Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives
Abstract
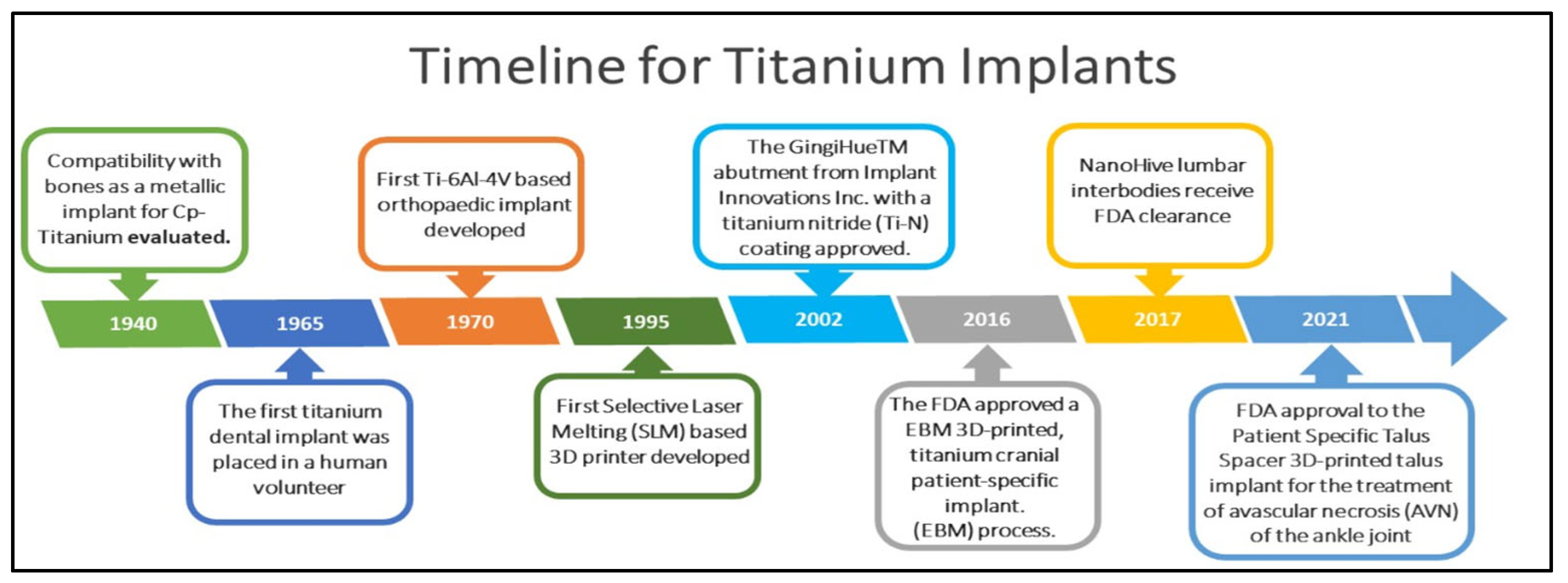
1. Introduction
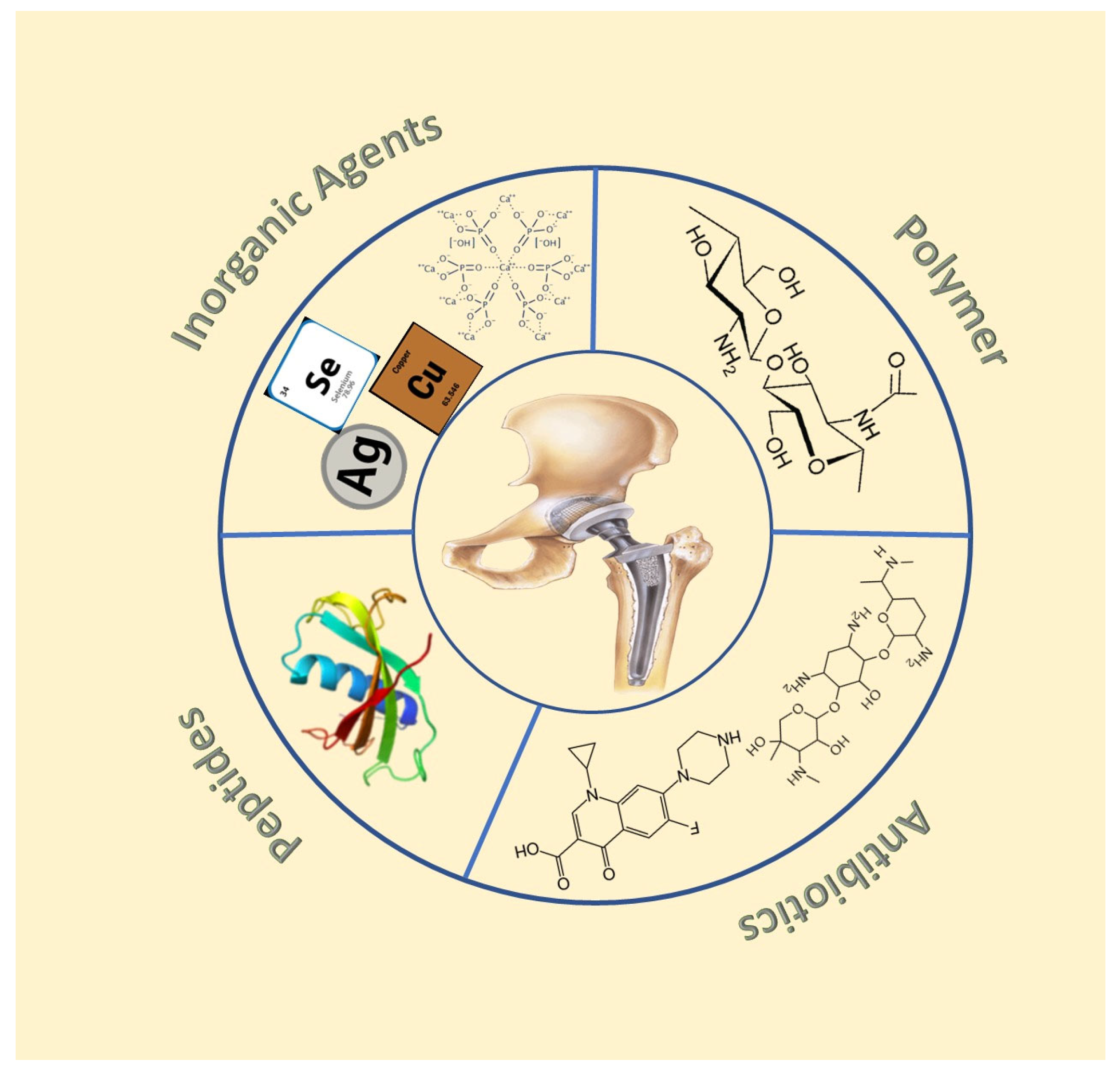
2. Antibacterial Coatings on Titanium Implants
2.1. Coatings with Inorganic Antibacterial Agents
2.1.1. Metal Doped Coatings
Silver
Copper, Zinc, and Selenium
Other Metals
2.1.2. Hydroxyapatite-Based Coatings
2.1.3. Iodine
2.1.4. Carbides and Nitrides
2.2. Coatings Loaded with Antibiotics
2.3. Polymer-Based Coatings
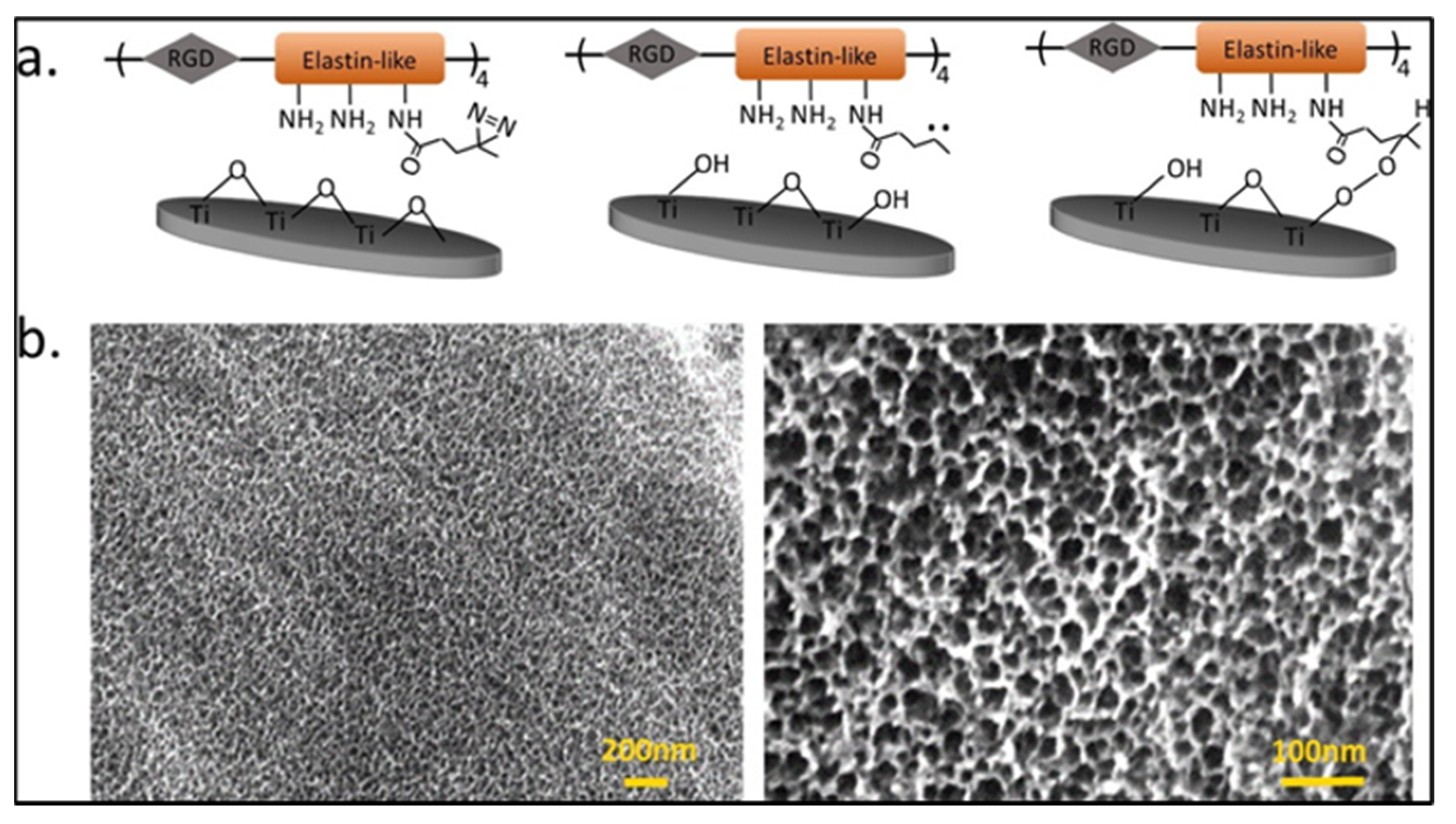
2.4. Antimicrobial Peptide-Based Coating
2.5. Multifunctional and Smart Coatings
3. Antimicrobial 3D Printed Titanium Implants
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Hodgins, D.; Bertsch, A.; Post, N.; Frischholz, M.; Volckaerts, B.; Spensley, J.; Wasikiewicz, J.; Higgins, H.; Von Stetten, F.; Kenney, L.; et al. Healthy Aims: Developing New Medical Implants and Diagnostic Equipment. IEEE Pervasive Comput. 2008, 7, 14–21. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.R.; Myles, P.S. The transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of Titanium dental implants. Dent. Mater. 2021, 37, 816–831. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef]
- Williams, D.F. Titanium for Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001; pp. 13–24. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.-M.; Lindahl, C.; Renvert, H.; Rutger Persson, G. Infection at Titanium implants with or without a clinical diagnosis of inflammation. Clin. Oral Implants Res. 2007, 18, 509–516. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation, and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar]
- Klein, E.Y.; Tseng, K.K.; Pant, S.; Laxminarayan, R. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob. Health 2019, 4, e001315. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm Formation on Dental Restorative and Implant Materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Kamath, S.; Bhattacharyya, D.; Padukudru, C.; Timmons, R.B.; Tang, L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. Part A 2008, 86, 617–626. [Google Scholar] [CrossRef]
- Prestat, M.; Thierry, D. Corrosion of Titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Egusa, H.; Ko, N.; Shimazu, T.; Yatani, H. Suspected association of an allergic reaction with Titanium dental implants: A clinical report. J. Prosthet. Dent. 2008, 100, 344–347. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Arciola, C.R.; Alvi, F.I.; An, Y.H.; Campoccia, D.; Montanaro, L. Implant Infection and Infection Resistant Materials: A Mini Review. Int. J. Artif. Organs 2005, 28, 1119–1125. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Cabral-Oliveira, G.G.; Oliveira, D.R.; Nogueira, B.A.; Pereira-Ribeiro, P.M.A.; Mattos-Guaraldi, A.L. Staphylococcus aureus biofilms: An opportunistic pathogen with multidrug resistance. Rev. Med. Microbiol. 2021, 32, 12–21. [Google Scholar] [CrossRef]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E.; et al. Biofilm formation on dental implant biomaterials by Staphylococcus aureus strains isolated from patients with cystic fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Cerioli, M.; Batailler, C.; Conrad, A.; Roux, S.; Perpoint, T.; Becker, A.; Triffault-Fillit, C.; Lustig, S.; Fessy, M.-H.; Laurent, F.; et al. Pseudomonas aeruginosa Implant-Associated Bone and Joint Infections: Experience in a Regional Reference Center in France. Front. Med. 2020, 7, 513242. [Google Scholar] [CrossRef]
- Meza-Siccha, A.S.; Aguilar-Luis, M.A.; Silva-Caso, W.; Mazulis, F.; Barragan-Salazar, C.; del Valle-Mendoza, J. In Vitro Evaluation of Bacterial Adhesion and Bacterial Viability of Streptococcus mutans, Streptococcus sanguinis, and Porphyromonas gingivalis on the Abutment Surface of Titanium and Zirconium Dental Implants. Int. J. Dent. 2019, 2019, 4292976. [Google Scholar] [CrossRef]
- Pretzen, G. Relevance, pathogenicity, and virulence of microorganisms in implant-related infections. Injury 1996, 27, SC9–SC15. [Google Scholar]
