Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Populations
2.2. Ethics Approval, Data Collection and Outcomes
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient and Infection Characteristics
3.2. Treatment Outcomes
3.3. Factors Associated with All-Cause 30-Day Mortality and 30-Day Survival Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, W.R.; Arias, C.A.; Murray, B.E. Enterococcal Infections. In Harrison’s Principles of Internal Medicine 21e; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- National Antimicrobial Resistance Surveillance Center Thailand. Available online: http://narst.dmsc.moph.go.th/ (accessed on 23 May 2022).
- McBride, S.J.; Upton, A.; Roberts, S.A. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia—A five-year retrospective review. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Granado, F.; Becerril, B.; Cuberos, L.; Bernabeu, M.; Cisneros, J.; Pachon, J.J.C.i.d. Attributable mortality rate and duration of hospital stay associated with enterococcal bacteremia. Clin. Infect. Dis. 2001, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Suppli, M.; Aabenhus, R.; Harboe, Z.B.; Andersen, L.P.; Tvede, M.; Jensen, J.U. Mortality in enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clin. Microbiol. Infect. 2011, 17, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Phang, S.H.; Gregson, D.B.; Pitout, J.D.D.; Ross, T.; Church, D.L.; Laupland, K.B.; Parkins, M.D. Incidence, Risk Factors, and Outcomes for Enterococcus spp. Blood Stream Infections: A Population-Based Study. Int. J. Infect. Dis. 2014, 26, 76–82. [Google Scholar] [CrossRef]
- Calik, S.; Ari, A.; Okur, O.; Tosun, S.; Yis, R. Nosocomial enterococcal bacteremia: Predictors of resistance and mortality. EJMI 2019, 3, 119–126. [Google Scholar] [CrossRef]
- Gray, J.; Marsh, P.J.; Stewart, D.; Pedler, S.J. Enterococcal bacteraemia: A prospective study of 125 episodes. J. Hosp. Infect. 1994, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Chater, M.; Maley, M.; van Hal, S.J. Glycopeptide use is associated with increased mortality in Enterococcus faecalis bacteraemia. J. Antimicrob. Chemother. 2014, 69, 2252–2257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumura, T.; Nagao, M.; Nakano, S.; Yamamoto, M.; Matsumura, Y.; Ichiyama, S. Enterococcal bacteraemia: Predictive and prognostic risk factors for ampicillin resistance. Epidemiol. Infect. 2018, 146, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Shie, S.S.; Cheng, C.W.; Yang, J.H.; Huang, P.Y.; Wu, T.S.; Lee, M.H.; Huang, C.T. Clinical characteristics and treatment outcomes of vancomycin-resistant Enterococcus faecium bacteremia. J. Microbiol. Immunol. Infect. 2018, 51, 705–716. [Google Scholar] [CrossRef]
- Hemapanpairoa, J.; Changpradub, D.; Thunyaharn, S.; Santimaleeworagun, W. Does Vancomycin Resistance Increase Mortality? Clinical Outcomes and Predictive Factors for Mortality in Patients with Enterococcus faecium Infections. Antibiotics 2021, 10, 105. [Google Scholar] [CrossRef]
- Hamada, Y.; Magarifuchi, H.; Oho, M.; Kusaba, K.; Nagasawa, Z.; Fukuoka, M.; Yamakuchi, H.; Urakami, T.; Aoki, Y. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: An eight-year retrospective comparison study. J. Infect. Chemother. 2015, 21, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Sandoe, J.A.T.; Witherden, I.R.; Au-Yeung, H.-K.C.; Kite, P.; Kerr, K.G.; Wilcox, M.H. Enterococcal intravascular catheter-related bloodstream infection: Management and outcome of 61 consecutive cases. J. Antimicrob. Chemother. 2002, 50, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bartoletti, M.; Gatti, M.; Viale, P. Advances in the therapy of bacterial bloodstream infections. Clin. Microbiol. Infect. 2020, 26, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Powers, C.E.; Bookstaver, P.B.; Caulder, C.; Bouknight, A.; Justo, J.A.; Kohn, J.; Winders, H.R.; Al-Hasan, M.N. Evaluation of early clinical failure criteria in Enterococcus species bloodstream infection. Infection 2022. [Google Scholar] [CrossRef]
- Clinical Practice Guideline for Acute Kidney Injury. 2012. Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 23 May 2022).
