-2 virus (Figure 3). RIBOTAC has two major constituents- a small molecule known as C5 (39) and an RNA attenuator hairpin (AH). This RNA attenuator hairpin binds to the RNA genome, and C5 (39) recruits endonucleases present in the cell and initiates the degradation of the viral genome (Figure 4) [53]. This strategy might provide solutions for various viral infections, and the only challenge is identifying and optimizing the appropriate attenuator sequence, which could bind toward a target of interest.
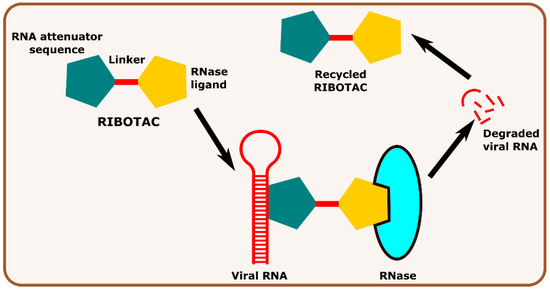
Figure 3.
Schematic representation of the mechanism of action of RIBOTAC molecules in targeting viral RNA.
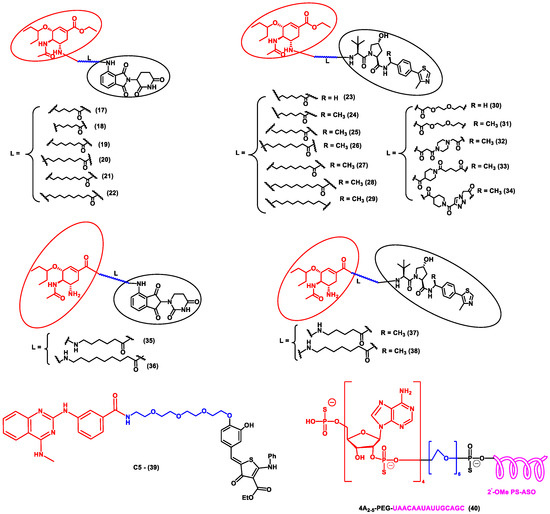
Figure 4.
Structures of the PROTAC molecules are used for the degradation of viral proteins. The red circle indicates the POI ligand; the blue wavy line indicates the linker; the black circles indicate the E3 ligand moiety.
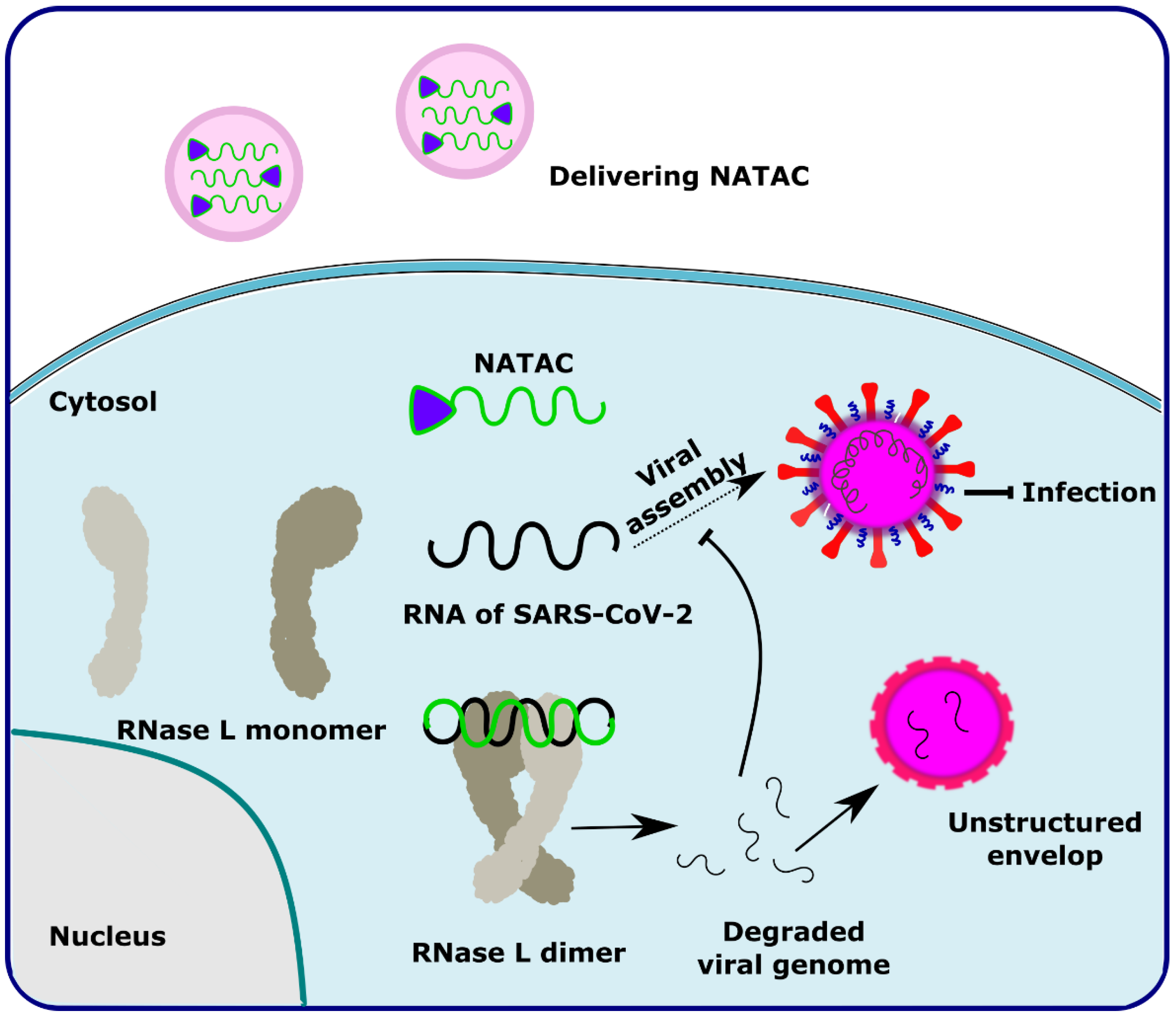
NATAC is another promising approach to PROTAC technology (Figure 5). NATAC explores the function of RNase L; RNase L is an innate RNA degrading enzyme that targets UN^N sites of the viral or single-stranded RNA. Tang and his group designed a NATAC molecule having a 5’ phosphorylated 2’-5’ polyA sequence that attracts RNase L, and another end had an antisense oligonucleotide strand targeting the spike protein of SARS-CoV-2 (Compound 40) (Figure 4). The knockdown efficiency of spike proteins by NATAC molecules was evaluated, and it was found that NATACs could significantly reduce the RNA sequence of the spike protein. Interestingly, it was also found that RNase L could also increase the mRNA level of IFN-β and IL-6 in the host cells, thereby increasing the production of interferon and further enhancing the anti-viral response in the host cell [54].
