Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review
Abstract
:1. Introduction
2. Foodborne Pathogenic Bacteria and Antibiotic Resistance
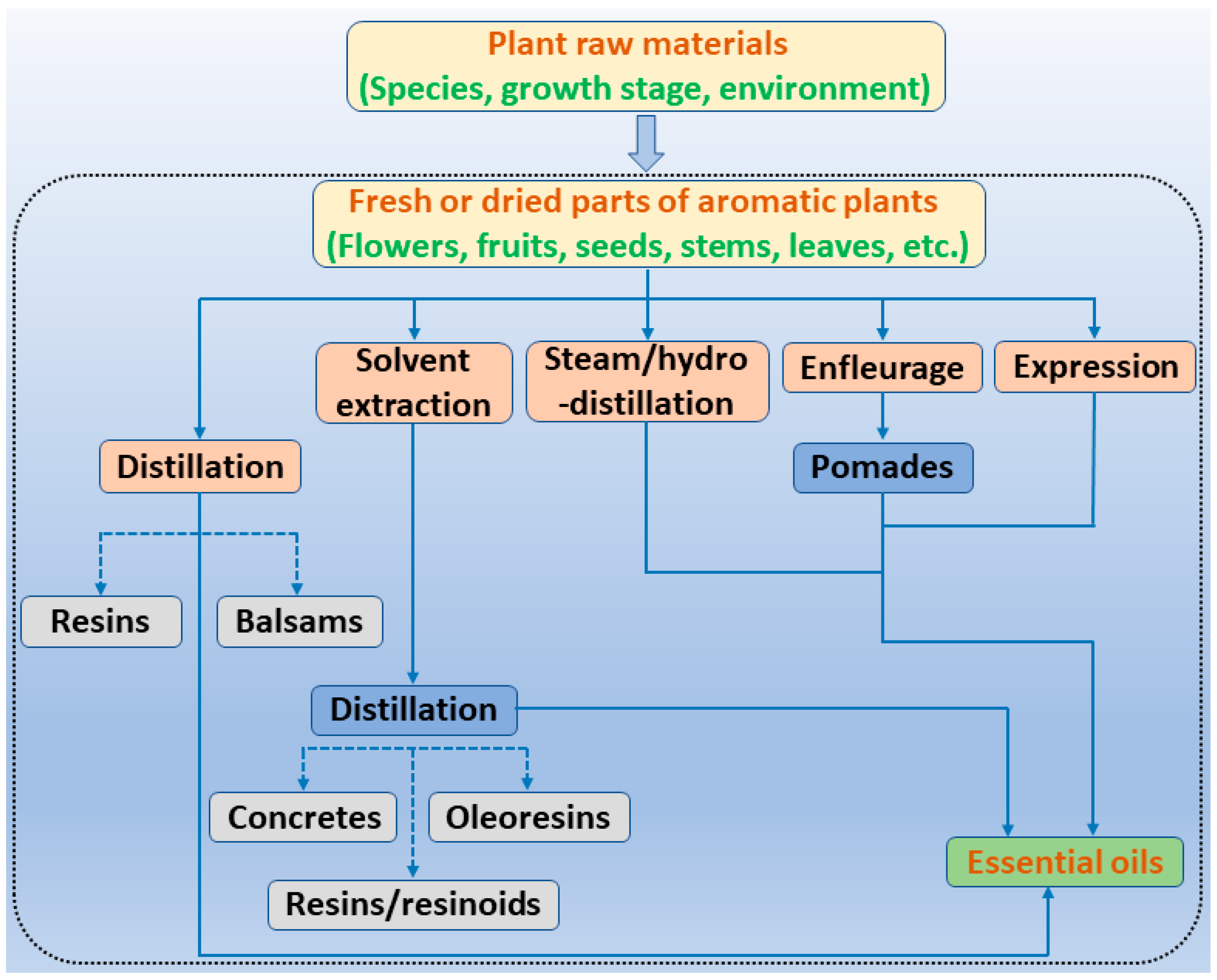
3. EOs and Their Individual Compounds Derived from Common Aromatic Plants and Their Antibacterial Actions
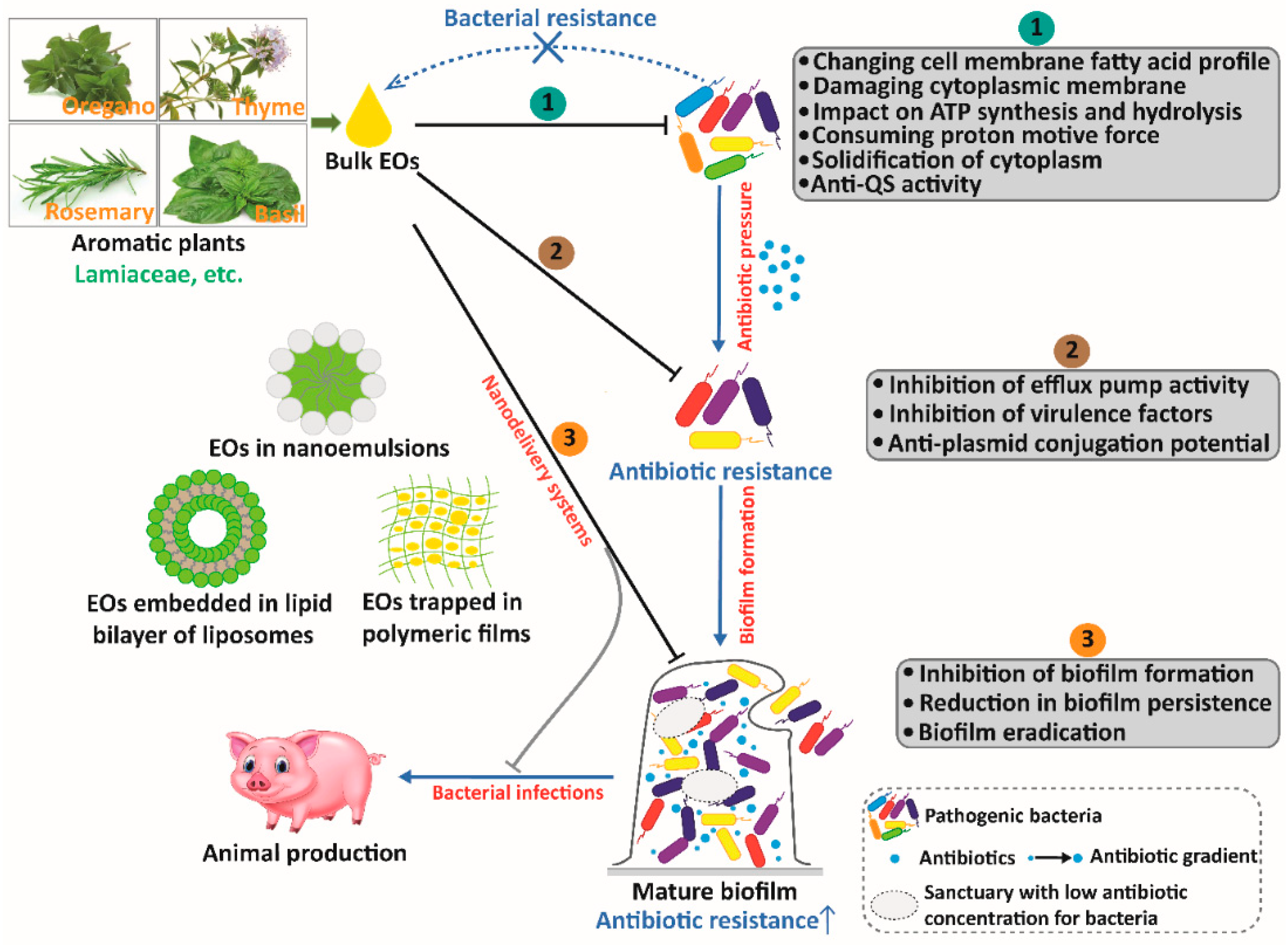
4. Antibiotic Resistance Genes and the Impact of EOs and Their Individual Compounds on Antibiotic Resistance
5. Nanoencapsulated EOs as a Promising Option for Animal Production against Antibiotic Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evangelista, A.G.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Luciano, F.B. The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5267–5283. [Google Scholar] [CrossRef] [PubMed]
- Fukase, E.; Martin, W. Economic growth, convergence, and world food demand and supply. World Dev. 2020, 132, 104954. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskam, J.L.; Lansink, A.G.J.M.O.; Saatkamp, H.W. The technical and economic impact of veterinary interventions aimed at reducing antimicrobial use on broiler farms. Poult. Sci. 2019, 98, 6644–6658. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Zhao, L. Grand challenges in understanding gut microbes. Front. Microbiol. 2021, 12, 752829. [Google Scholar] [CrossRef] [PubMed]
- Hagbø, M.; Ravi, A.; Angell, I.L.; Sunde, M.; Ludvigsen, J.; Diep, D.B.; Foley, S.L.; Vento, M.; Collado, M.C.; Perez-Martinez, G.; et al. Experimental support for multidrug resistance transfer potential in the preterm infant gut microbiota. Pediatr. Res. 2020, 88, 57–65. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Hao, X.; Gan, M.; Lin, P.; Shang, X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021, 344, 128674. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Snoussi, A.; Chouaibi, M.; Koubaier, H.B.H.; Bouzouita, N. Encapsulation of Tunisian thyme essential oil in O/W nanoemulsions: Application for meat preservation. Meat Sci. 2022, 188, 108785. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiihonen, K.; Kettunen, H.; Bento, M.H.; Saarinen, M.; Lahtinen, S.; Ouwehand, A.C.; Schulze, H.; Rautonen, N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010, 51, 381–392. [Google Scholar] [CrossRef]