- Sriskandan, S.; Cohen, J. GRAM-POSITIVE SEPSIS: Mechanisms and Differences from Gram-Negative Sepsis. Infect. Dis. Clin. N. Am. 1999, 13, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Kram, S.; Sarubbi, C.; Anderson, D.; Kram, B. Effectiveness of Vancomycin or Beta-Lactam Therapy in Ampicillin-Susceptible Enterococcus spp. Bloodstream Infections. J. Pharm. Pract. 2018, 32, 089719001775120. [Google Scholar] [CrossRef]
- Katip, W.; Oberdorfer, P. A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics 2021, 13, 1378. [Google Scholar] [CrossRef] [PubMed]
- Jumah, M.T.B.; Vasoo, S.; Menon, S.R.; De, P.P.; Neely, M.; Teng, C.B. Pharmacokinetic/Pharmacodynamic Determinants of Vancomycin Efficacy in Enterococcal Bacteremia. Antimicrob. Agents Chemother. 2018, 62, e01602-17. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, I.; Sakakura, K.; Imanishi, K.; Sako, R.; Yamazaki, K. Association between vancomycin pharmacokinetic/pharmacodynamic parameters, patient characteristics, and mortality in patients with bacteremia caused by vancomycin-susceptible Enterococcus faecium: A single-center retrospective study. J. Pharm. Health Care Sci. 2019, 5, 8. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Thirty-Second Information Supplement. CLSI Document M100; CLSI: Wayne, PA, USA, 2022. [Google Scholar]
- Kim, S.H.; Kim, K.H.; Kim, H.B.; Kim, N.J.; Kim, E.C.; Oh, M.D.; Choe, K.W. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2008, 52, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Apellaniz, G.; Valdés, M.; Perez, R.; Martin, F.; Soria, F.; Garcia, A.; Gòmez, J.; Vicente, T. Comparison of the Effectiveness of Various Antibiotics in the Treatment of Methicillin-Susceptible Staphylococcus aureus Experimental Infective Endocarditis. J. Chemother. 1991, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fernández Guerrero, M.L.; de Górgolas, M. Comparative activity of cloxacillin and vancomycin against methicillin-susceptible Staphylococcus aureus experimental endocarditis. J. Antimicrob. Chemother. 2006, 58, 1066–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Houlihan, H.H.; Stokes, D.P.; Rybak, M.J. Pharmacodynamics of vancomycin and ampicillin alone and in combination with gentamicin once daily or thrice daily against Enterococcus faecalis in an in vitro infection model. J. Antimicrob. Chemother. 2000, 46, 79–86. [Google Scholar] [CrossRef][Green Version]
- Hoellman, D.B.; Visalli, M.A.; Jacobs, M.R.; Appelbaum, P.C. Activities and time-kill studies of selected penicillins, beta-lactamase inhibitor combinations, and glycopeptides against Enterococcus faecalis. Antimicrob. Agents Chemother. 1998, 42, 857–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Graninger, W.; Ragette, R. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin. Infect. Dis. 1992, 15, 49–57. [Google Scholar] [CrossRef]