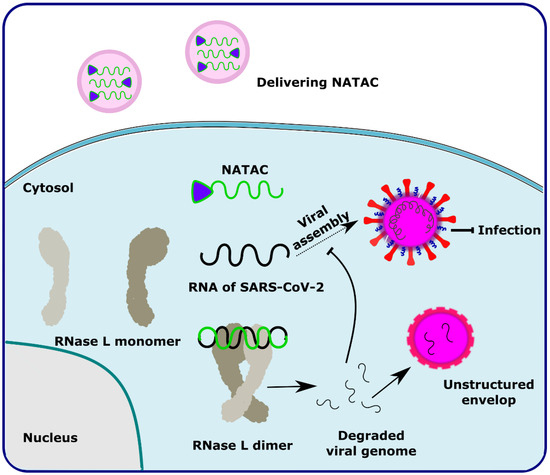
Figure 5.
Schematic representation of the mechanism of action of NATAC molecules in targeting the SARS-CoV-2 virus. Adapted from [54].
2.1.2. Degradation of Human Host Protein
In this section, PROTACs that target human host cell proteins, which are involved with viral proteins to enhance their virulence, are listed (Table 3). For instance, human cytomegalovirus (HCMV) is one of the major pathogens that cause the herpes disease. It was found that HCMV protein kinase pUL97, a viral cyclin-dependent kinase (vCDK), plays a crucial role in the generation of nuclear capsid and viral replication. During HMCV infection, viral proteins upregulate the expression of various CDK-cyclin complexes that initiate pseudomitosis, which is favorable for viral replication. Thus, CDK inhibitors targeting CDKs could be a solution for HCMV infection. In this study, THAL-SNS032, which is a protein kinase inhibitor, was used to target CDK9, and thalidomide was the E3 ligase targeting ligand. In vitro cytotoxicity studies showed that the THAL-SNS032 (EC50 0.025 ± 0.001 µM) was nearly fourfold more efficient than the nonPROTAC parent compound SNS032 (EC50 = 0.105 ± 0.004 µM) [55]. Thus, it could be concluded that these PROTAC molecules could be a possible candidate for treating herpes disease.

Table 3.
Comprehensive information on the targeted degradation of human host protein using PROTACs.
The research community was doomed due to the pandemic SARS-CoV-2 and researchers across the globe were trying to provide solutions for COVID-19. This led to the discovery of a repurposing drug, i.e., indomethacin (IMN) (Compound 41), which is an anti-inflammatory drug and was found to have anti-viral activity against the Coronaviridae family (Figure 6). Desantis et al. designed four PROTAC molecules (42–45) using indomethacin for targeting human prostaglandin E synthase type 2 (PGE-2). PGE-2 interacts with the NSP7 protein of SARS-CoV-2. NSF7 proteins are essential for SARS-CoV-2 replication. However, the exact mode of action of indomethacin is unknown. Furthermore, it is confirmed that degradation of PGE-2 inhibits repression of viral protein synthesis by ds-RNA-dependent protein kinase R (PKR)-mediated pathway. Since this PROTAC degrades human cellular protein, it could be classified as PROTACs targeting host protein for degradation. These four synthesized PROTAC compounds (43) and (45) showed the highest activity, with EC50 values of 18.1 and 21.5 μM, which was nearly five times more effective than indomethacin (EC50 = 94.4 μM). Molecular docking studies also described that using 6 methylene units in (43) and a piperazine group in (45) as a linker seems to be perfect for the molecule’s binding interaction with the VHL ligand. Moreover, compounds (43) and (45) exhibited anti-viral activity against β-coronavirus (i.e., HCoV-OC43), with EC50 values of 4.7 and 2.5 µM, respectively. These compounds were specific in that they did not show cytotoxicity against MRC-5 cells (normal uninfected cells), indicating that these PROTACs are able to specifically target infected cells. PROTACs showed effective broad-spectrum action in inhibiting two different SARS-CoV-2 strains, i.e., β-coronavirus HCoV and the α-coronavirus HCoV-229E. Due to the broad-spectrum nature of the PROTACs, these molecules could be a potential target for COVID-19 disease [56]. Y et al. developed a novel PROTAC targeting HUWE1 E3 ligase and ORF3 MERS-CoV accessory protein. ORF3 restricts apoptosis in the host cell, which could be beneficial for the virus to replicate. Hence, degradation of the ORF3 protein will induce apoptosis in the host cell, thereby restricting the spread of MERS-CoV infection. HUWE1 E3 ligase was specific towards the degradation of ORF3 since PROTACs targeting other E3 ligases such as UBR5, TRIM33, Cullin5, Cullin3, and UBR4 had no effect on the stability of ORF3. Thus, it indicates that HUWE1 E3 ligase could be a better E3 ligase for targeting ORF3 of MERS-CoV. Subsequently, MERS-CoV belongs to the same family as SARS-CoV-2; studies on SARS-CoV-2 infected cells could be another potential area of research for targeting COVID-19 [57].
