Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Type of Study
2.2.2. Type of Population
2.2.3. Type of Interventions
2.2.4. Type of Outcomes
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Extraction
2.7. Geometry of the Network
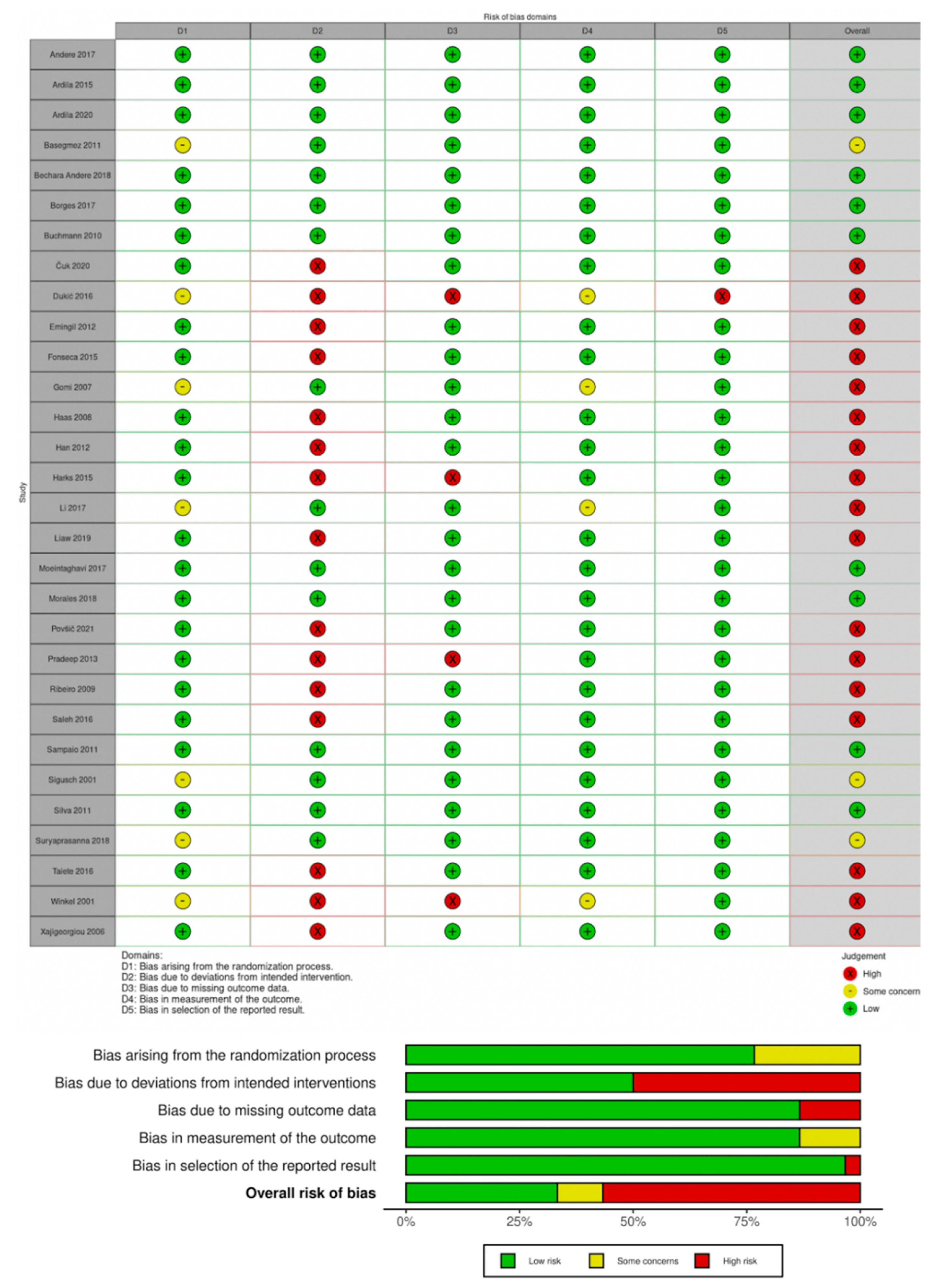
2.8. Risk of Bias within Individual Studies
2.9. Data Synthesis
2.10. Assessment of Inconsistency