- Cetin, E.; Yibar, A.; Yesilbag, D.; Cetin, I.; Cengiz, S.S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 2016, 57, 780–787. [Google Scholar] [CrossRef]
- Nouri, A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential oils and aromatic plants in animal feeding–a European perspective. A review. Flavour Frag. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [Green Version]
- Janz, J.A.; Morel, P.C.; Wilkinson, B.H.; Purchas, R.W. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007, 75, 350–355. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J.P.; Kim, H.J.; Meng, Q.W.; Ao, X.; Hong, S.M.; Kim, I.H. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower-finisher pigs. Livest. Sci. 2010, 128, 115–122. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Woolhouse, M.E.; Ward, M.J. Sources of antimicrobial resistance. Science 2013, 341, 1460–1461. [Google Scholar] [CrossRef]
- Rahman, M.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.J.; Hungate, B.A.; Price, L.B. Food-animal production and the spread of antibiotic resistance: The role of ecology. Front. Ecol. Environ. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Heikkilä, A.M.; Nousiainen, J.I.; Pyörälä, S. Costs of clinical mastitis with special reference to premature culling. J. Dairy Sci. 2012, 95, 139–150. [Google Scholar] [CrossRef]
- Cheng, Z.; Palma-Vera, S.; Buggiotti, L.; Salavati, M.; Becker, F.; Werling, D.; Wathes, D.C.; GplusE Consortium. Transcriptomic analysis of circulating leukocytes obtained during the recovery from clinical mastitis caused by Escherichia coli in Holstein dairy cows. Animals 2022, 12, 2146. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental bovine mastitis pathogens: Prevalence, antimicrobial susceptibility, and sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. essential oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Ludbey, P.A.; Sahibzada, S.; Annandale, C.H.; Robertson, I.D.; Waichigo, F.K.; Tufail, M.S.; Valenzuela, J.L.; Aleri, J.W. A pilot study on bacterial isolates associated with purulent vaginal discharge in dairy cows in the south-west region of Western Australia. Aust. Vet. J. 2022, 100, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.C.; Leite, E.L.; Araújo, W.J.; Silva, N.; Saraiva, M.; Filho, L.S.; Neto, O.C.F.; Givisiez, P.; Oliveira, C. Draft genome sequence of mcr-1-mediated colistin-resistant Escherichia coli ST359 from chicken carcasses in Northeastern Brazil. J. Glob. Antimicrob. Resist. 2020, 23, 135–136. [Google Scholar] [CrossRef]