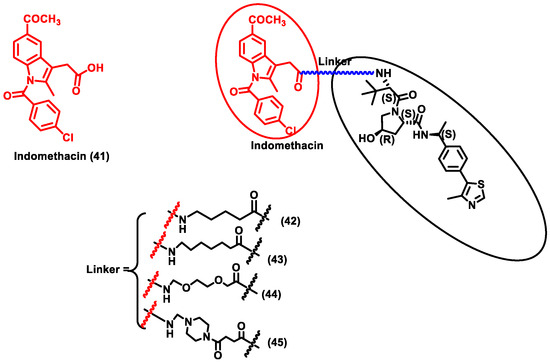
Figure 6.
Structures of the PROTAC molecules are used for the degradation of host proteins. The red circle indicates the POI ligand; the blue wavy line indicates the linker; the black circle indicates the E3 ligand moiety.
3. Prokaryotic System
Similar to the ubiquitin-based proteolytic protein degradation system, prokaryotes also have their protein degradation system, known as the Caseinolytic protease proteolytic (ClpCP) system [58]. In Gram-positive bacteria, protein arginine phosphorylation has a crucial role in bacterial cell homeostasis [59]. Phosphoarginine-based signaling has high relevance in gram-positive bacteria like Bacillus subtilis and Staphylococcus aureus. In a protein structure, arginine side chains are phosphorylated by McsB and dephosphorylated by the YwIE enzyme. McsB and YwIE are phosphorylases and dephosphorylase enzymes of bacterium, which play a crucial role in proteolysis. This phosphorylated arginine acts as a tag or marker for the degradation of a protein. Hence, these phosphorylated arginine residues are identified by the pArg-specific reader domain, which is present in the ClpCP proteolytic complex. This leads to the degradation of the pArg-tagged protein [60]. Thus, scientists have explored bacterial proteolysis systems and developed novel PROTACs targeting the ClpCP mechanism, which are known as BacPROTACs, for degrading bacterial disease-related proteins (Table 4).
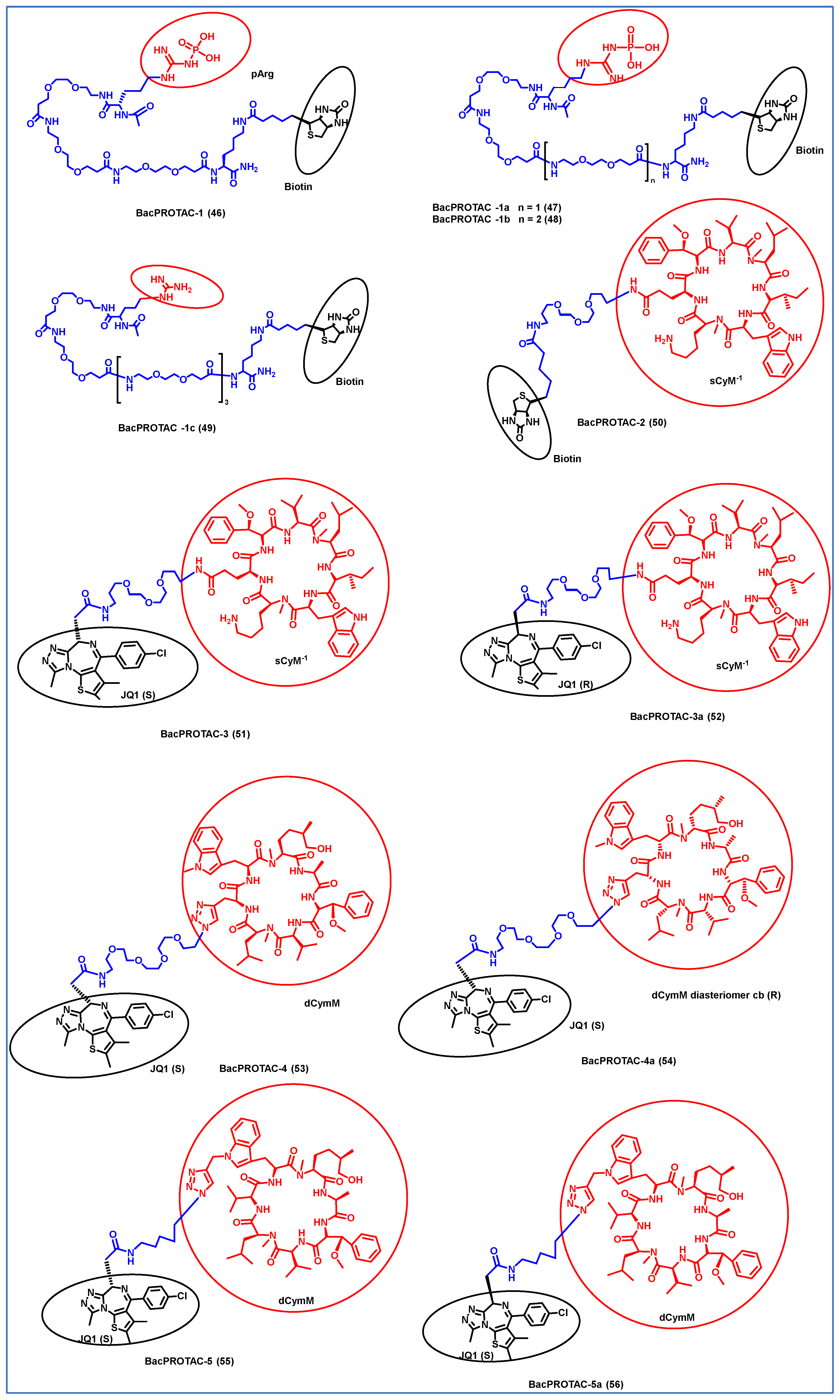
Morreale et al. synthesized four different BacPROTACs (46–49) using the arginine peptide linker and the targeted POI was biotin (Figure 7) [61]. ClpC protease-mediated degradation is enabled by the BacPROTAC-1 (46) consisting of monomeric streptavidin that targets biotin and a ClpCNTD anchor which has phosphorylated arginine residues mimicking bacterial degradation tag. The characterization of the designed BacPROTAC was performed using isothermal titration calorimetry (ITC), and the results revealed that it has a high affinity towards the biotin moiety (KD = 0.69 µM). The BacPROTAC-1 was further modified to obtain three more compounds, one of which (BacPROTAC-1c, 49 had nonphosphorylated arginine residue as linker, while BacPROTAC -1a and BacPROTAC-1b (47 and 48) had modification with the chain length spacing between the biotin and the phosphorylated arginine residue. However, these variations did not affect the degradation efficiency of these four molecules.