- Bartoletti, M.; Tedeschi, S.; Scudeller, L.; Pascale, R.; Rosselli del Turco, E.; Trapani, F.; Tumietto, F.; Virgili, G.; Marconi, L.; Ianniruberto, S.; et al. Impact on Mortality of a Bundle for the Management of Enterococcal Bloodstream Infection. Open Forum Infect. Dis. 2019, 6, ofz473. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Arensman, K.; Shields, M.; Beganovic, M.; Miller, J.L.; LaChance, E.; Anderson, M.; Dela-Pena, J. Fluoroquinolone versus Beta-Lactam Oral Step-Down Therapy for Uncomplicated Streptococcal Bloodstream Infections. Antimicrob. Agents Chemother. 2020, 64, e01515-20. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.M.; James, E. Switch over from intravenous to oral therapy: A concise overview. J. Pharmacol. Pharmacother. 2014, 5, 83–87. [Google Scholar] [CrossRef]
- Sakka, V.; Tsiodras, S.; Galani, L.; Antoniadou, A.; Souli, M.; Galani, I.; Pantelaki, M.; Siafakas, N.; Zerva, L.; Giamarellou, H. Risk-factors and predictors of mortality in patients colonised with vancomycin-resistant enterococci. Clin. Microbiol. Infect. 2008, 14, 14–21. [Google Scholar] [CrossRef]
- Gouliouris, T.; Warne, B.; Cartwright, E.J.P.; Bedford, L.; Weerasuriya, C.K.; Raven, K.E.; Brown, N.M.; Török, M.E.; Limmathurotsakul, D.; Peacock, S.J. Duration of exposure to multiple antibiotics is associated with increased risk of VRE bacteraemia: A nested case-control study. J. Antimicrob. Chemother. 2018, 73, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Shorman, M.; Al-Tawfiq, J.A. Risk factors associated with vancomycin-resistant enterococcus in intensive care unit settings in saudi arabia. Interdiscip. Perspect. Infect. Dis. 2013, 2013, 369674. [Google Scholar] [CrossRef]
| Characteristic | Number (%) |
|---|---|
| Male | 86 (59.3) |
| Age (years), median (IQR) | 75 (62–81) |
| ACCI, median (IQR) | 6 (4–7) |
| Immunocompromised status | 23 (14.4) |
| Time from admission to bacteremia onset (days), median (IQR) | 4 (0–18.75) |
| Hospital acquired bacteremia | 91 (56.9) |
| Intensive-care unit | 35 (21.9) |
| Mechanical ventilation | 58 (36.3) |
| Septic shock | 37 (23.1) |
| PBS, median (IQR) | 2 (0–4) |
| Sites of infection | |
| Primary bacteremia | 47 (29.4) |
| Intraabdominal infection | 35 (21.9) |
| Intravascular catheter infection | 26 (16.3) |
| Urinary system infection | 21 (13.1) |
| Infective endocarditis | 12 (7.5) |
| Skin and soft tissue infection | 9 (5.6) |
| Bone and joint infection | 6 (3.8) |
| Lower respiratory tract infection (Empyema thoracis) | 3 (1.9) |
| Reproductive tract infection | 1 (0.6) |
| Polymicrobial bacteremia | 41 (25.6) |
| Polymicrobial infection | 49 (30.6) |
| Time to appropriate therapy (days), median (IQR) | 1 (0–3) |