- Montanaro, L.; Speziale, P.; Campoccia, D.; Ravaioli, S.; Cangini, I.; Pietrocola, G.; Giannini, S.; Arciola, C.R. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011, 6, 1329–1349. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first four weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Luo, J.; Mamat, B.; Yue, Z.; Zhang, N.; Xu, X.; Li, Y.; Su, Z.; Ma, C.; Zang, F.; Wang, Y. Multi-metal ions doped hydroxyapatite coatings via electrochemical methods for antibacterial and osteogenesis. Colloid Interface Sci. Commun. 2021, 43, 100435. [Google Scholar] [CrossRef]
- Graziani, G.; Barbaro, K.; Fadeeva, I.V.; Ghezzi, D.; Fosca, M.; Sassoni, E.; Vadalà, G.; Cappelletti, M.; Valle, F.; Baldini, N.; et al. Ionized jet deposition of antimicrobial and stem cell friendly Silver-substituted tricalcium phosphate nanocoatings on Titanium alloy. Bioact. Mater. 2021, 6, 2629–2642. [Google Scholar] [CrossRef]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated Titanium. Bone Joint J. 2021, 103-B, 522–529. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Ai, J.; Bu, S.; Liu, H. Preparation of Coating on the Titanium Surface by Micro-Arc Oxidation to Improve Corrosion Resistance. Coatings 2021, 11, 230. [Google Scholar] [CrossRef]
- Xu, N.; Fu, J.; Zhao, L.; Chu, P.K.; Huo, K. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater. 2020, 9, 2000681. [Google Scholar] [CrossRef]
- Ständert, V.; Borcherding, K.; Bormann, N.; Schmidmaier, G.; Grunwald, I.; Wildemann, B. Antibiotic-loaded amphora-shaped pores on a Titanium implant surface enhance osteointegration and prevent infections. Bioact. Mater. 2021, 6, 2331–2345. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antibiotics Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhu, Y.; Yu, B.; Sun, Y.; Ding, X.; Xu, C.; Wu, Y.-W.; Tang, Z.; Xu, F.-J. Antimicrobial and Antifouling Polymeric Agents for Surface Functionalization of Medical Implants. Biomacromolecules 2018, 19, 2805–2811. [Google Scholar] [CrossRef]
- Saveleva, M.; Vladescu, A.; Cotrut, C.; Van der Meeren, L.; Surmeneva, M.; Surmenev, R.; Parakhonskiy, B.; Skirtach, A.G. The effect of hybrid coatings based on hydrogel, biopolymer, and inorganic components on the corrosion behavior of Titanium bone implants. J. Mater. Chem. B 2019, 7, 6778–6788. [Google Scholar] [CrossRef]
- Mouriñ, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Inkielewicz-Stepniak, I. Modified Nanoparticles as Potential Agents in Bone Diseases: Cancer and Implant-Related Complications. Nanomaterials 2020, 10, 658. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Iodine-supported Titanium implants have good antimicrobial attachment effects. J. Orthop. Sci. 2019, 24, 548–551. [Google Scholar] [CrossRef]
- Zhao, Z. Nanosurface modification of Ti64 implant by anodic fluorine-doped alumina/TiO2 for orthopedic application. Mater. Chem. Phys. 2022, 281, 125867. [Google Scholar] [CrossRef]
- Chen, C.-S.; Chang, J.-H.; Srimaneepong, V.; Wen, J.-Y.; Tung, O.-H.; Yang, C.-H.; Lin, H.-C.; Lee, T.-H.; Han, Y.; Huang, H.-H. Improving the in vitro cell differentiation and in vivo osseointegration of Titanium dental implant through oxygen plasma immersion ion implantation treatment. Surf. Coat. Technol. 2020, 399, 126125. [Google Scholar] [CrossRef]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Low voltage environmentally friendly plasma electrolytic oxidation process for Titanium alloys. Sci. Rep. 2022, 12, 6037. [Google Scholar] [CrossRef]
- Batebi, K.; Abbasi Khazaei, B.; Afshar, A. Characterization of sol-gel derived Silver/fluor-hydroxyapatite composite coatings on Titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef]
- Kalaivani, S.; Singh, R.K.; Ganesan, V.; Kannan, S. Effect of Copper(Cu2+) inclusion on the bioactivity and antibacterial behavior of calcium silicate coatings on Titanium metal. J. Mater. Chem. B 2014, 2, 846–858. [Google Scholar] [CrossRef]