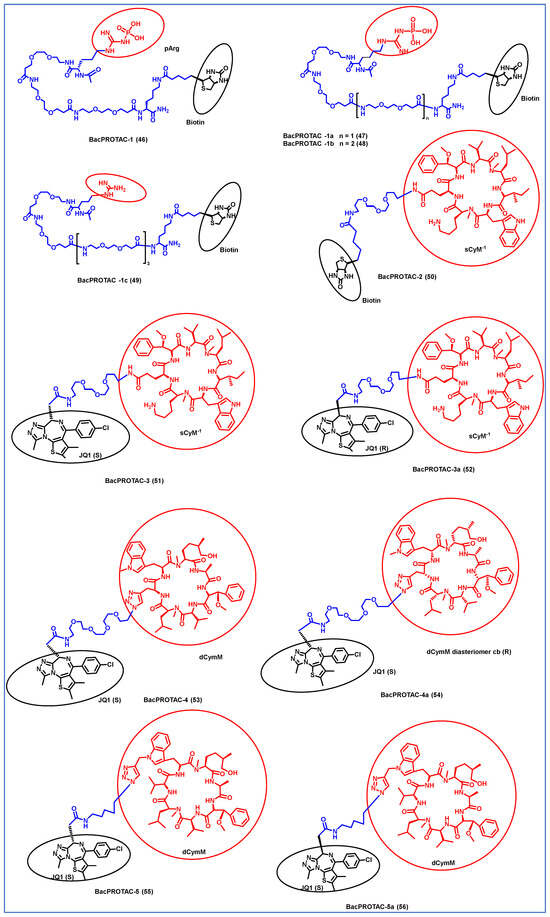
Figure 7.
Structures of the PROTAC molecules are used for the degradation of proteins in prokaryotic cells using a bacterial proteolysis system. The red circle indicates the POI ligand. The Blue wavy line indicates the linker, and the black circle indicates the E3 ligand moiety.
Furthermore, the same group has developed new BacPROTAC molecules to target Mycobacterium tuberculosis, a pathogen that causes tuberculosis [61]. However, the phosphorylated arginine-mediated signaling pathway for protein degradation is absent in mycobacteria. Interestingly, the ClpC1P1P2 protease of mycobacteria is another similar degradation pathway that initiates degradation in the presence of phospho-guanidinium residues in the ClpCNTD domain [62]. Cyclomarin A (CymA) is known to penetrate the cell membrane of mycobacteria and could be used to construct a specific protein degrader [63]. CymA-based degraders were developed by solid phase peptide synthesis, and a set of cyclic peptides (sCym-1) were synthesized to target the bromodomain 1 (BD-1). JQ1 is a small molecule inhibitor that binds to BD-1, which is used as one of the ligands to target the BD-1 protein. BacPROTAC-2 (compound 50) was made by linking the sCym-1 with the biotin moiety. Another set of BacPROTACs, BacPROTAC-3 and 3a (51 and 52), were made by linking the JQ1 ligand with the stereochemical modifications, i.e., S and R forms, respectively. BacPROTAC-4 and 4a (53 and 54) were made with the diastereomers of dCymM linked with the JQ1(S) ligand. BacPROTAC-5 and 5a (55 and 56) were constructed using a different linker group, connecting dCymM with JQ1 ligands in S and R forms, respectively. These synthesized BacPROTACs showed the efficient degradation of the targeted protein in the mycobacteria, and compound 50 showed the highest binding affinity. Furthermore, compounds 51 and 55 showed only partial degradation towards the targeted protein ever at higher concentrations [61]. Thus, it could be concluded that compound 50 could be a potential target for curbing tuberculosis; however, further preclinical studies are essential to evaluate its effectiveness. Junk et al. developed a similar BacPROTAC approach towards the mycobacteria by inducing the ClpC1-mediated degradation. A simplified desoxycyclomarin derivative was used as the active compound for the development of BacPROTAC against the tuberculosis H37Rv strains residing on the THP-1 macrophages. Homo-BacPROTAC molecule was made by dimerizing the cyclomarin molecules, and it was known to show high degradation efficiency at the sub-nM level [64].

Table 4.
Comprehensive information on targeted degradation of bacterial protein in the prokaryotic system using PROTAC.
Table 4.
Comprehensive information on targeted degradation of bacterial protein in the prokaryotic system using PROTAC.
| S. No. | Pathogen | Protein of Interest (POI) | POI Ligand | E3 Ligase Ligand | Research Outcome | Ref. |
|---|---|---|---|---|---|---|
| 1 | Bacillus subtilis | ClpCP protease | Phosphorylated arginine residue | Biotin |
| [61] |
| 2 | Mycobacterium tuberculosis | ClpC1P1P2 protease | Cyclomarin A (CymA) | Biotin JQ1(R) and JQ1(S) |
| [61] |
| 3 | Mycobacterium tuberculosis | ClpC1-NTD protease | Desoxycyclomarin derivative | - |
| [64] |
Another interesting area of research is the utilization of the anti-tuberculosis antibiotic pyrazinamide as a degradation tag of its bacterial target. Pyrazinamide pro-drug converted into pyrazinoic acid by pyrazinamidase, an enzyme found in M. tuberculosis. It has been observed that pyrazinoic acid binds to PanD, aspartate decarboxylase, an essential and unique bacterial enzyme. These bindings induce conformational changes, which results in the degradation of PanD via the ClpP1P2 degradation pathway. Thus, this idea could be used to design novel bacterial protein degraders [65]. Long et al. developed a PROTAC-based strategy for protein degradation using tert-butyl carbamate-protected arginine. It was discovered that inhibitors linked to Boc3Arg could effectively degrade the inhibitor-bound protein. Thus, using this strategy, the degradation of GST-α1 (glutathione) in cancer cells was proved. Similarly, Boc3Arg-bound trimethoprim (TMP) showed reducing of Escherichia coli dihydrofolate reductase (eDGFR). Trimethoprim is an inhibitor of DGFR. It was observed that the degradation process is rapid, that within 5 h of administration, there is a decrease in the progress level by 30–80% [66]. Thus, it could be concluded that by harnessing the power of bacterial proteolytic systems, various PROTAC molecules have been created. These novel protein degraders have the potential to change the future in the field of antibiotics.