- Trongjit, S.; Angkittitrakul, S.; Chuanchuen, R. Occurrence and molecular characteristics of antimicrobial resistance of Escherichia coli from broilers, pigs and meat products in Thailand and Cambodia provinces. Microbiol. Immunol. 2016, 60, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, Y.; Yang, L.; Wu, X.; Wu, Y.; Shao, B. Prevalence of Escherichia coli and antibiotic resistance in animal-derived food samples—Six districts, Beijing, China, 2020. China CDC Wkly. 2021, 3, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Österberg, J.; Wingstrand, A.; Jensen, A.N.; Kerouanton, A.; Cibin, V.; Barco, L.; Denis, M.; Aabo, S.; Bengtsson, B. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE 2016, 11, e0157049. [Google Scholar] [CrossRef] [Green Version]
- Saeed, E.; Amer, A.A.E.; Keshta, H.G.; Hafez, E.E.; Sultan, R.M.S.; Khalifa, E. Prevalence, antibiotic sensitivity profile, and phylogenetic analysis of Escherichia coli isolated from raw dromedary camel milk in Matrouh Governorate, Egypt. J. Adv. Vet. Anim. Res. 2022, 9, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Messele, Y.E.; Abdi, R.D.; Yalew, S.T.; Tegegne, D.T.; Emeru, B.A.; Werid, G.M. Molecular determination of antimicrobial resistance in Escherichia coli isolated from raw meat in Addis Ababa and Bishoftu, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. A preliminary study: Antibiotic resistance of Escherichia coli and Staphylococcus aureus from the meat and feces of various South African wildlife species. Food Sci. Anim. Resour. 2021, 41, 135–144. [Google Scholar] [CrossRef]
- Taylor, E.A.; Ossa-Trujillo, C.; Vinasco, J.; Jordan, E.R.; Buitrago, J.A.G.; Hagevoort, R.; Norman, K.N.; Lawhon, S.D.; Piñeiro, J.M.; Levent, G.; et al. Use of critically important antimicrobial classes early in life may adversely impact bacterial resistance profiles during adult years: Potential co-selection for plasmid-borne fluoroquinolone and macrolide resistance via extended-spectrum beta-lactam use in dairy cattle. Lett. Appl. Microbiol. 2021, 72, 220–224. [Google Scholar] [PubMed]