- Panda, S.; Behera, B.P.; Bhutia, S.K.; Biswas, C.K.; Paul, S. Rare transition metal doped hydroxyapatite coating prepared via microwave irradiation improved corrosion resistance, biocompatibility and anti-biofilm property of Titanium alloy. J. Alloys Compd. 2022, 918, 165662. [Google Scholar] [CrossRef]
- Dhayal, M.; Kapoor, R.; Sistla, P.G.; Pandey, R.R.; Kar, S.; Saini, K.K.; Pande, G. Strategies to prepare Titanium thin films, doped with transition metal ions, that exhibit specific physicochemical properties to support osteoblast cell adhesion and proliferation. Mater. Sci. Eng. C 2014, 37, 99–107. [Google Scholar] [CrossRef]
- Pihl, M.; Galli, S.; Jimbo, R.; Andersson, M. Osseointegration and antibacterial effect of an antimicrobial peptide releasing mesoporous TiO2 implant. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1787–1795. [Google Scholar] [CrossRef]
- Gunputh, U.F.; Le, H.; Handy, R.D.; Tredwin, C. Anodised Titanium nanotubes as a scaffold for antibacterial Silver nanoparticles on Titanium implants. Mater. Sci. Eng. C 2018, 91, 638–644. [Google Scholar] [CrossRef]
- Avcu, E.; Yıldıran Avcu, Y.; Baştan, F.E.; Rehman, M.A.U.; Üstel, F.; Boccaccini, A.R. Tailoring the surface characteristics of electrophoretically deposited chitosan-based bioactive glass composite coatings on Titanium implants via grit blasting. Prog. Org. Coat. 2018, 123, 362–373. [Google Scholar] [CrossRef]
- Stevanović, M.; Đošić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Sakač, M.C.; Rhee, K.Y.; Mišković-Stanković, V. Gentamicin-Loaded Bioactive Hydroxyapatite/Chitosan Composite Coating Electrodeposited on Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, M.; Gao, W.; Yang, P.; Meng, F.; Liu, Q.; Chang, H. Functional Silver nanoparticles as broad-spectrum antimicrobial agents. New J. Chem. 2022, 46, 16387–16393. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of Silver nanoparticles: The emerging trends and prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of Silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of Silver nanoparticles—Nanoparticle or Silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of Silver ions and Silver nanoparticles on bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981. [Google Scholar] [CrossRef]
- Thukkaram, M.; Cools, P.; Nikiforov, A.; Rigole, P.; Coenye, T.; Van Der Voort, P.; Du Laing, G.; Vercruysse, C.; Declercq, H.; Morent, R.; et al. Antibacterial activity of a porous Silver doped Titanium coating on Titanium substrates synthesized by plasma electrolytic oxidation. Appl. Surf. Sci. 2020, 500, 144235. [Google Scholar] [CrossRef]
- Oleshko, O.; Liubchak, I.; Husak, Y.; Korniienko, V.; Yusupova, A.; Oleshko, T.; Banasiuk, R.; Szkodo, M.; Matros-Taranets, I.; Kazek-Kęsik, A.; et al. In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials 2020, 13, 4359. [Google Scholar] [CrossRef]
- Wang, L.-J.; Ni, X.-H.; Zhang, F.; Peng, Z.; Yu, F.-X.; Zhang, L.-B.; Li, B.; Jiao, Y.; Li, Y.-K.; Yang, B.; et al. Osteoblast Response to Copper-Doped Microporous Coatings on Titanium for Improved Bone Integration. Nanoscale Res. Lett. 2021, 16, 146. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Shao, S.-Y.; Xu, L.-N.; Chen, W.-Q.; Yu, X.-Y.; Tang, K.-M.; Tang, Z.-H.; Zhang, F.; Qiu, J. Enhanced corrosion resistance of zinc-containing nanowires-modified Titanium surface under exposure to oxidizing microenvironment. J. Nanobiotechnol. 2019, 17, 55. [Google Scholar] [CrossRef]
- Gao, C.; Li, C.; Wang, C.; Qin, Y.; Wang, Z.; Yang, F.; Liu, H.; Chang, F.; Wang, J. Advances in the induction of osteogenesis by Zinc surface modification based on Titanium alloy substrates for medical implants. J. Alloys Compd. 2017, 726, 1072–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Xiao, D.; Zhao, Q.; Chen, S.; Yang, F.; Liu, J.; Duan, K. Cu2+ Release from Polylactic Acid Coating on Titanium Reduces Bone Implant-Related Infection. J. Funct. Biomater. 2022, 13, 78. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X. The osteogenic, anti-oncogenic and antibacterial activities of Selenium-doped Titanium dioxide coatings on Titanium. Surf. Coat. Technol. 2020, 403, 126408. [Google Scholar] [CrossRef]