4. Patent Analysis and Future Perspectives
Su, Xiangdong, et al. invented and patented an anti-viral PROTAC molecule having the general formula LGP-LK-LGE, wherein LGP is a ligand for binding a deoxyribonucleic acid (DNA) polymerase; LGE is a ligand for binding an E3 ubiquitin ligase, and LK is a bridging chain linking the two above ligands. These compounds degrade deoxyribonucleic acid (DNA) polymerase and hence inhibit virus replication and kill viruses such as hepatitis B and HIV [67]. Haibing et al. made a novel oseltamivir-based PROTACs and filed a patent, which can degrade influenza virus neuraminidase and, thus, display the activity of inhibiting the replication of the influenza virus [68].
Although anti-viral PROTACs or bacPROTACs have the potential to become therapeutic agents, efficient delivery methodologies are required. Proteolysis-targeting chimeras (PROTACs) must be cell permeable to reach their target proteins, especially to cross the dense bacterial cell wall. The incorporation of PROTAC-like molecules with nanoparticles will precisely take these degrader molecules to the diseased site, where the off-target effects and the lower cell permeability could be greatly minimized [69]. Though there are 600 E3 ligases encoded in the human genome, only 4 of them are targeted in PROTAC-based degradation. Thus, the exploration of ligands for other E3 ligases might improve the efficacy of PROTACs [70]. Current knowledge of protein the degradation mechanism in pathogens is very limited compared with oncology applications; hence, a comprehensive understanding of the E3 ligases expressed in the host cell as well as in the infectious pathogen is required to design effective anti-infective PROTACs. Combining the PROTAC technology with some other therapeutic techniques, such as photodynamic therapy (PDT), could open a different way in the targeted protein degradation in a more efficient, cost-effective, and controlled manner [71]. The field of targeted protein degradation in bacteria and viruses will grow in the next future, leading to the development of chemically induced knockdowns of disease-causing proteins. Finally, anti-viral PROTACs or bacPROTACs will open a plethora of opportunities to develop next-generation antimicrobial therapies.
5. Conclusions
The emergence of many drug-resistant, mutant strains of pathogens has become a serious challenge to human health. It creates a need for anti-microbial drugs that follow a novel mode of action. In the current scenario, the efficacy of antibiotics is highly compromised due to the mutation in the microbial genome and the emergence of drug-resistance micro-organisms. Targeted protein degradation is a technology that could specifically degrade disease-related proteins, such as endotoxins, surface protein markers, or even viral RNA using the ubiquitin–proteasome system (UPS) and/or prokaryotic proteolysis system. Both pathways are the innate system that could eliminate these disease-related proteins. Targeted protein degradation for the elimination of proteins by hijacking the cell’s own innate mechanism is a promising technique in the case of oncogenic proteins. The same approach has been extended for the development of anti-microbials. Targeted protein degradation using PROTACs, RIBOTAC, and NATACs for the development of anti-microbial drugs could be the next-generation solution for microbial infections. These techniques are capable of degradation of infectious pathogen-derived proteins. Thus, it could be predicted that PROTACs could be a futuristic solution for microbial infections.
Author Contributions
Conceptualization, J.V., D.M. and L.R.; writing—original draft preparation, D.M. and J.V.; supervision L.R. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Department of Biotechnology, New Delhi, for the DBT Ramalingaswami Re-Entry Fellowship project (BT/RLF/Re-entry/44/2018) and Science and Engineering Research Board (SERB), New Delhi, for a Core Research Grant (CRG) (CRG/2020/001213); the Board of Research in Nuclear Sciences (BRNS) 54/14/03/2022-BRNS/10207 and VIT SEED GRANT 2020–2021, 2021–2023 are kindly acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AH | Attenuator hairpin |
| BacPROTAC | Bacterial PROTAC |
| CDK | Cyclin-dependent kinase |
| CLpCP | Caseinolytic protease proteolytic |
| CRBN | Cereblon |
| CymA | Cyclomarin A |
| DNA | Deoxyribonucleic acid |
| eDGFR | Escherichia coli dihydrofolate reductase |
| HA | Hemagglutinin |
| HBV | Hepatitis B virus |
| HCC | Hepato cellular carcinoma |
| HCMV | Human cytomegalovirus |
| HIF | hypoxia-inducible factor |
| HyT | hydrophobic tagging |
| IFN-β | Interferon beta |
| IL-6 | Interleukin-6 |
| IMN | Indomethacin |
| LID | Ligand-induced degradation |
| LYTAC | Lysosome-targeting chimeras |
| MDCK cells | Madin–Darby canine kidney cells |
| MERS-CoV | Middle East respiratory syndrome |
| mRNA | Messenger RNA |
| NA | Neuraminidase |
| NATAC | Nucleic acid targeting chimeras |
| ODD | Oxygen-dependent degradation |
| ORF3 | Open reading frame 3 |
| PDT | Photo dynamic therapy |
| PGE-2 | Prostaglandin E2 |
| PKR | Protein kinase R |
| POI | Protein of interest |
| PROTAC | Proteolysis-targeting chimeras |
| PTG | Pentacyclic triterpenoid group |
| RIBOTAC | Ribonuclease targeting chimeras |
| RNA | Ribonucleic acid |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TMP | Trimethoprim |
| UPS | Ubiquitin–proteasome-system |
| VHL | Von Hippel-Lindau |
References