2.11. Subgroup Analyses
2.12. Sensitivity Analyses
2.13. Assessment of Publication Bias
2.14. Assessment of Body of Evidence Sertainty
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.2.1. General Characteristics
3.2.2. Intervention Characteristics
3.2.3. Reported Outcome and the Outcome Measurements
3.3. Risk of Bias
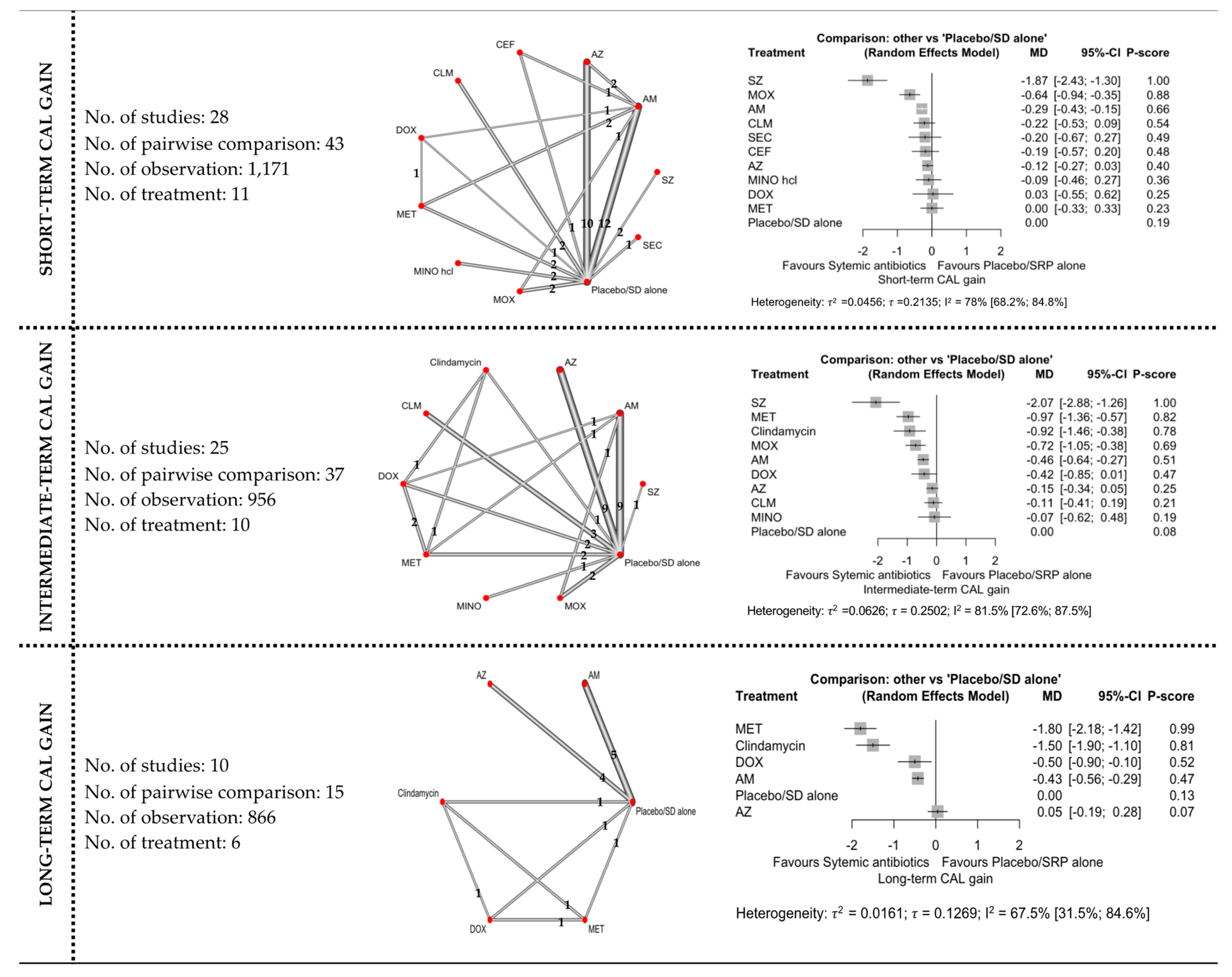