- Wolf-Brandstetter, C.; Beutner, R.; Hess, R.; Bierbaum, S.; Wagner, K.; Scharnweber, D.; Gbureck, U.; Moseke, C. Multifunctional calcium phosphate-based coatings on Titanium implants with integrated trace elements. Biomed. Mater. 2020, 15, 025006. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lee, Y.-T.; Huang, T.-C.; Lin, G.-S.; Chen, Y.-W.; Lee, B.-S.; Tung, K.-L. In Vitro Bioactivity and Antibacterial Activity of Strontium-, Magnesium-, and Zinc-Multidoped Hydroxyapatite Porous Coatings Applied via Atmospheric Plasma Spraying. ACS Appl. Bio Mater. 2021, 4, 2523–2533. [Google Scholar] [CrossRef]
- Dong, J.; Fang, D.; Zhang, L.; Shan, Q.; Huang, Y. Gallium-doped TiO2 nanotubes elicit anti-bacterial efficacy in vivo against Escherichia coli and Staphylococcus aureus biofilm. Materialia 2019, 5, 100209. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Bismuth-doped nanohydroxyapatite coatings on Titanium implants for improved radiopacity and antimicrobial activity. Nanomaterials 2019, 9, 1696. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, B.; Chang, X.; Yang, H.; Lan, J.; Wang, S.; Qiao, H.; Lin, H.; Han, S. A functionalized Sm/Sr doped Titanium nanotube array on Titanium implant enables exceptional bone-implant integration and also self-antibacterial activity. Ceram. Int. 2020, 46, 14796–14807. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Ramya, S.; Shinyjoy, E.; Kavitha, L.; Gopi, D.; Vijayalakshmi, U. Structural, morphological and biological evaluations of cerium incorporated hydroxyapatite sol–gel coatings on Ti–6Al–4V for orthopaedic applications. J. Mater. Res. Technol. 2021, 12, 1319–1338. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Shi, J.; Yao, X.; Chen, W.; Wei, X.; Zhang, X.; Chu, P.K. Near-infrared light II-assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials 2021, 269, 120634. [Google Scholar] [CrossRef]
- Katunar, M.R.; Pastore, J.I.; Cisilino, A.; Merlo, J.; Alonso, L.S.; Baca, M.; Haddad, K.; Cere, S.; Ballarre, J. Early osseointegration of Strontium-doped coatings on Titanium implants in an osteoporotic rat model. Surf. Coat. Technol. 2022, 433, 128159. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, L.; Jiang, L.; Ma, Y.; Lin, H.; Dong, J. Surface functionalization of Titanium with zinc/Strontium-doped Titanium dioxide microporous coating via microarc oxidation. Nanomedicine 2019, 16, 149–161. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N. Ivanković; H Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Arcos, D. Vallet-Regí; M Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Ohtsu, N.; Kakuchi, Y.; Ohtsuki, T. Antibacterial effect of Zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition. Appl. Surf. Sci. 2018, 445, 596–600. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on Titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Terauchi, R.; Tsuchida, S.; Mizoshiri, N.; Mori, Y.; Takeuchi, A.; Hayashi, K.; Yamamoto, N.; Ikoma, K.; et al. A retrospective study of antibacterial Iodine-coated implants for postoperative infection. Medicine 2019, 98, e17932. [Google Scholar] [CrossRef]
- Altaf, I.; Nadeem, M.F.; Hussain, N.; Nawaz, M.; Raza, S.; Shabbir, M.A.B.; Ashraf, M.A.; Ali, M.A.; Hassan, S.; Aziz, M.W.; et al. An in vitro antiviral activity of Iodine complexes against SARS-CoV-2. Arch. Microbiol. 2021, 203, 4743–4749. [Google Scholar] [CrossRef]
- Efimov, N.N.; Loginov, D.A.; Sharipov MYu Nazarov, A.A.; Nelyubina, Y.V.; Perekalin, D.S. Unexpected antifungal activity of half-sandwich complexes with metal−Iodine bonds. J. Organomet. Chem. 2020, 916, 121272. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Pawar, V.; Topkar, H.; Srivastava, R. Chitosan nanoparticles and povidone Iodine containing alginate gel for prevention and treatment of orthopedic implant associated infections. Int. J. Biol. Macromol. 2018, 115, 1131–1141. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Le, P.T.M.; Shintani, S.A.; Takadama, H.; Ito, M.; Ferraris, S.; Spriano, S. Iodine-loaded calcium titanate for bone repair with sustainable antibacterial activity prepared by solution and heat treatment. Nanomaterials 2021, 11, 2199. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Fini, M.; Longo, G.; Ioannidu, C.A.; Scotto D’Abusco, A.; Superti, F.; Panzini, G.; Misiano, C.; Palattella, A.; et al. Osseointegration is improved by coating Titanium implants with a nanostructured thin film with Titanium carbide and Titanium oxides clustered around graphitic carbon. Mater. Sci. Eng. C 2017, 70, 264–271. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Roy, T.; Iv, P.H.C.; Fares, C.; Ren, F.; Clark, A.E.; Esquivel-Upshaw, J.F. Novel coatings to minimize bacterial adhesion and promote osteoblast activity for Titanium implants. J. Funct. Biomater. 2020, 11, 42. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on Titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 470–480. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2017, 36, 22–32. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implants Res 2014, 25, 82–90. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, P.; Vishwamitra, B.; Singh, G. Review on Surface Treatment for Implant Infection via Gentamicin and Antibiotic Releasing Coatings. Coatings 2021, 11, 1006. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Schrader, C.; Gras, F.; Mückley, T.; Schmidt, J.; Zankovych, S.; Bossert, J.; Jandt, K.; Völpel, A.; Sigusch, B.; et al. Gentamicin coating of plasma chemical oxidized Titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 2016, 101, 156–164. [Google Scholar] [CrossRef]
- Xi, W.; Hegde, V.; Zoller, S.D.; Park, H.Y.; Hart, C.M.; Kondo, T.; Hamad, C.D.; Hu, Y.; Loftin, A.H.; Johansen, D.O.; et al. Point-of-care antimicrobial coating protects orthopaedic implants from bacterial challenge. Nat. Commun. 2021, 12, 5473. [Google Scholar] [CrossRef]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z.; et al. Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 2021, 263, 124432. [Google Scholar] [CrossRef]
- Humayun, A.; Luo, Y.; Mills, D.K. Electrophoretic deposition of gentamicin-loaded znhnts-chitosan on Titanium. Coatings 2020, 10, 944. [Google Scholar] [CrossRef]
- He, L.-J.; Hao, J.-C.; Dai, L.; Zeng, R.-C.; Li, S.-Q. Layer-by-layer assembly of gentamicin-based antibacterial multilayers on Ti alloy. Mater. Lett. 2020, 261, 127001. [Google Scholar] [CrossRef]
- Chen, S.-T.; Chien, H.-W.; Cheng, C.-Y.; Huang, H.-M.; Song, T.-Y.; Chen, Y.-C.; Wu, C.-H.; Hsueh, Y.-H.; Wang, Y.-H.; Ou, S.-F. Drug-release dynamics and antibacterial activities of chitosan/cefazolin coatings on Ti implants. Prog. Org. Coat. 2021, 159, 106385. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Lyu, C.; Hu, Y.; Zou, D.; He, Y.-S.; Lu, J. Synergistic antibacterial effect of graphene-coated Titanium loaded with levofloxacin. Colloids Surf. B Biointerfaces 2021, 208, 112090. [Google Scholar] [CrossRef]
- Ozdil, D.; Aydin, H.M. Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Doymus, B.; Kerem, G.; Yazgan Karatas, A.; Kok, F.N.; Önder, S. A functional coating to enhance antibacterial and bioactivity properties of Titanium implants and its performance in vitro. J. Biomater. Appl. 2021, 35, 655–669. [Google Scholar] [CrossRef]
- Guo, C.; Cui, W.; Wang, X.; Lu, X.; Zhang, L.; Li, X.; Li, W.; Zhang, W.; Chen, J. Poly-l-lysine/Sodium Alginate Coating Loading NanoSilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 5, 10562–10571. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable Titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef]
- Kaleli-Can, G.; Özgüzar, H.F.; Kahriman, S.; Türkal, M.; Göçmen, J.S.; Yurtçu, E.; Mutlu, M. Improvement in antimicrobial properties of Titanium by diethyl phosphite plasma-based surface modification. Mater. Today Commun. 2020, 25, 101565. [Google Scholar] [CrossRef]
- Peng, J.; Liu, P.; Peng, W.; Sun, J.; Dong, X.; Ma, Z.; Gan, D.; Liu, P.; Shen, J. Poly(hexamethylene biguanide) (PHMB) as high-efficiency antibacterial coating for Titanium substrates. J. Hazard. Mater. 2021, 411, 125110. [Google Scholar] [CrossRef]