- Qi, S.-M.; Dong, J.; Xu, Z.-Y.; Cheng, X.-D.; Zhang, W.-D.; Qin, J.-J. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front. Pharmacol. 2021, 12, 692574. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sun, X.; Rao, Y. PROTAC Technology: Opportunities and Challenges. ACS Med. Chem. Lett. 2020, 11, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.K.; Kuppuswamy, V.; Chinnakkannu, P. Administration of Grape Seed Extract Alleviates Age-Associated Decline in Ubiquitin-Proteasome System and Cardiomyocyte Apoptosis in Rats. Adv. Biol. Chem. 2013, 3, 253–263. [Google Scholar] [CrossRef]
- Yesbolatova, A.; Tominari, Y.; Kanemaki, M.T. Ligand-Induced Genetic Degradation as a Tool for Target Validation. Drug Discov. Today Technol. 2019, 31, 91–98. [Google Scholar] [CrossRef]
- Choi, S.R.; Wang, H.M.; Shin, M.H.; Lim, H.-S. Hydrophobic Tagging-Mediated Degradation of Transcription Coactivator SRC-1. Int. J. Mol. Sci. 2021, 22, 6407. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Schneekloth, A.R.; Pucheault, M.; Tae, H.S.; Crews, C.M. Targeted Intracellular Protein Degradation Induced by a Small Molecule: En Route to Chemical Proteomics. Bioorganic Med. Chem. Lett. 2008, 18, 5904–5908. [Google Scholar] [CrossRef]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great Opportunities for Academia and Industry. Sig. Transduct. Target 2019, 4, 1–33. [Google Scholar] [CrossRef]
- Mayor-Ruiz, C.; Winter, G.E. Identification and Characterization of Cancer Vulnerabilities via Targeted Protein Degradation. Drug Discov. Today Technol. 2019, 31, 81–90. [Google Scholar] [CrossRef]
- Protacdb Server. Available online: http://cadd.zju.edu.cn/protacdb/ (accessed on 11 November 2022).
- Alabi, S.B.; Crews, C.M. Major Advances in Targeted Protein Degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021, 296, 100647. [Google Scholar] [CrossRef]
- Protein Degradation with PROTAC Protein Degraders. Available online: https://www.arvinas.com/ (accessed on 2 November 2022).
- Arvinas and Pfizer Announce Global Collaboration to Develop and Commercialize PROTAC® Protein Degrader ARV-471 | Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/arvinas-and-pfizer-announce-global-collaboration-develop (accessed on 2 November 2022).
- Accutar Biotech. Available online: https://www.accutarbio.com/workflow/ (accessed on 2 November 2022).
- Understanding Protein Degradation–Bristol Myers Squibb. Available online: https://www.bms.com/media/media-library/scientific-media-resources/understanding-protein-degradation-and-resources.html (accessed on 2 November 2022).
- Dialectic. Available online: https://www.dtsciences.com/ (accessed on 2 November 2022).
- Foghorntx Therapeutics Pipeline. Available online: https://foghorntx.com/pipeline/ (accessed on 2 November 2022).
- Therapeutic Pipeline. Available online: https://www.kymeratx.com/pipeline/ (accessed on 2 November 2022).
- Nurix Pipeline. Available online: https://www.nurixtx.com/pipeline/ (accessed on 2 November 2022).
- Targeted Protein Degradation–C4 Therapeutics. Available online: https://c4therapeutics.com/our-science/targeted-protein-degradation (accessed on 2 November 2022).
- Cullgen. Available online: https://www.cullgen.com (accessed on 3 November 2022).
- Mullard, A. Targeted Protein Degraders Crowd into the Clinic. Nat. Rev. Drug Discov. 2021, 20, 247–250. [Google Scholar] [CrossRef]
- Jensen, S.M.; Potts, G.K.; Ready, D.B.; Patterson, M.J. Specific MHC-I Peptides Are Induced Using PROTACs. Front. Immunol. 2018, 9, 2697. [Google Scholar] [CrossRef] [PubMed]
- Mares, A.; Miah, A.H.; Smith, I.E.D.; Rackham, M.; Thawani, A.R.; Cryan, J.; Haile, P.A.; Votta, B.J.; Beal, A.M.; Capriotti, C.; et al. Extended Pharmacodynamic Responses Observed upon PROTAC-Mediated Degradation of RIPK2. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gu, Z.; Lin, S.; Chen, D.; Wang, J.; Zhao, Y.; Li, Y.; Liu, T.; Li, Y.; Wang, Y.; et al. Attenuation of NLRP3 Inflammasome Activation by Indirubin-Derived PROTAC Targeting HDAC6. ACS Chem. Biol. 2021, 16, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Wang, W.; Sun, D.; Zheng, M.; Zhou, Y.; Liang, J.; Zhu, M.; Li, H.; Chen, L. PROTAC Technology as a Novel Tool to Identify the Target of Lathyrane Diterpenoids. Biol. Med. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y. Proteolysis-Targeting Chimera (PROTAC) for Targeted Protein Degradation and Cancer Therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Khan, S.; He, Y.; Zhang, X.; Yuan, Y.; Pu, S.; Kong, Q.; Zheng, G.; Zhou, D. PROteolysis TArgeting Chimeras (PROTACs) as Emerging Anticancer Therapeutics. Oncogene 2020, 39, 4909–4924. [Google Scholar] [CrossRef]
- Kargbo, R.B. PROTAC Molecules for the Treatment of Autoimmune Disorders. ACS Med. Chem. Lett. 2019, 10, 276–277. [Google Scholar] [CrossRef]
- Kumar, D.; Hassan, M.I. Targeted Protein Degraders March towards the Clinic for Neurodegenerative Diseases. Ageing Res. Rev. 2022, 78, 101616. [Google Scholar] [CrossRef]
- Powell, M.; Blaskovich, M.A.T.; Hansford, K.A. Targeted Protein Degradation: The New Frontier of Antimicrobial Discovery? ACS Infect. Dis. 2021, 7, 2050–2067. [Google Scholar] [CrossRef]
- Desantis, J.; Goracci, L. Proteolysis Targeting Chimeras in Antiviral Research. Future Med. Chem. 2022, 14, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Trial of ARV-110 in Patients with Metastatic Castration Resistant Prostate Cancer-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03888612 (accessed on 8 November 2022).