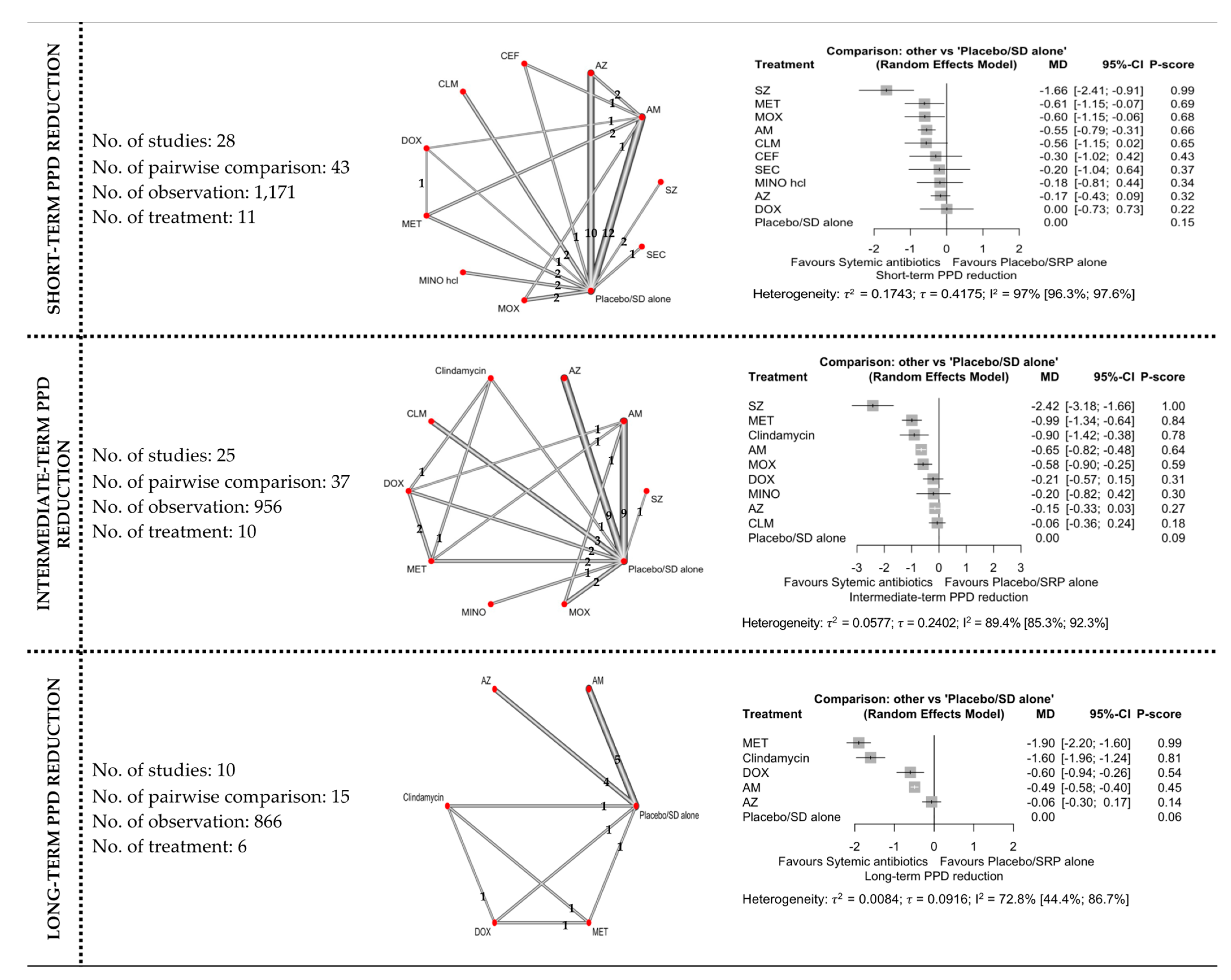
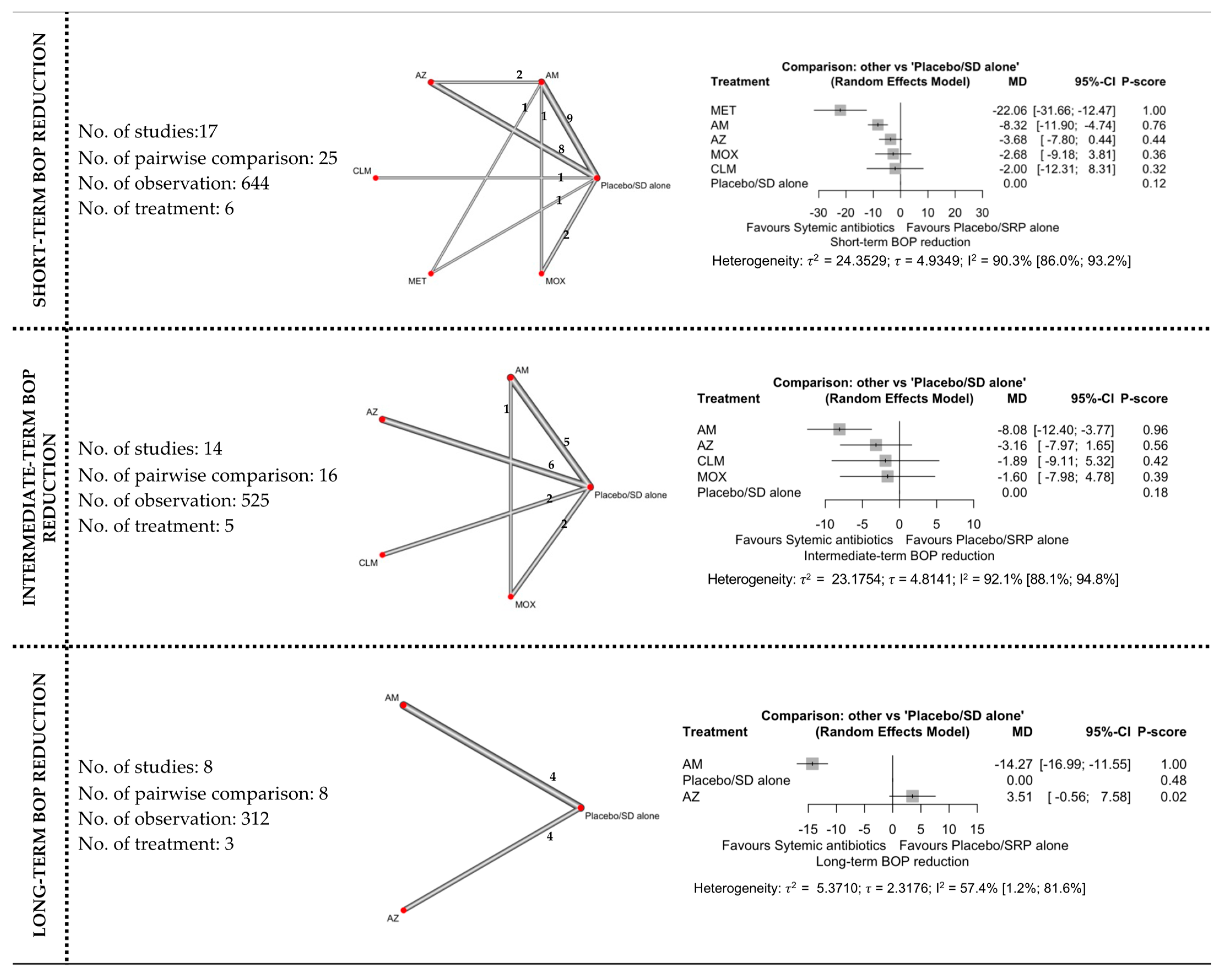
3.4. Network Meta-Analyses
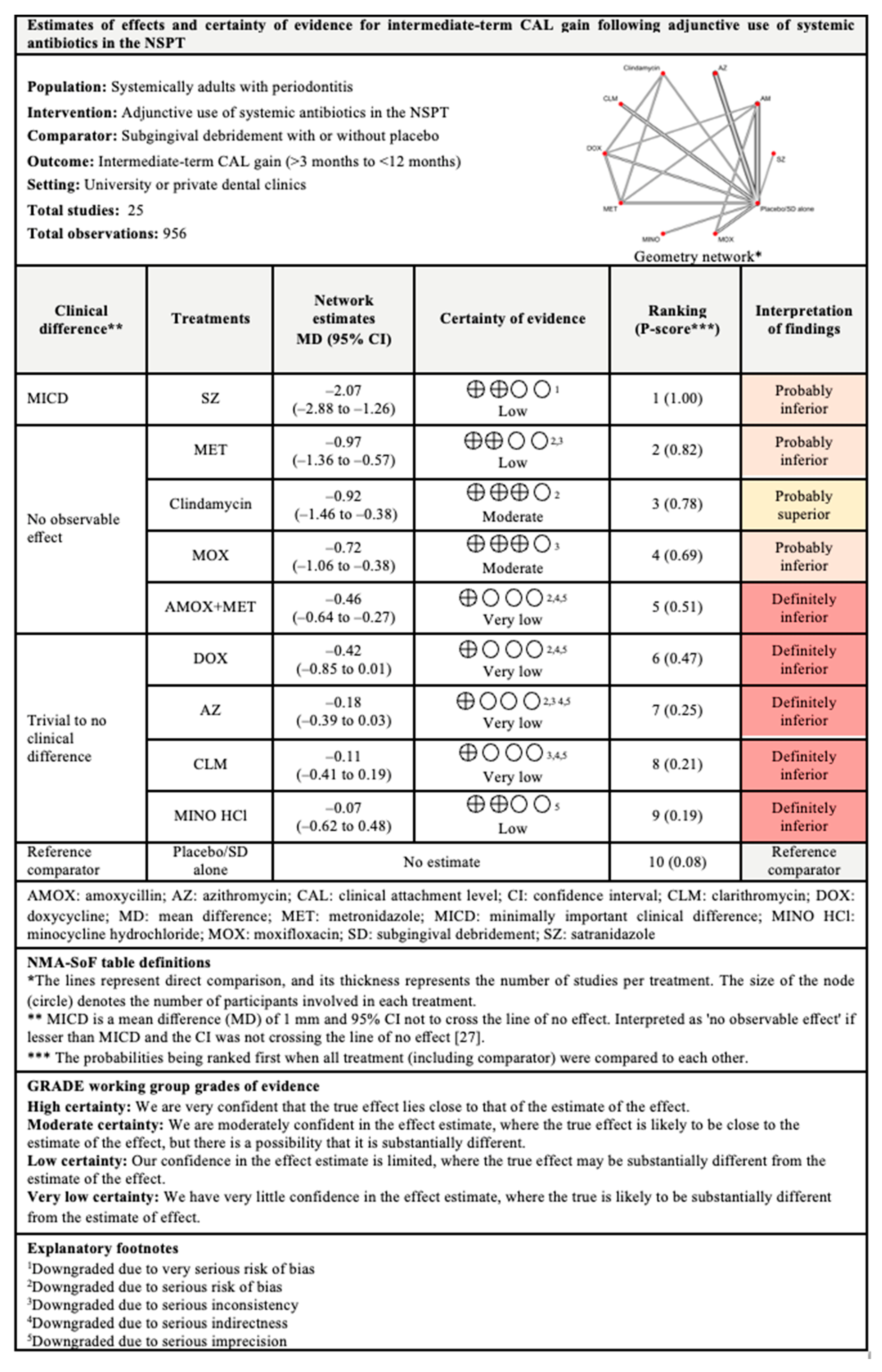
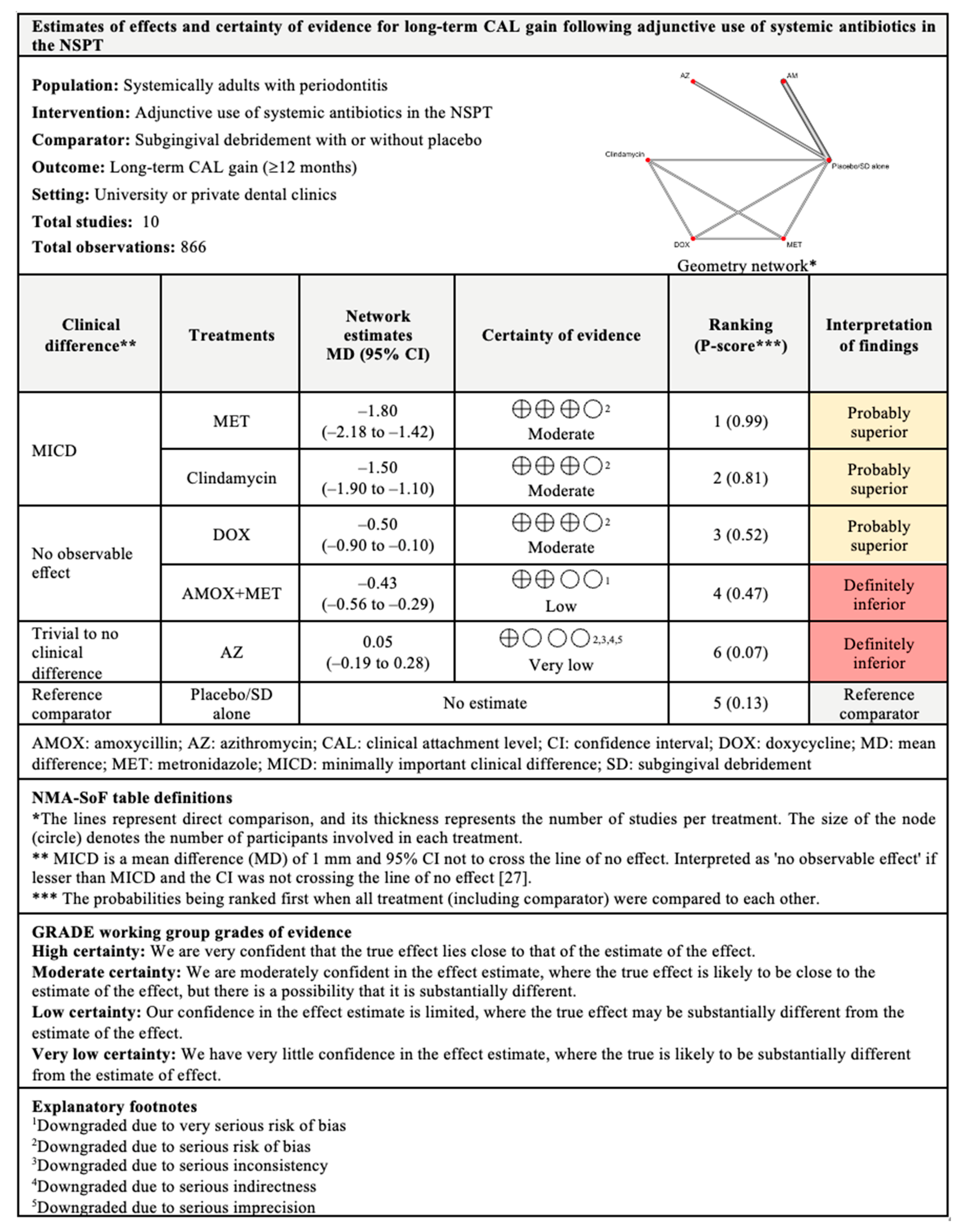
3.4.1. CAL Gain
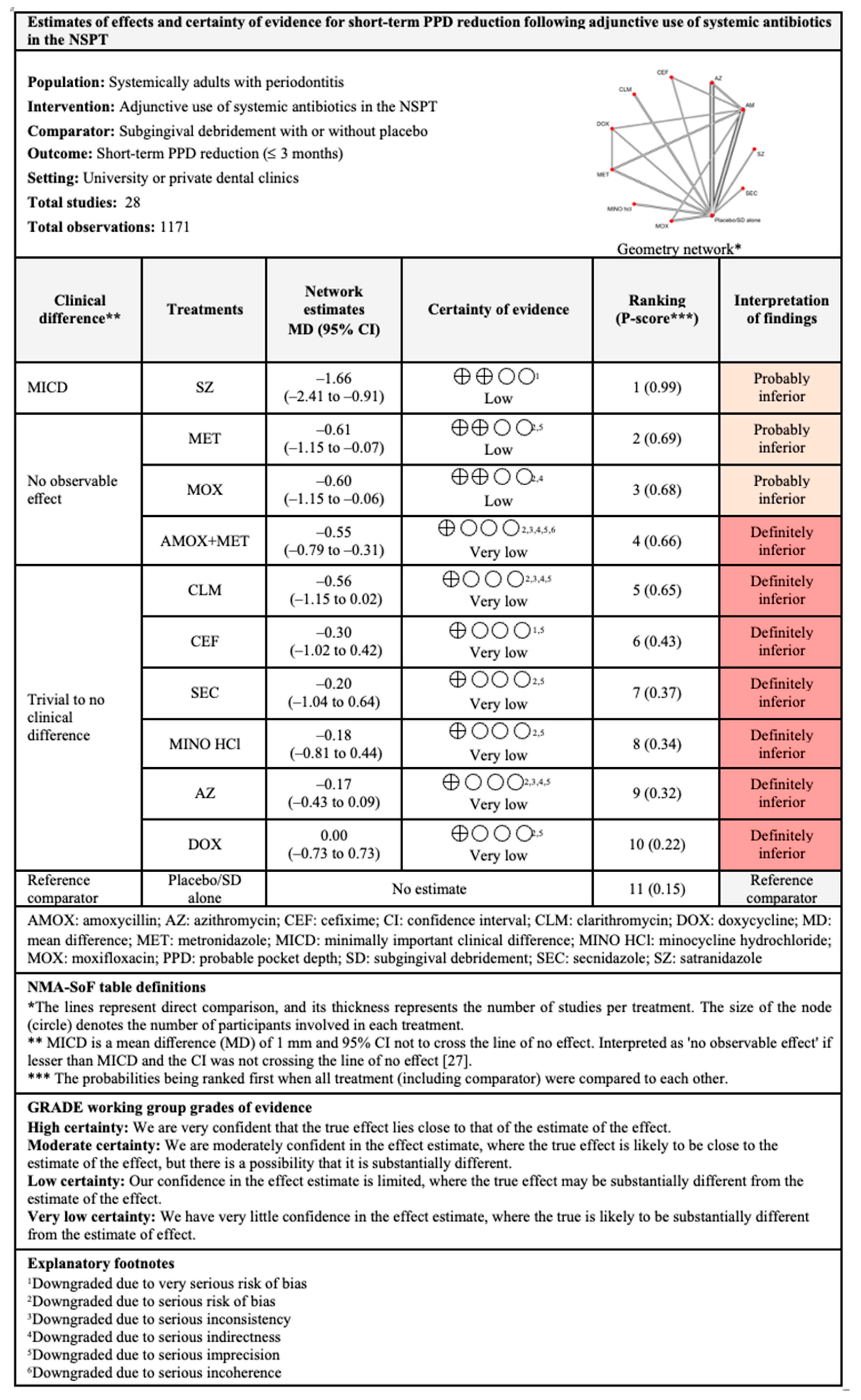
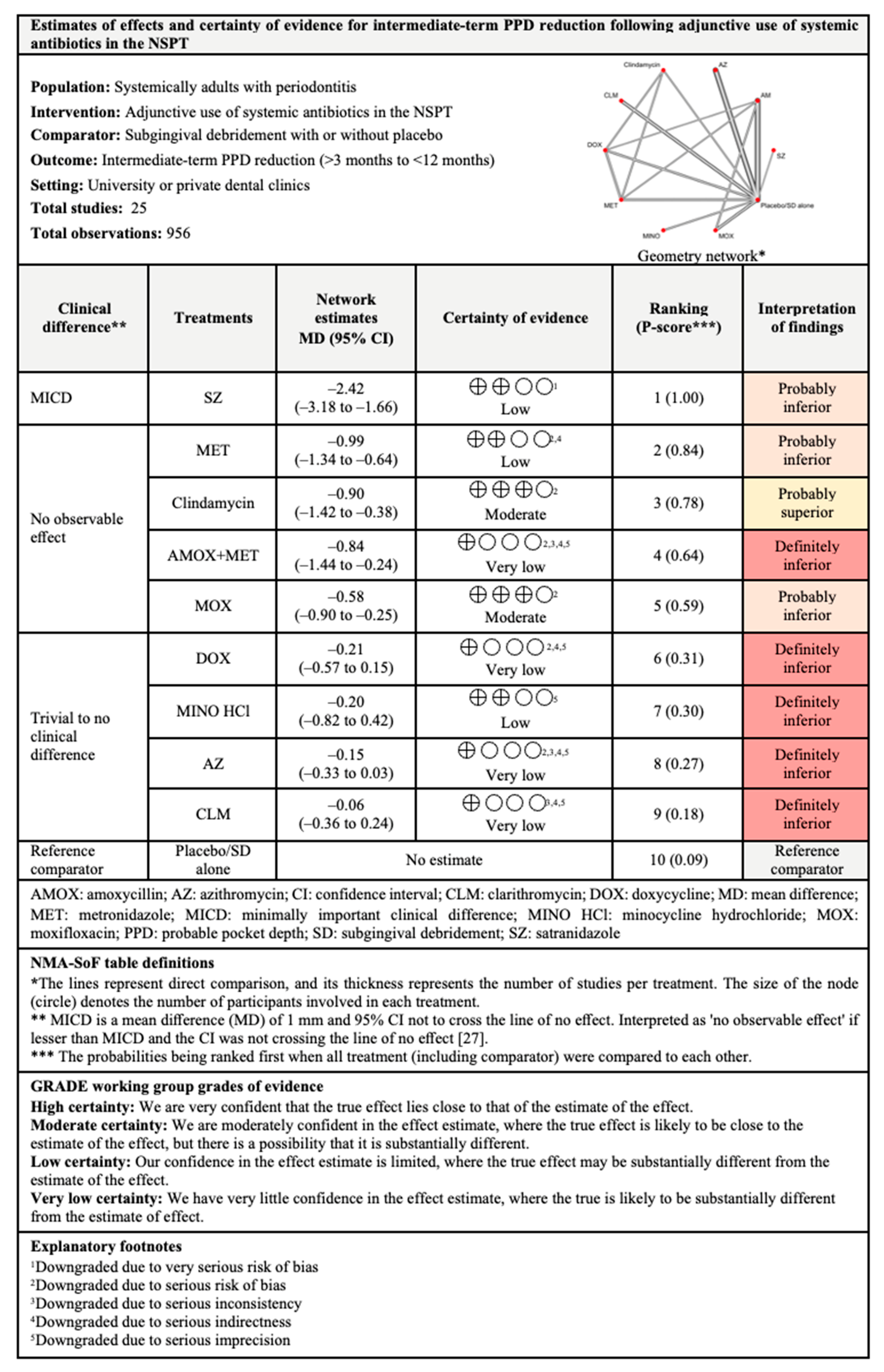
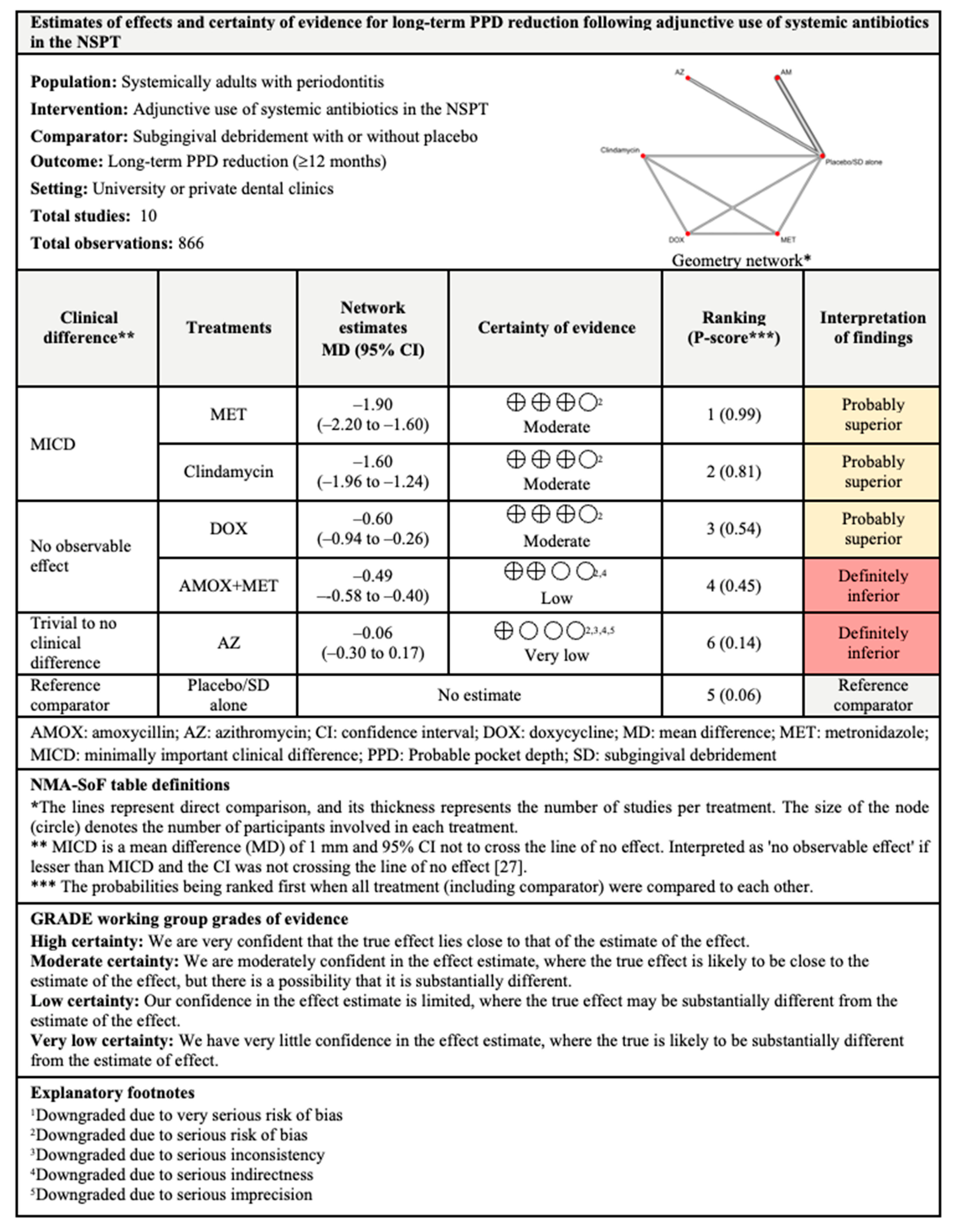
3.4.2. PPD Reduction
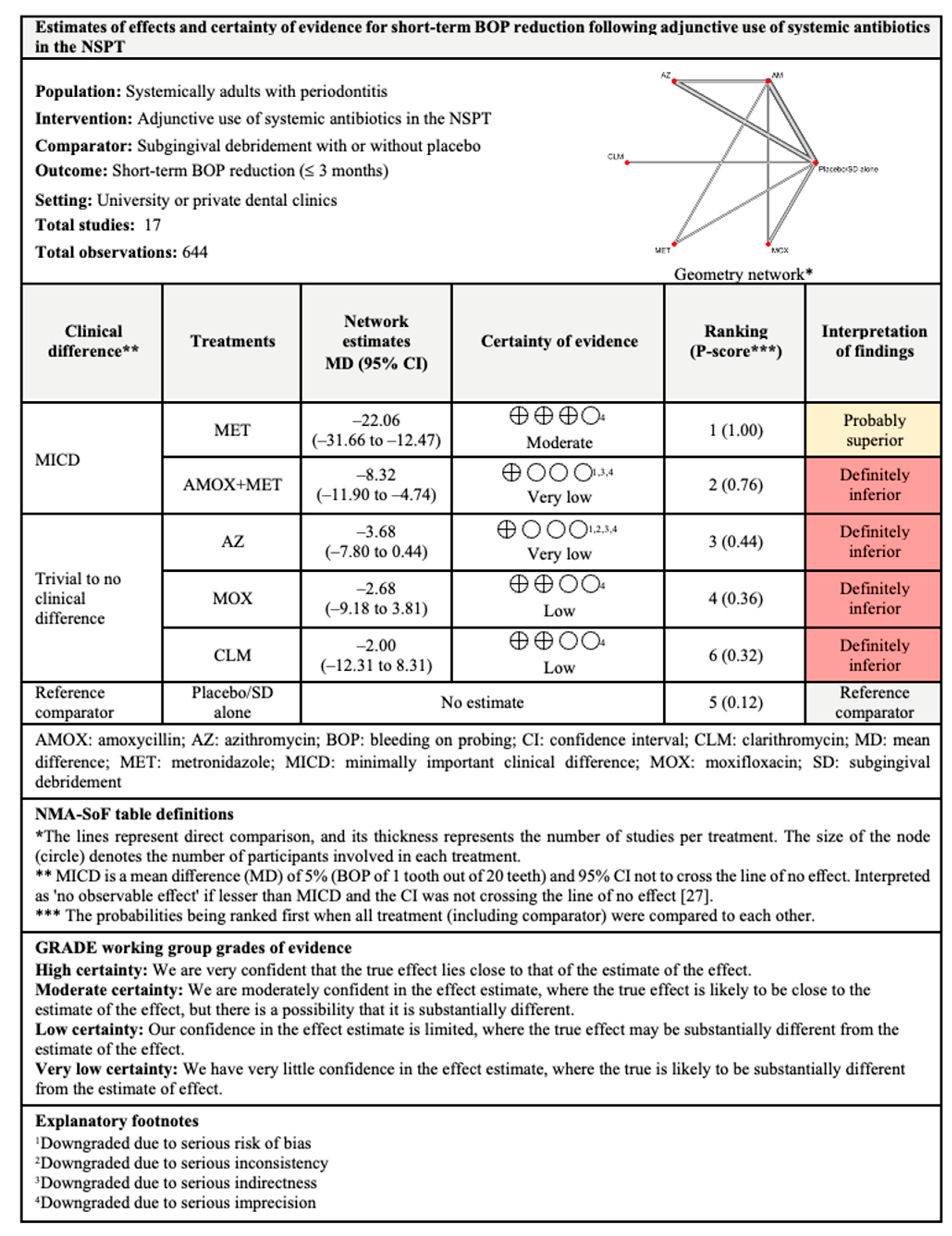