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards Robust Delivery of Antimicrobial Peptides to Combat Bacterial Resistance. Molecules 2020, 25, 3048. [Google Scholar] [CrossRef]
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Y.; Gopalakrishnan, S.; Li, K.; Han, Y.; Rotello, V.M. Antimicrobial Peptide-Loaded Pectolite Nanorods for Enhancing Wound-Healing and Biocidal Activity of Titanium. ACS Appl. Mater. Interfaces 2021, 13, 28764–28773. [Google Scholar] [CrossRef]
- Ghilini, F.; Fagali, N.; Pissinis, D.E.; Benítez, G.; Schilardi, P.L. Multifunctional Titanium Surfaces for Orthopedic Implants: Antimicrobial Activity and Enhanced Osseointegration. ACS Appl. Bio Mater. 2021, 4, 6451–6461. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Z.; Yang, Y.; Shi, J.; Lv, J.; Fang, Y.; Shen, Z.; Lv, Z.; Li, P.; Yao, X.; et al. A multifunctional antibacterial coating on bone implants for osteosarcoma therapy and enhanced osteointegration. Chem. Eng. J. 2022, 428, 131155. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhu, Y.; Chen, Y.; Gao, M.; Gao, H.; Liu, L.; Wang, L.; Mao, C.; Wang, Y. On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Theranostics 2020, 10, 109–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Xing, X.; Zhang, J.; Zhang, M.-R.; Ma, X.; Shi, R.; Zhang, L. Smart Titanium Coating Composed of Antibiotic Conjugated Peptides as an Infection-Responsive Antibacterial Agent. Macromol. Biosci. 2021, 21, 2000194. [Google Scholar] [CrossRef]
- Lin, J.; Hu, J.; Wang, W.; Liu, K.; Zhou, C.; Liu, Z.; Kong, S.; Lin, S.; Deng, Y.; Guo, Z. Thermo and light-responsive strategies of smart Titanium-containing composite material surface for enhancing bacterially anti-adhesive property. Chem. Eng. J. 2021, 407, 125783. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Q.; Wei, Y.; Meng, W.; Wang, R.; Liu, J.; Nie, Y.; Luo, R.; Wang, Y.; Shen, B. Surface modification of Titanium implants by pH-Responsive coating designed for Self-Adaptive antibacterial and promoted osseointegration. Chem. Eng. J. 2022, 435, 134802. [Google Scholar] [CrossRef]
- Pavlović, M.R.P.; Stanojević, B.P.; Pavlović, M.M.; Mihailović, M.D.; Stevanović, J.S.; Panić, V.V.; Ignjatović, N.L. Anodizing/anaphoretic electrodeposition of nano-calcium phosphate/chitosan lactate multifunctional coatings on Titanium with advanced corrosion resistance, bioactivity, and antibacterial properties. ACS Biomater. Sci. Eng. 2021, 7, 3088–3102. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef]
- Jakubowski, M.; Voelkel, A.; Sandomierski, M. Crystalline Zeolite Layers on the Surface of Titanium Alloys in Biomedical Applications: Current Knowledge and Possible Directions of Development. Crystals 2022, 12, 1520. [Google Scholar] [CrossRef]
- Maher, S.; Linklater, D.; Rastin, H.; Liao, S.T.-Y.; Martins de Sousa, K.; Lima-Marques, L.; Kingshott, P.; Thissen, H.; Ivanova, E.P.; Losic, D. Advancing of 3D-Printed Titanium Implants with Combined Antibacterial Protection Using Ultrasharp Nanostructured Surface and Gallium-Releasing Agents. ACS Biomater. Sci. Eng. 2022, 8, 314–327. [Google Scholar] [CrossRef]
- Bassous, N.J.; Jones, C.L.; Webster, T.J. 3-D printed Ti-6Al-4V scaffolds for supporting osteoblast and restricting bacterial functions without using drugs: Predictive equations and experiments. Acta Biomater. 2019, 96, 662–673. [Google Scholar] [CrossRef]
- Nano Hive Anterior Lumbar Implants. Available online: https://nanohive.com/interbody-fusion-cages/#alif (accessed on 1 November 2022).
- Benmassaoud, M.M.; Kohama, C.; Kim, T.W.B.; Kadlowec, J.A.; Foltiny, B.; Mercurio, T.; Ranganathan, S.I. Efficacy of eluted antibiotics through 3D printed femoral implants. Biomed. Microdevices 2019, 21, 51. [Google Scholar] [CrossRef]
- Griseti, Q.; Jacquet, C.; Sautet, P.; Abdel, M.P.; Parratte, S.; Ollivier, M.; Argenson, J.-N. Antimicrobial properties of antibiotic-loaded implants. Bone Joint J. 2020, 102-B, 158–162. [Google Scholar]