- A Phase 1/2 Trial of ARV-471 Alone and in Combination with Palbociclib (IBRANCE®) in Patients with ER+/HER2- Locally Advanced or Metastatic Breast Cancer-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04072952 (accessed on 8 November 2022).
- Accutar Biotechnology Inc. A Phase I Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Anti-Tumor Activity of AC682 in Patients with Estrogen Receptor Positive/Human Epidermal Growth Factor Receptor 2 Negative (ER+/HER2-) Locally Advanced or Metastatic Breast Cancer. 2022. Available online: https://clinicaltrials.gov/ (accessed on 2 November 2022).
- A Study of ARV-766 Given by Mouth in Men with Metastatic Castration-Resistant Prostate Cancer Who Have Progressed on Prior Approved Systemic Therapies-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT05067140 (accessed on 8 November 2022).
- Study to Evaluate the Safety and Tolerability of CC-94676 in Participants with Metastatic Castration-Resistant Prostate Cancer-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04428788 (accessed on 8 November 2022).
- A Study of DT2216 in Relapsed/Refractory Malignancies-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04886622 (accessed on 8 November 2022).
- FHD-609 in Subjects with Advanced Synovial Sarcoma or Advanced SMARCB1-Loss Tumors-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04965753 (accessed on 9 November 2022).
- A Single and Multiple Ascending Dose Trial of KT-474 in Healthy Adult Volunteers and Patients with Atopic Dermatitis (AD) or Hidradenitis Suppurativa (HS)-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04772885 (accessed on 9 November 2022).
- Kymera Therapeutics, Inc. A Phase 1, Multicenter, Open-Label, Dose Escalation and Expansion Study to Evaluate the Safety, Tolerability, PK/PD, and Clinical Activity of Intravenously Administered KT-413 in Adult Patients with Relapsed or Refractory B-Cell NHL. 2022. Available online: https://clinicaltrials.gov/ (accessed on 2 November 2022).
- Nurix Therapeutics, Inc. A Phase 1, Dose Escalation, Safety and Tolerability Study of NX-2127, a Bruton’s Tyrosine Kinase (BTK) Degrader, in Adults with Relapsed/Refractory B-Cell Malignancies. 2022. Available online: https://clinicaltrials.gov/ (accessed on 2 November 2022).
- A Study of NX-5948 in Adults with Relapsed/Refractory B-Cell Malignancies-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT05131022 (accessed on 9 November 2022).
- A Study to Assess the Safety and Tolerability of CFT8634 in Locally Advanced or Metastatic SMARCB1-Perturbed Cancers, Including Synovial Sarcoma and SMARCB1-Null Tumors-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT05355753 (accessed on 9 November 2022).
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar] [PubMed]
- Gopal, P.; Dick, T. Targeted Protein Degradation in Antibacterial Drug Discovery? Prog. Biophys. Mol. Biol. 2020, 152, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Montrose, K.; Krissansen, G.W. Design of a PROTAC That Antagonizes and Destroys the Cancer-Forming X-Protein of the Hepatitis B Virus. Biochem. Biophys. Res. Commun. 2014, 453, 735–740. [Google Scholar] [CrossRef]
- Lin, C. HCV NS3-4A Serine Protease. In Hepatitis C Viruses: Genomes and Molecular Biology; Tan, S.-L., Ed.; Horizon Bioscience: Norfolk, UK, 2006; ISBN 978-1-904933-20-5. [Google Scholar]
- de Wispelaere, M.; Du, G.; Donovan, K.A.; Zhang, T.; Eleuteri, N.A.; Yuan, J.C.; Kalabathula, J.; Nowak, R.P.; Fischer, E.S.; Gray, N.S.; et al. Small Molecule Degraders of the Hepatitis C Virus Protease Reduce Susceptibility to Resistance Mutations. Nat. Commun. 2019, 10, 3468. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Pang, X.; Liu, Z.; Li, Q.; Yi, D.; Zhang, Y.; Fang, X.; Zhang, T.; Zhou, R.; et al. An Anti-Influenza A Virus Microbial Metabolite Acts by Degrading Viral Endonuclease PA. Nat. Commun. 2022, 13, 2079. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Ma, W.; Cheng, B.; Yi, Y.; Ma, X.; Xiao, S.; Zhang, L.; Zhou, D. Discovery of Pentacyclic Triterpenoid PROTACs as a Class of Effective Hemagglutinin Protein Degraders. J. Med. Chem. 2022, 65, 7154–7169. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Ma, X.; Zou, W.; Chen, Q.; Chen, F.; Deng, X.; Liang, J.; Dong, C.; Lan, K.; et al. Discovery of Oseltamivir-Based Novel PROTACs as Degraders Targeting Neuraminidase to Combat H1N1 Influenza Virus. Cell Insight 2022, 1, 100030. [Google Scholar] [CrossRef]
- Haniff, H.S.; Tong, Y.; Liu, X.; Chen, J.L.; Suresh, B.M.; Andrews, R.J.; Peterson, J.M.; O’Leary, C.A.; Benhamou, R.I.; Moss, W.N.; et al. Targeting the SARS-COV-2 RNA Genome with Small Molecule Binders and Ribonuclease Targeting Chimera (RiboTAC) Degraders. ACS Cent. Sci. 2020, 6, 1713–1721. [Google Scholar] [CrossRef]