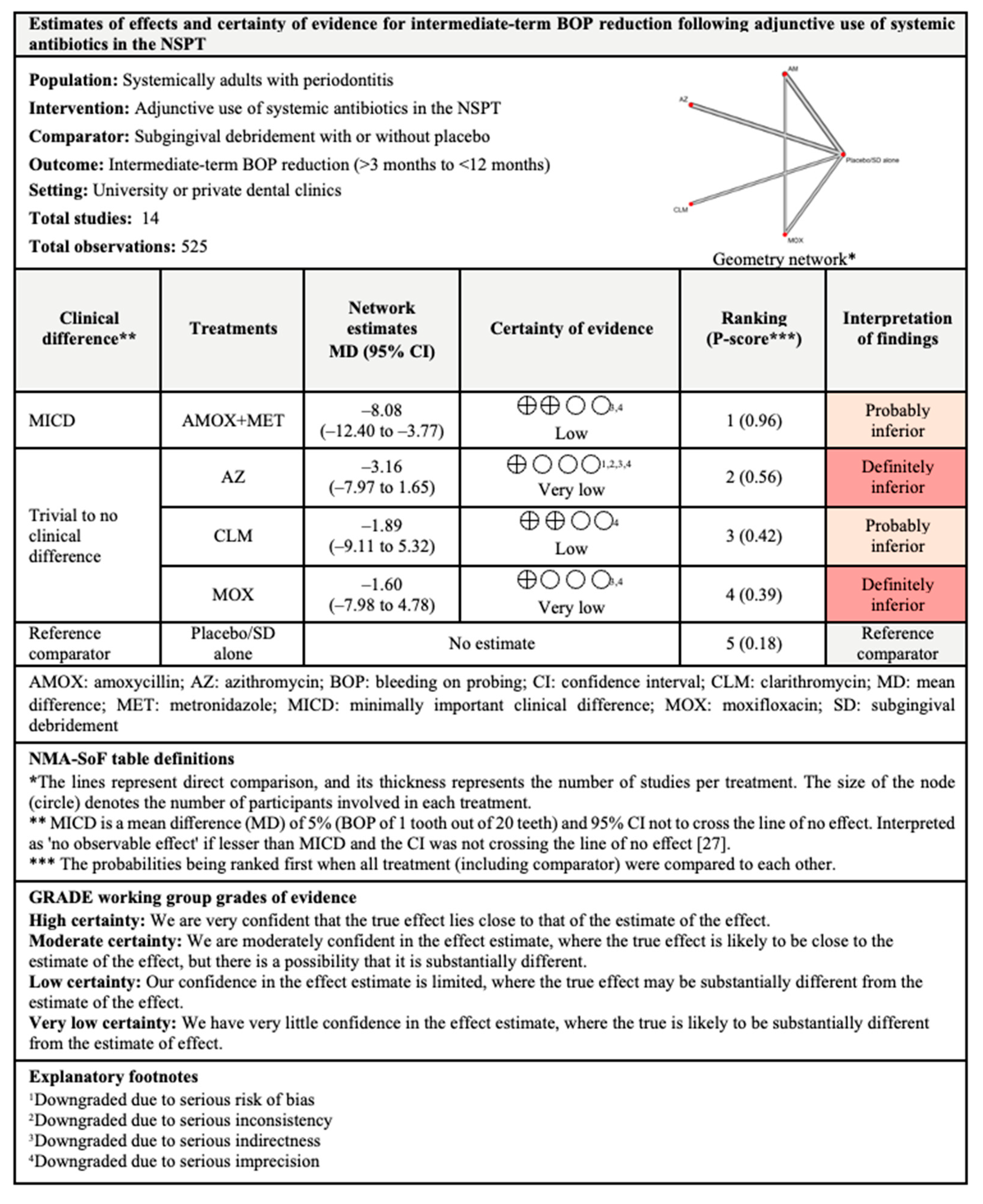
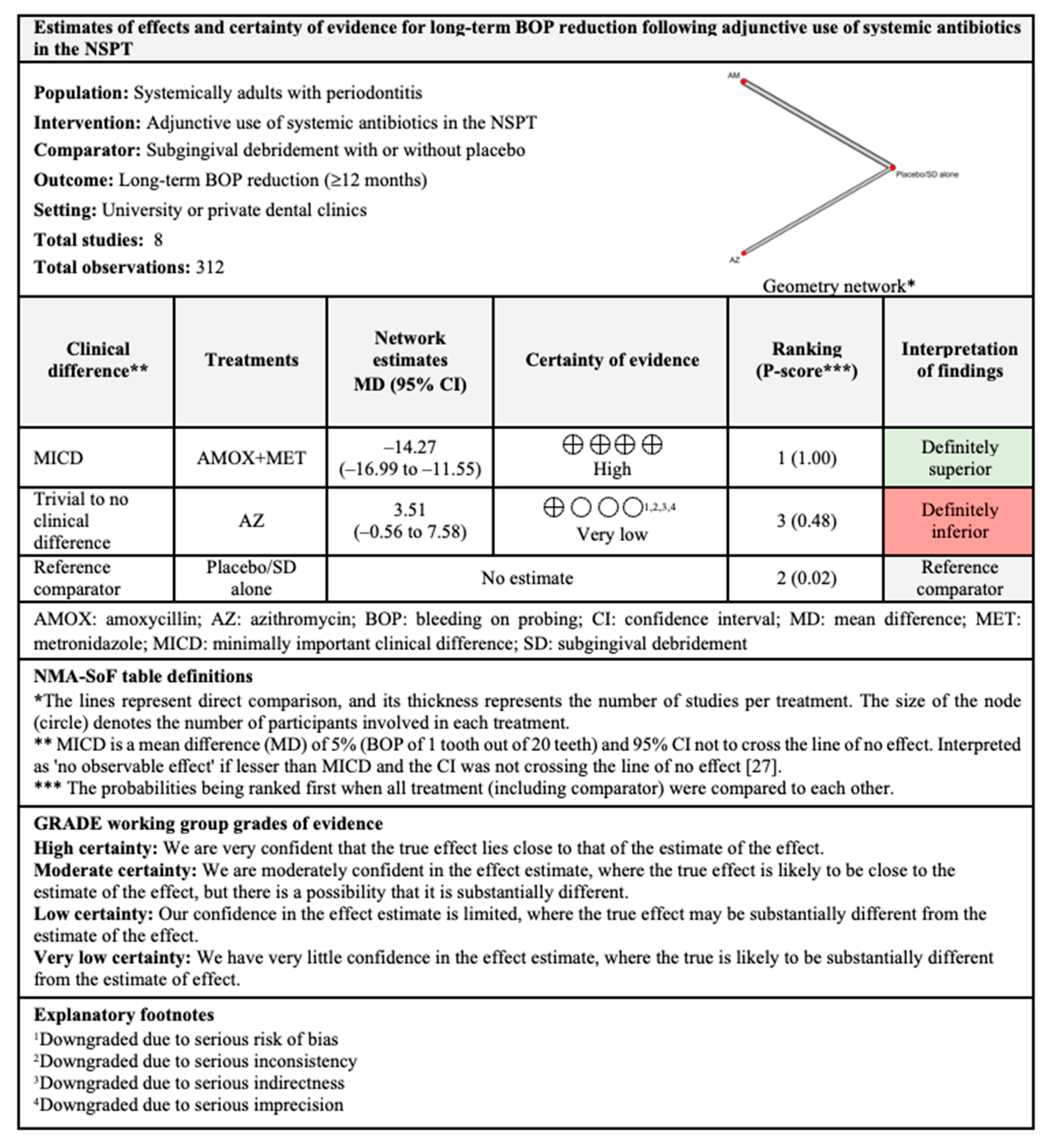
3.4.3. BOP Reduction
3.4.4. Adverse Events
3.5. Assessment of Inconsistency
3.6. Subgroup Analyses
3.7. Sensitivity Analyses
3.7.1. CAL Gain
3.7.2. PPD Reduction
3.7.3. BOP Reduction
3.8. Publication Bias
3.9. Summary and Certainty of Evidence
4. Discussion
4.1. Summary of Findings
4.2. Comparisons with Previous NMA
4.3. Strengths and Limitations
- There was substantial (I2 > 50%) between-studies heterogeneity in most of the network estimates. To overcome this limitation, subgroup analyses were conducted by splitting the studies into less heterogenous groups and separate analyses were performed for each group to explore potential reasons for differences.
- Numerous studies included parameters (outliers and influential cases, imputed/transformed missing data, and high risk of bias) that may have affected the estimates of treatment effect. To address this limitation, sensitivity analyses were conducted by re-running the NMA after excluding studies with such parameters, to ensure the robustness of the treatment-effect estimates.