- Le, P.Q.; Awasthi, S.P.; Hatanaka, N.; Hinenoya, A.; Hassan, J.; Ombarak, R.A.; Iguchi, A.; Tran, N.; Dao, K.; Vien, M.Q.; et al. Prevalence of mobile colistin resistance (mcr) genes in extended-spectrum β-lactamase-producing Escherichia coli isolated from retail raw foods in Nha Trang, Vietnam. Int. J. Food Microbiol. 2021, 346, 109164. [Google Scholar] [CrossRef]
- Ferreira, A.; Pavelquesi, S.; Monteiro, E.; Rodrigues, L.; Silva, C.; Silva, I.; Orsi, D.C. Prevalence and antimicrobial resistance of Salmonella spp. in aquacultured Nile Tilapia (Oreochromis niloticus) commercialized in Federal District, Brazil. Foodborne Pathog. Dis. 2021, 18, 778–783. [Google Scholar] [CrossRef]
- Lay, K.S.; Vuthy, Y.; Song, P.; Phol, K.; Sarthou, J.L. Prevalence, numbers and antimicrobial susceptibilities of Salmonella serovars and Campylobacter spp. in retail poultry in Phnom Penh, Cambodia. J. Vet. Med Sci. 2011, 73, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Huang, J.; Wu, Q.; Zhang, J.; Liu, S.; Guo, W.; Cai, S.; Yu, S. Prevalence, Antimicrobial resistance and genetic diversity of Salmonella isolated from retail ready-to-eat foods in China. Food Control 2016, 60, 50–56. [Google Scholar] [CrossRef]
- Abd-Elghany, S.M.; Sallam, K.I.; Abd-Elkhalek, A.; Tamura, T. Occurrence, genetic characterization and antimicrobial resistance of Salmonella isolated from chicken meat and giblets. Epidemiol. Infect. 2015, 143, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, R.; Salari, S.; Najimi, M.; Rashki, A. Determination of frequency, multiple antibiotic resistance index and resistotype of Salmonella spp. in chicken meat collected from southeast of Iran. Vet. Med. Sci. 2022, 8, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Suárez, E.J.; Palós-Guitérrez, T.; Ruíz-López, F.A.; Pérez, C.F.H.; Ballesteros-Nova, N.E.; Soberanis-Ramos, O.; Méndez-Medina, R.D.; Allard, M.W.; Rubio-Lozano, M.S. Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant non-typhoidal Salmonella in Mexico. PLoS ONE 2021, 16, e0243681. [Google Scholar] [CrossRef]