- Su, X.; Ma, W.; Feng, D.; Cheng, B.; Wang, Q.; Guo, Z.; Zhou, D.; Tang, X. Efficient Inhibition of SARS-CoV-2 Using Chimeric Antisense Oligonucleotides through RNase L Activation**. Angew. Chem. -Int. Ed. 2021, 60, 21662–21667. [Google Scholar] [CrossRef]
- Hahn, F.; Hamilton, S.T.; Wangen, C.; Wild, M.; Kicuntod, J.; Brückner, N.; Follett, J.E.L.; Herrmann, L.; Kheimar, A.; Kaufer, B.B.; et al. Development of a PROTAC-Based Targeting Strategy Provides a Mechanistically Unique Mode of Anti-Cytomegalovirus Activity. IJMS 2021, 22, 12858. [Google Scholar] [CrossRef] [PubMed]
- Desantis, J.; Mercorelli, B.; Celegato, M.; Croci, F.; Bazzacco, A.; Baroni, M.; Siragusa, L.; Cruciani, G.; Loregian, A.; Goracci, L. Indomethacin-Based PROTACs as Pan-Coronavirus Antiviral Agents. Eur. J. Med. Chem. 2021, 226, 113814. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, R.; Liu, S.; Disoma, C.; Du, A.; Li, S.; Chen, Z.; Dong, Z.; Zhang, Y.; Li, S.; et al. Host E3 Ligase HUWE1 Attenuates the Proapoptotic Activity of the MERS-CoV Accessory Protein ORF3 by Promoting Its Ubiquitin-Dependent Degradation. J. Biol. Chem. 2022, 298. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cinos, C.; Goossens, K.; Salado, I.G.; Van Der Veken, P.; De Winter, H.; Augustyns, K. ClpP Protease, a Promising Antimicrobial Target. Int. J. Mol. Sci. 2019, 20, 2232. [Google Scholar] [CrossRef] [PubMed]
- Trentini, D.B.; Suskiewicz, M.J.; Heuck, A.; Kurzbauer, R.; Deszcz, L.; Mechtler, K.; Clausen, T. Arginine Phosphorylation Marks Proteins for Degradation by a Clp Protease. Nature 2016, 539, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Suskiewicz, M.J.; Hajdusits, B.; Beveridge, R.; Heuck, A.; Vu, L.D.; Kurzbauer, R.; Hauer, K.; Thoeny, V.; Rumpel, K.; Mechtler, K.; et al. Structure of McsB, a Protein Kinase for Regulated Arginine Phosphorylation. Nat. Chem. Biol. 2019, 15, 510–518. [Google Scholar] [CrossRef]
- Morreale, F.E.; Kleine, S.; Leodolter, J.; Ovchinnikov, S.; Kley, J.; Kurzbauer, R.; Hoi, D.M.; Meinhart, A.; Hartl, M.; Haselbach, D.; et al. BacPROTACs Mediate Targeted Protein Degradation in Bacteria. Cell 2022, 185, 2338–2353.e18. [Google Scholar] [CrossRef] [PubMed]
- Weinhäupl, K.; Brennich, M.; Kazmaier, U.; Lelievre, J.; Ballell, L.; Goldberg, A.; Schanda, P.; Fraga, H. The Antibiotic Cyclomarin Blocks Arginine-Phosphate-Induced Millisecond Dynamics in the N-Terminal Domain of ClpC1 from Mycobacterium Tuberc. J. Biol. Chem. 2018, 293, 8379–8393. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.K.; Riwanto, M.; Sambandamurthy, V.; Roggo, S.; Miault, C.; Zwingelstein, C.; Krastel, P.; Noble, C.; Beer, D.; Rao, S.P.S.; et al. The Natural Product Cyclomarin Kills Mycobacterium Tuberculosis by Targeting the ClpC1 Subunit of the Caseinolytic Protease. Angew. Chem. Int. Ed. Engl. 2011, 50, 5889–5891. [Google Scholar] [CrossRef]
- Junk, L.; Schmiedel, V.; Guha, S.; Greb, P.; Fischel, K.; Rumpel, K.; Kaur, P.; Krishnamurthy, R.; Narayanan, S.; Kofink, C.; et al. BacPROTAC-Induced Degradation of ClpC1 as a New Strategy against Drug-Resistant Mycobacteria. Chemrxiv 2022. [Google Scholar] [CrossRef]
- Gopal, P.; Sarathy, J.; Yee, M.; Ragunathan, P.; Shin, J.; Bhushan, S.; Zhu, J.; Akopian, T.; Kandror, O.; Lim, T.K.; et al. Pyrazinamide Triggers Degradation of Its Target Aspartate Decarboxylase. Nat. Commun. 2019, 11, 674416. [Google Scholar] [CrossRef]
- Long, M.J.C.; Gollapalli, D.R.; Hedstrom, L. Inhibitor Mediated Protein Degradation. Chem. Biol. 2012, 19, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lu, F.; Qi, F.; Wen, T.; Bai, M.; Wang, J. Compound for Degrading Deoxyribonucleic Acid (DNA) Polymerase, and Use Thereof. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2022156764&_gid=202230 (accessed on 24 November 2022).
- Zhou, H.; Wu, S.; Xu, Z. A kind of Oseltamivir PROTAC Compound and its Preparation Method and Application in Anti-Influenza Virus Drug. CN112592331B, 22 October 2021. Available online: https://patents.google.com/patent/CN112592331B/en (accessed on 17 November 2022).
- Gao, J.; Hou, B.; Zhu, Q.; Yang, L.; Jiang, X.; Zou, Z.; Li, X.; Xu, T.; Zheng, M.; Chen, Y.-H.; et al. Engineered Bioorthogonal POLY-PROTAC Nanoparticles for Tumour-Specific Protein Degradation and Precise Cancer Therapy. Nat. Commun. 2022, 13, 4318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Picaud, S.; Filippakopoulos, P.; D’Angiolella, V.; Bullock, A.N. Structural Basis for Recruitment of DAPK1 to the KLHL20 E3 Ligase. Structure 2019, 27, 1395–1404.e4. [Google Scholar] [CrossRef]
- Silva, M.C.; Ferguson, F.M.; Cai, Q.; Donovan, K.A.; Nandi, G.; Patnaik, D.; Zhang, T.; Huang, H.-T.; Lucente, D.E.; Dickerson, B.C.; et al. Targeted Degradation of Aberrant Tau in Frontotemporal Dementia Patient-Derived Neuronal Cell Models. eLife 2019, 8, e45457. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).