- Zetao, C.; Travis, K.; Rachael, M.; Ross, C.; Jiang, C.; Chengtie, W.; Yin, X. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Hu, J.; Ding, Y.; Tao, B.; Yuan, Z.; Yang, Y.; Xu, K.; Li, X.; Liu, P.; Cai, K. Surface modification of Titanium substrate via combining photothermal therapy and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection. Bioact. Mater. 2022, 18, 228–241. [Google Scholar] [CrossRef]



| Coating Type | Composition | Major Technique | Antibacterial Efficiency (In Vitro Studies) | Reference |
|---|---|---|---|---|
| Inorganic ions/elements | Ag-doped TiO2 | Plasma Electrolytic Oxidation (PEO) | Significant reduction (p < 0.05) in cell numbers of Escherichia coli and Staphylococcus aureus and metabolic activity. | [55] |
| High silver content samples showed a 6-log reduction of Staphylococcus aureus | ||||
| Ag nanoparticles (Ag NP)-loaded calcium–phosphate solution | Plasma Electrolytic Oxidation | Antibacterial action aginst Staphylococcus aureus (strain B 918) | [56] | |
| Zn doped HAP | Solution Precursor Plasma Spray (SPPS) process | Antibacterial action aginst Escherichia coli and Staphylococcus aureus | [58] | |
| Selenium incorporated onto microporous titanium dioxide coatings with Ca and P on titanium substrates | Micro-arc oxidation (MAO) | A minimum of 94% killing activity after 28 days was observed for Escherichia coli and Staphylococcus aureus in in vitro studies | [62] | |
| Zn/Sr-doped titanium dioxide | Micro-arc oxidation | Fewer surviving colonies of Staphylococcus aureus was observed in spread plate analysis | [71] | |
| Bismuth doped nanohydroxyapatite | Alkali-thermal treatment | Large zone of inhibiton was observed for Escherichia coli and Staphylococcus aureus | [66] | |
| Samarium and Strontium on TiO2 nanotubes | Anodization | Nearly 100% antibacterial activity was observed for Escherichia coli and Staphylococcus aureus in plate counting experiments | [67] | |
| Zone of inhibition was larger for Staphylococcus aureus than Escherichia coli. | ||||
| TiO2 nanotubes doped with gallium | Anodization and solvent casting | Low concentration of live Staphylococcus aureus and Escherichia coli was observed in Live/Dead cell assay | [65] | |
| Ag–HAP-fluoride | Sol-gel | 96% reduction in Escherichia coli was observed after 6 h in spread plate results for Ag—HAP-fluoride coating which had 0.3% w/v of Ag and P/F ratio of 6 | [40] | |
| ZnO-HAP | Spin coating | Drastic reduction in the colonies of Escherichia coli and Staphylococcus aureus was observed after 4 h of incubation | [76] | |
| Niobium doped hydroxyapatite | Microwave irradiation | Large zone of inhibition was observed for Escherichia coli and Bacillus subtilis | [43] | |
| Cerium incorporated collagen-HAP | Immersion of the titanium substrate in supersaturated calcification solution (Ce-SCS) | After 24 h incubation 92.61% Escherichia coli and 73.59% Staphylococcus aureus were eradicated | [77] | |
| Calcium Titanate-Iodine coating | Solution and heat treatment method that controllably incorporates 0.7% to 10.5% of Iodine into Titanium | About 99% of bactericidal activity was observed for Methicillin-resistant Staphylococcus aureus, Staphylococcus aureus, Escherichia coli, and Staphylococcus epidermidis | [84] | |
| TiN and SiC coating | Magnetron sputtering, Plasma-enhanced chemical vapor deposition system (PECVD) | Reduction in number of live Porphyromonas gingivalis was observed after 4 h of incubation. | [86] | |
| Antibiotic based | Gentamicin loaded zinc–incorporated Halloysites (ZnHNTs)–Chitosan | Electrodeposition (EPD) | Inhibition zones of 3.11 ± 0.79 cm2/unit area of the sample was observed in disc diffusion assay for Staphylococcus aureus | [96] |
| Gentamicin and polyacrylic acid (PAA) | Layer-by-layer assembly | About 99% of bactericidal activity was observed for Staphylococcus aureus and Escherichia coli | [97] | |
| Chitosan/cefazolin | Electrophoretic deposition | Nearly 100% bactericidal activity against Escherichia coli was shown by the coating which has the highest drug concentration | [98] | |