| Definitive antibiotic regimens | |
| Anti-enterococcal beta-lactams | 123 (76.9) |
| Vancomycin | 13 (8.1) |
| Anti-enterococcal beta-lactams—Gentamicin | 14 (8.8) |
| Anti-enterococcal beta-lactams—Ceftriaxone | 3 (1.9) |
| Anti-enterococcal beta-lactams—Vancomycin | 6 (3.8) |
| Anti-enterococcal beta-lactams—Tigecycline | 1 (0.6) |
| Switched therapy from IV to oral | 22 (13.8) |
| Source control | 50 (31.3) |
| Treatment outcomes | |
| All-cause 30-day mortality | 46 (28.8) |
| All-cause in hospital mortality | 67 (41.9) |
| Clinical stability | 114 (71.3) |
| Time to clinical stability (days); median (IQR) | 3 (2–6) |
| Length of stay after infection (days); median (IQR) | 19 (13–34) |
| Relapsed ASE bacteremia; median (IQR) = 29 (24–38) days | 7 (4.4) |
| Microbiological clearance within 7 days (n = 104) | 76 (73.1) |
| Adverse outcomes | |
| Vancomycin-resistant enterococci * | 5 (3.1) |
| Acute kidney injury | 26 (16.3) |
| Characteristic | Death (n = 46) | Survival (n = 114) | p Value | OR | 95% CI |
|---|---|---|---|---|---|
| Mal | 25 (54.3) | 71 (62.3) | 0.354 | 0.72 | 0.36–1.44 |
| Age (years) | 77 (65–85) | 74 (61–80) | 0.052 * | ||
| ACCI; median (IQR) | 6.5 (5–8) | 5 (4–7) | <0.001 * | ||
| ACCI 0–2 (n = 21) | 0 | 21 | |||
| ACCI 3–5 (n = 58) | 15 (32.9) | 43 (37.7) | |||
| ACCI 6–13 (n = 81) | 31 (67.4) | 50 (43.9) | |||
| Immunocompromised | 9 (19.6) | 14 (12.3) | 0.235 | 1.74 | 0.69–4.35 |
| Time from admission to bacteremia (days); median (IQR) | 10 (2.5–31.5) | 0 (0–12) | 0.001 * | ||
| HA-bacteremia | 35 (76.1) | 56 (49.1) | 0.002 | 3.3 | 1.52–7.12 |
| Intensive care unit | 18 (39.1) | 17 (14.9) | 0.001 | 3.67 | 1.67–8.04 |
| Mechanical ventilation | 26 (56.5) | 32 (28.1) | 0.001 | 3.33 | 1.64–6.79 |
| Septic shock | 20 (43.5) | 17 (14.9) | <0.001 | 4.39 | 2.02–9.56 |
| PBS; median (IQR) | 3.5 (2–5) | 1 (0–3) | <0.001 * | ||
| PBS 0–3 (n = 113) | 22 (47.8) | 91 (79.8) | |||
| PBS 4–7 (n = 41) | 20 (43.5) | 21 (18.4) | |||
| PBS 8–11 (n= 6) | 4 (8.7) | 2 (1.8) | |||
| Site of infection | |||||
| Primary bacteremia | 17 (37) | 30 (26.3) | 0.181 | 1.64 | 0.79–3.4 |
| Intraabdominal infection | 13 (28.3) | 22 (19.3) | 0.215 | 1.65 | 0.75–3.64 |
| Intravascular catheter infection | 6 (13) | 20 (17.5) | 0.485 | 0.71 | 0.26–1.89 |
| Urinary system infection | 6 (13) | 15 (13.2) | 0.985 | 1 | 0.36–2.73 |
| Infective endocarditis | 2 (4.3) | 10 (8.8) | 0.511 | 0.47 | 0.1–2.25 |
| Skin and soft tissue infection | 1 (2.2) | 8 (7) | 0.448 | 0.29 | 0.04–2.42 |
| Bone and joint infection | 0 | 6 (5.3) | 0183 | - | - |
| Lower respiratory tract infection (Empyema thoracis) | 1 (2.2) | 2 (1.8) | 1.0 | 1.24 | 0.11–14.07 |
| Reproductive tract infection | 0 | 1 (0.9) | 1.0 | - | - |