- More than half of the included studies were at high risk of bias. In addition to removing such studies during the sensitivity analysis, the certainty of evidence provided by studies with a high risk of bias was downgraded to avoid misleading interpretation of the network estimates.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Smith, A.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition, and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990-2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Sheiham, A.; Watt, R.G. The common risk factor approach: A rational basis for promoting oral health. Community Dent. Oral. Epidemiol. 2000, 28, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.G. Strategies and approaches in oral disease prevention and health promotion. Bull. World Health Organ. 2005, 83, 711–718. [Google Scholar]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 7, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 8, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Time to take periodontitis seriously. BMJ 2014, 348, g2645. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal. Res. 2017, 52, 651–665. [Google Scholar] [CrossRef]
- Lawrence, H.P.; Thomson, W.M.; Broadbent, J.M.; Poulton, R. Oral health-related quality of life in a birth cohort of 32-years old. Community Dent. Oral Epidemiol. 2008, 36, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol. 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.; Abdulbaqi, H.; Gul, S.; Milward, M.; Chasib, N.; Alhashimi, R. Classic vs. Novel antibacterial approaches for eradicating dental biofilm as adjunct to periodontal debridement: An evidence-based overview. Antibiotics 2022, 11, 9. [Google Scholar] [CrossRef]
- Kwon, T.; Levin, L. Cause-related therapy: A review and suggested guidelines. Quintessence Int. 2014, 45, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Salem, D.M.; Levin, L. Nonsurgical periodontal therapy based on the principles of cause-related therapy: Rationale and case series. Quintessence Int. 2019, 50, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Bhuvaraghan, A.; King, R.; Larvin, H.; Aggarwal, V.R. Antibiotic Use and Misuse in Dentistry in India-A Systematic Review. Antibiotics 2021, 10, 1459. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e5. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 17 November 2022).
- Mshana, S.E.; Sindato, C.; Matee, M.I.; Mboera, L.E.G. Antimicrobial Use and Resistance in Agriculture and Food Production Systems in Africa: A Systematic Review. Antibiotics 2021, 10, 976. [Google Scholar] [CrossRef]
- Durrance-Bagale, A.; Jung, A.-S.; Frumence, G.; Mboera, L.; Mshana, S.E.; Sindato, C.; Clark, T.G.; Matee, M.; Legido-Quigley, H. Framing the Drivers of Antimicrobial Resistance in Tanzania. Antibiotics 2021, 10, 991. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Antibiotic resistance of human periodontal pathogen parvimonas micra over 10 years. Antibiotics 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Burden of AMR Collaborative Group; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Agossa, K.; Sy, K.; Mainville, T.; Gosset, M.; Jeanne, S.; Grosgogeat, B.; Siepmann, F.; Loingeville, F.; Dubar, M. Antibiotic Use in Periodontal Therapy among French Dentists and Factors Which Influence Prescribing Practices. Antibiotics 2021, 10, 303. [Google Scholar] [CrossRef]
- Mohsen, S.; Dickinson, J.A.; Somayaji, R. Update on the adverse effects of antimicrobial therapies in community practice. Can. Fam. Physician 2020, 66, 651–659. [Google Scholar]
- Khattri, S.; Kumbargere, N.S.; Arora, A.; Eachempati, P.; Kusum, C.K.; Bhat, K.G.; Johnson, T.M.; Lodi, G. Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis. Cochrane Database Syst. Rev. 2020, 11, CD012568. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Ciarrocchi, I.; Masci, C.; Spadaro, A. Adjunctive systemic antimicrobials in the treatment of chronic periodontitis: A systematic review and network meta-analysis. J. Periodontal. Res. 2021, 56, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 257–281. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available online: www.training.cochrane.org/handbook (accessed on 22 February 2021).
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Chapple, I.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 8, S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. Available online: https://www.york.ac.uk/crd/SysRev/!SSL!/WebHelp/SysRev3.htm#1_1_CORE_PRINCIPLES_GETTING_STARTED.htm (accessed on 22 February 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.T.; Lin, L.; Chu, H.; Tong, T. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, D.; Wan, X.; Liu, Y.; Liu, J.; Bian, Z.; Tong, T. Detecting the Skewnesso Data from the Sample Size and the Five-Number Summary. arXiv 2020, arXiv:2010.05749. Available online: https://arxiv.org/pdf/2010.05749.pdf (accessed on 22 February 2021).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol 2014, 14, 135. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R [Software]. Available online: http://www.rstudio.com/ (accessed on 27 February 2022).
- Rücker, G.; Krahn, U.; König, J.; Efthimiou, O.; Davies, A.; Papakonstantinou, T.; Schwarzer, G. Netmeta: Network Meta-Analysis Using Frequentist Methods, R Package Version 2.1-0 2022; R: Vienna, Austria. Available online: https://cran.r-project.org/web/packages/netmeta/netmeta.pdf (accessed on 27 February 2022).
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Eds.; Updated February 2021. Available online: www.training.cochrane.org/handbook (accessed on 27 February 2022).
- König, J.; Krahn, U.; Binder, H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat. Med. 2013, 32, 5414–5429. [Google Scholar] [CrossRef]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Broź ek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2 (accessed on 27 February 2022).
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2010, 22, 276–282. [Google Scholar] [CrossRef]
- Basegmez, C.; Berber, L.; Yalcin, F. Clinical and biochemical efficacy of minocycline in nonsurgical periodontal therapy: A randomized controlled pilot study. J. Clin. Pharmacol. 2011, 51, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Emingil, G.; Han, B.; Ozdemir, G.; Tervahartiala, T.; Vural, C.; Atilla, G.; Baylas, H.; Sorsa, T. Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. J. Periodontal. Res. 2012, 47, 729–739. [Google Scholar] [CrossRef]
- Gomi, K.; Yashima, A.; Nagano, T.; Kanazashi, M.; Maeda, N.; Arai, T. Effects of full-mouth scaling and root planing in conjunction with systemically administered azithromycin. J. Periodontol. 2007, 78, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Emingil, G.; Özdemir, G.; Tervahartiala, T.; Vural, C.; Atilla, G.; Baylas, H.; Sorsa, T. Azithromycin as an adjunctive treatment of generalized severe chronic periodontitis: Clinical, microbiologic, and biochemical parameters. J. Periodontol. 2012, 83, 1480–1491. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Jin, Q.; Pang, J.; Xu, Z. The efficacy of proanthocyanidins and secnidazole in the treatment of chronic periodontitis after scaling and root planing therapy. J. Biol. Regul. Homeost. Agents 2017, 31, 93–97. [Google Scholar]
- Moeintaghavi, A.; Talebi-ardakani, M.R.; Haerian-ardakani, A.; Zandi, H.; Taghipour, S.; Fallahzadeh, H.; Pakzad, A.; Fahami, N. Adjunctive effects of systemic amoxicillin and metronidazole with scaling and root planing: A randomized, placebo controlled clinical trial. J. Contemp. Dent. Pract. 2007, 8, 51–59. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Priyanka, N.; Kalra, N.; Naik, S.B. A randomized controlled clinical trial on the clinical and microbiological efficacy of systemic satranidazole in the treatment of chronic periodontitis. J. Int. Acad. Periodontol. 2013, 15, 43–50. [Google Scholar]
- Suryaprasanna, J.; Radhika, P.L.; Karunakar, P.; Rekharani, K.; Faizuddin, U.; Manojkumar, M.G.; Jammula, S. Evaluating the effectiveness of clarithromycin as an adjunct to scaling and root planing: A randomized clinical trial. J. Indian Soc. Periodontol. 2018, 22, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Andere, N.; Castro Dos Santos, N.C.; Araujo, C.F.; Mathias, I.F.; Taiete, T.; Casarin, R.; Jardini, M.; Shaddox, L.M.; Santamaria, M.P. Clarithromycin as an Adjunct to One-Stage Full-Mouth Ultrasonic Periodontal Debridement in Generalized Aggressive Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Flórez-Flórez, J.; Castañeda-Parra, L.D.; Guzmán, I.C.; Bedoya-García, J.A. Moxifloxacin versus amoxicillin plus metronidazole as adjunctive therapy for generalized aggressive periodontitis: A pilot randomized controlled clinical trial. Quintessence Int. 2020, 51, 612–621. [Google Scholar] [CrossRef]