| Levofloxacin loaded graphene coating | Sandblasting, large-grit, acid-etching and salinization | Large bacteriostatic circle diameters were observed for Staphylococcus aureus and Escherichia coli | [99] | |
| Chitosan, coated with a thin layer of melittin and loaded with the antibiotics vancomycin and Oxacillin | Spin coating and casting method | The coatings were able to eradicate Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin Resistant Staphylococcus aureus (VRSA) at the early stages | [95] | |
| poly-L-lysine (PLL)/sodium alginate (SA)/Silver nanoparticles | Electrostatic self-assembly, dip coating | A distinct ring was observed in zone of inhibition test for Staphylococcus aureus and Staphylococcus mutans | [102] | |
| N-halamine based porous coating (Ti-PAA-NCl) | Alkali-heat treatment, surface grafting and N-Cl functionalization | Bactericidal rate of 96% for Staphylococcus aureus and 91% Porphyromonas gingivalis was observed in contact killing assay | [103] | |
| Diethyl phosphite (DEP) coated Titanium (pp (DEP)-Ti) | Plasma polymerization | The number of Staphylococcus aureus and Candida albicans colonies decreased after 24 h | [104] | |
| Phosphonate/active ester block copolymers (pDEMMP-b-pNHSMA) and PHMB | Reversible addition-fragmentation chain transfer (RAFT) polymerization. | Nearly 100% antibacterial activity was observed for Staphylococcus aureus and Escherichia coli | [105] | |
| Antimicrobial Peptide based | A recombinant elastin like peptide coating with cell- adhesive RGD sequences with a covalently attached AMP, RRP9W4 | Covalent immobilization of AMPs to titanium surface | The number dead Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa cells increased after 48 h | [107] |
| RRP9W4N incorporated into mesoporous TiO2. | Spin-coating | Bactericidal action against Staphylococcus epidermidis | [45] | |
| Pectolite nanorod (NCS) with AMP-loaded collagen shell | Microarc oxidation (MAO), Spin coating | Contact killing efficiency was almost 100% for Staphylococcus aureus | [108] | |
| Multifunctional Coatings | poly (quaternary ammonium salts-co-methacrylic acid) (PQA) | Anodic Oxidation and Spray coating | Efficient antibacterial action was observed against Escherichia coli and Staphylococcus aureus | [114] |
| P (vinylcaprolactam (VCL)–co-polyethylene glycol methacrylate (PEGMA)–co-alkyl-dimethyl tertiary amine (QAS)–co-vinyltrimethoxysilane (VTMO)) copolymer/ TiO2 nanotube | Layer-by-layer (LbL) self-assembly method | Antibacterial action was observed at lower pH for Staphylococcus aureus and Escherichia coli | [115] | |
| Nano amorphous calcium phosphate (ACP) and titanium dioxide with chitosan oligosaccharide lactate (ChOL) | Anodization and anaphoretic electrodeposition | Three-to-four-fold reduction in the number of Staphylococcus aureus and Pseudomonas aeruginosa colonies was observed after 420 min | [116] | |
| poly (methacrylic acid) (PMAA) loaded onto TiO2 nanotubes (Ti-NTs) with HHC36 peptides, with a sequence of KRWWKWWRR | Anodization | 99% of bactericidal activity was observed for Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus | [112] | |
| Yb and Er doped Ti nano -shovel/quercetin/L-arginine (TiO2@UCN/Qr/LA) | Phototherapy | Above 90% bactericidal action against Staphylococcus aureus | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. https://doi.org/10.3390/antibiotics11121719
Akshaya S, Rowlo PK, Dukle A, Nathanael AJ. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics. 2022; 11(12):1719. https://doi.org/10.3390/antibiotics11121719
Chicago/Turabian StyleAkshaya, S., Praveen Kumar Rowlo, Amey Dukle, and A. Joseph Nathanael. 2022. "Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives" Antibiotics 11, no. 12: 1719. https://doi.org/10.3390/antibiotics11121719
APA StyleAkshaya, S., Rowlo, P. K., Dukle, A., & Nathanael, A. J. (2022). Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics, 11(12), 1719. https://doi.org/10.3390/antibiotics11121719