| Species of enterococci | |||||
| E. faecalis (139) | 41 (89.1) | 97 (85.8) | 0.591 | 1.34 | 0.46–3.9 |
| E. faecium (15) | 5 (10.9) | 9 (8) | 0.546 | 1.42 | 0.45–4.5 |
| Other species (7) | 0 | 7 (6.2) | 0.194 | - | - |
| Polymicrobial bacteremia | 11 (23.9) | 30 (26.3) | 0.75 | 0.88 | 0.4–1.95 |
| Polymicrobial infection | 13 (28.3) | 36 (31.6) | 0.68 | 0.85 | 0.4–1.81 |
| Time to appropriate therapy (days); median (IQR) | 1 (0–2) | 1 (0–3) | 0.337 * | ||
| Received appropriate therapy within 1 day | 33 (71.7) | 68 (60.2) | 0.170 | 1.68 | 0.8–3.54 |
| Definitive antibiotic regimens | 0.024 | ||||
| Anti-enterococcal beta-lactams | 32 (69.6) | 91 (79.8) | 0.164 | 0.58 | 0.27–1.26 |
| Anti-enterococcal beta-lactams combination | 6 (13) | 18 (15.8) | 0.66 | 0.8 | 0.3–2.16 |
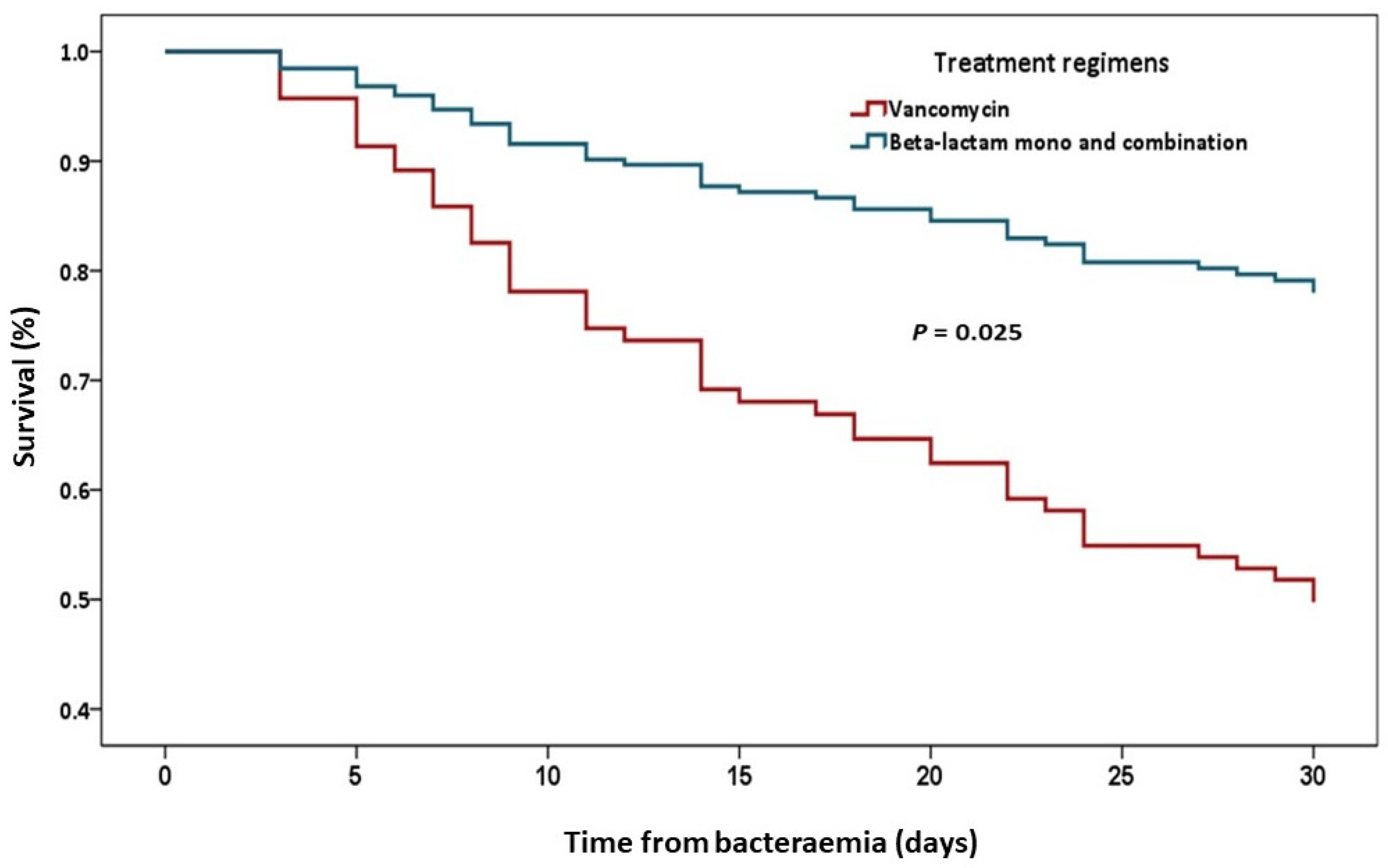
| Vancomycin | 8 (17.4) | 5 (4.4) | 0.011 | 4.59 | 1.42–14.89 |
| Source control | 8 (17.4) | 42 (36.8) | 0.016 | 0.36 | 0.15–0.85 |
| Regimens between Group 1 and Group 2 1 | p Value | OR | 95%CI |
|---|---|---|---|
| Vancomycin vs. Anti-enterococcal beta-lactams | 0.02 | 4.55 | 1.39–14.92 |
| Vancomycin vs. Anti-enterococcal beta-lactams combination | 0.039 | 4.8 | 1.13–20.46 |
| Anti-enterococcal beta-lactams vs. Anti-enterococcal beta-lactams combination | 0.917 | 1.06 | 0.38–2.89 |
| Variable | p Value | OR | 95% CI |
|---|---|---|---|
| Age (years) | 0.963 | 1.0 | 0.96–1.04 |
| Time from admission to bacteremia (days) | 0.628 | 0.99 | 0.98–1.01 |
| HA-bacteremia | 0.48 | 0.69 | 0.25–1.91 |
| ACCI (each increase of 1 point) | <0.001 | 1.34 | 1.14–1.58 |
| PBS (each increase of 1 point) | <0.001 | 1.44 | 1.20–1.71 |
| Intensive-care unit | 0.154 | 2.28 | 0.73–7.12 |
| Mechanical ventilation | 0.575 | 0.67 | 0.17–2.7 |
| Septic shock | 0.403 | 1.72 | 0.48–6.21 |
| Vancomycin monotherapy | 0.047 | 4.07 | 1.02–16.22 |
| Source control | 0.135 | 0.44 | 0.15–1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemapanpairoa, J.; Changpradub, D.; Santimaleeworagun, W. Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections. Antibiotics 2022, 11, 1698. https://doi.org/10.3390/antibiotics11121698
Hemapanpairoa J, Changpradub D, Santimaleeworagun W. Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections. Antibiotics. 2022; 11(12):1698. https://doi.org/10.3390/antibiotics11121698
Chicago/Turabian StyleHemapanpairoa, Jatapat, Dhitiwat Changpradub, and Wichai Santimaleeworagun. 2022. "Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections" Antibiotics 11, no. 12: 1698. https://doi.org/10.3390/antibiotics11121698
APA StyleHemapanpairoa, J., Changpradub, D., & Santimaleeworagun, W. (2022). Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections. Antibiotics, 11(12), 1698. https://doi.org/10.3390/antibiotics11121698