- Ardila, C.M.; Martelo-Cadavid, J.F.; Boderth-Acosta, G.; Ariza-Garcés, A.A.; Guzmán, I.C. Adjunctive moxifloxacin in the treatment of generalized aggressive periodontitis patients: Clinical and microbiological results of a randomized, triple-blind, and placebo-controlled clinical trial. J. Clin. Periodontol. 2015, 42, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bechara Andere, N.; Dos Santos, N.; Araujo, C.F.; Mathias, I.F.; Rossato, A.; de Marco, A.C.; Santamaria, M.; Jardini, M., Jr.; Santamaria, M.P. Evaluation of the local effect of nonsurgical periodontal treatment with and without systemic antibiotic and photodynamic therapy in generalized aggressive periodontitis. A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 24, 115–120. [Google Scholar] [CrossRef]
- Borges, I.; Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Retamal-Valdes, B.; Montenegro, S.; Feres, M. Different antibiotic protocols in the treatment of severe chronic periodontitis: A 1-year randomized trial. J. Clin. Periodontol. 2017, 44, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.C.; Cortelli, J.R.; Cortelli, S.C.; Miranda Cota, L.O.; Machado Costa, L.C.; Moreira Castro, M.V.; Oliveira Azevedo, A.M.; Costa, F.O. Clinical and Microbiologic Evaluation of Scaling and Root Planing per Quadrant and One-Stage Full-Mouth Disinfection Associated with Azithromycin or Chlorhexidine: A Clinical Randomized Controlled Trial. J. Periodontol. 2015, 86, 1340–1351. [Google Scholar] [CrossRef]
- Haas, A.N.; de Castro, G.D.; Moreno, T.; Susin, C.; Albandar, J.M.; Oppermann, R.V.; Rösing, C.K. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J. Clin. Periodontol. 2008, 35, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Contador, R.; Bravo, J.; Carvajal, P.; Silva, N.; Strauss, F.J.; Gamonal, J. Clinical effects of probiotic or azithromycin as an adjunct to scaling and root planning in the treatment of stage III periodontitis: A pilot randomized controlled clinical trial. BMC Oral Health 2021, 21, 12. [Google Scholar] [CrossRef]
- Ribeiro, E.; Bittencourt, S.; Zanin, I.C.; Bovi Ambrosano, G.M.; Sallum, E.A.; Nociti, F.H.; Gonçalves, R.B.; Casati, M.Z. Full-mouth ultrasonic debridement associated with amoxicillin and metronidazole in the treatment of severe chronic periodontitis. J. Periodontol. 2009, 80, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, E.; Rocha, M.; Figueiredo, L.C.; Faveri, M.; Duarte, P.M.; Gomes Lira, E.A.; Feres, M. Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2011, 38, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Feres, M.; Sirotto, T.A.; Soares, G.M.; Mendes, J.A.; Faveri, M.; Figueiredo, L.C. Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2011, 38, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Taiete, T.; Casati, M.Z.; Ribeiro, É.; Sallum, E.A.; Nociti Júnior, F.H.; Casarin, R.C. Amoxicillin/metronidazole associated with nonsurgical therapy did not promote additional benefits in immunologic parameters in generalized aggressive periodontitis: A randomized controlled clinical trial. Quintessence Int. 2016, 47, 281–292. [Google Scholar] [CrossRef]
- Buchmann, R.; Conrads, G.; Sculean, A. Short-term effects of systemic antibiotics during periodontal healing. Quintessence Int. 2010, 41, 303–312. [Google Scholar]
- Čuk, K.; Povšič, K.; Milavec, S.; Seme, K.; Gašperšič, R. Influence of adjunctive azithromycin on microbiological and clinical outcomes in periodontitis patients: 6-month results of randomized controlled clinical trial. BMC Oral Health 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Dukić, S.; Matijević, S.; Daković, D.; Cutović, T. Comparison of cefixime and amoxicillin plus metronidazole in the treatment of chronic periodontitis. Vojnosanit. Pregl. 2016, 73, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Harks, I.; Koch, R.; Eickholz, P.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Doering, S.; et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015, 42, 832–842. [Google Scholar] [CrossRef]
- Povšič, K.; Čuk, K.; Milavec, S.; Erčulj, V.; Seme, K.; Gašperšič, R. Systemic azithromycin as an adjunct to scaling and root planing in patients with stage III/IV periodontitis: 12-month results of a randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5997–6006. [Google Scholar] [CrossRef]
- Sigusch, B.; Beier, M.; Klinger, G.; Pfister, W.; Glockmann, E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J. Periodontol. 2001, 72, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Winkel, E.G.; Van Winkelhoff, A.J.; Timmerman, M.F.; Van der Velden, U.; Van der Weijden, G.A. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. J. Clin. Periodontol. 2001, 28, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Xajigeorgiou, C.; Sakellari, D.; Slini, T.; Baka, A.; Konstantinidis, A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J. Clin. Periodontol. 2006, 33, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Miller, C.; Nimmo, A. Comparing the periodontal tissue response to non-surgical scaling and root planing alone, adjunctive azithromycin, or adjunctive amoxicillin plus metronidazole in generalized chronic moderate-to-severe periodontitis: A preliminary randomized controlled trial. Aust. Dent. J. 2019, 64, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Rincon, J.; Tan, A.; Firth, M. Comparison of adjunctive azithromycin and amoxicillin/metronidazole for patients with chronic periodontitis: Preliminary randomized control trial. Aust. Dent. J. 2016, 61, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Durr-E-Sadaf. How to apply evidence-based principles in clinical dentistry. J. Multidiscip. Healthc. 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef]
- Armijo-Olivo, S. The importance of determining the clinical significance of research results in physical therapy clinical research. Braz. J. Phys. Ther. 2018, 22, 175–176. [Google Scholar] [CrossRef]
- National Center for Advancing Translational Sciences. Satranidazole. Available online: https://drugs.ncats.io/drug/4N7G8A6439 (accessed on 18 November 2022).
- MIMS. Satranidazole. Available online: https://www.mims.com/malaysia/drug/info/satranidazole?mtype=generic (accessed on 18 November 2022).
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Chereau, F.; Opatowski, L.; Tourdjman, M.; Vong, S. Risk assessment for antibiotic resistance in Southeast Asia. BMJ 2017, 358, j3393. [Google Scholar] [CrossRef]
- Grimshaw, J.M.; Eccles, M.P.; Lavis, J.N.; Hill, S.J.; Squires, J.E. Knowledge translation of research findings. Implement. Sci. 2012, 7, 50. [Google Scholar] [CrossRef]
- Naeemmudeen, N.M.; Mohd Ghazali, N.; Bahari, H.; Ibrahim, R.; Samsudin, A.D.; Jasni, A.S. Trends in antimicrobial resistance in Malaysia. Med. J. Malays. 2021, 76, 698–705. [Google Scholar]
- Prietto, N.R.; Martins, T.M.; Santinoni, C.; Pola, N.M.; Ervolino, E.; Bielemann, A.M.; Leite, F. Treatment of experimental periodontitis with chlorhexidine as adjuvant to scaling and root planing. Arch. Oral Biol. 2020, 110, 104600. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.C.; Feres, M.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.K.; Chambrone, L. Systemic antibiotics in the treatment of aggressive periodontitis. A systematic review and a Bayesian Network meta-analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 4, S1–S8. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kherul Anuwar, A.H.; Saub, R.; Safii, S.H.; Ab-Murat, N.; Mohd Taib, M.S.; Mamikutty, R.; Ng, C.W. Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis. Antibiotics 2022, 11, 1716. https://doi.org/10.3390/antibiotics11121716
Kherul Anuwar AH, Saub R, Safii SH, Ab-Murat N, Mohd Taib MS, Mamikutty R, Ng CW. Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis. Antibiotics. 2022; 11(12):1716. https://doi.org/10.3390/antibiotics11121716
Chicago/Turabian StyleKherul Anuwar, Ainol Haniza, Roslan Saub, Syarida Hasnur Safii, Norintan Ab-Murat, Mohd Syukri Mohd Taib, Rokiah Mamikutty, and Chiu Wan Ng. 2022. "Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis" Antibiotics 11, no. 12: 1716. https://doi.org/10.3390/antibiotics11121716
APA StyleKherul Anuwar, A. H., Saub, R., Safii, S. H., Ab-Murat, N., Mohd Taib, M. S., Mamikutty, R., & Ng, C. W. (2022). Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis. Antibiotics, 11(12), 1716. https://doi.org/10.3390/antibiotics11121716