- Patchanee, P.; Tansiricharoenkul, K.; Buawiratlert, T.; Wiratsudakul, A.; Angchokchatchawal, K.; Yamsakul, P.; Yano, T.; Boonkhot, P.; Rojanasatien, S.; Tadee, P. Salmonella in pork retail outlets and dissemination of its pulsotypes through pig production chain in Chiang Mai and surrounding areas, Thailand. Prev. Vet. Med. 2016, 130, 99–105. [Google Scholar] [CrossRef]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroning, I.S.; Iglesias, M.A.; Mendonça, K.S.; Lopes, G.V.; Silva, W.P. Presence of classical enterotoxin genes, agr typing, antimicrobial resistance, and genetic diversity of Staphylococcus aureus from milk of cows with mastitis in southern Brazil. J. Food Prot. 2018, 81, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Wu, Z.; Lv, J.; Ren, Q.; Chen, W. Investigation of clonal diversity, virulence genes, and antibiotic resistance of Staphylococcus aureus recovered from raw cow milk in southern Xinjiang, China. Folia Microbiol. 2022, 67, 245–252. [Google Scholar] [CrossRef]
- Thongratsakul, S.; Usui, M.; Higuchi, H.; Takahashi, T.; Sato, T.; Poolkhet, C.; Tamura, Y. Prevalence and characterization of Staphylococcus aureus isolated in raw milk from cows in Hokkaido, Japan. Trop. Anim. Health Prod. 2020, 52, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Kanungpean, D.; Takai, S.; Kakuda, T. Contamination and antimicrobial susceptibility testing of Staphylococcus aureus isolated from pork in fresh markets, Nongchok district, Thailand. Vet. Med. Int. 2021, 2021, 6646846. [Google Scholar] [CrossRef]
- Ge, B.; Mukherjee, S.; Hsu, C.H.; Davis, J.A.; Tran, T.; Yang, Q.; Abbott, J.W.; Ayers, S.L.; Young, S.R.; Crarey, E.T.; et al. MRSA and multidrug-resistant Staphylococcus aureus in U.S. retail meats, 2010–2011. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef]
- Oliveira, C.J.; Tiao, N.; de Sousa, F.G.; de Moura, J.F.; Filho, L.S.; Gebreyes, W.A. Methicillin-resistant Staphylococcus aureus from Brazilian dairy farms and identification of novel sequence types. Zoonoses Public Health 2016, 63, 97–105. [Google Scholar] [CrossRef]
- Yi, Y.; Su, L.; Li, B.; Li, S.; Zhang, B.; Su, Y. Analysis of the genetic diversity in methicillin-resistant Staphylococcus aureus isolates from bovine subclinical mastitis case in Xinjiang, China. Foodborne Pathog. Dis. 2018, 15, 568–575. [Google Scholar] [CrossRef]
- Li, H.; Andersen, P.S.; Stegger, M.; Sieber, R.N.; Ingmer, H.; Staubrand, N.; Dalsgaard, A.; Leisner, J.J. Antimicrobial resistance and virulence gene profiles of methicillin-resistant and -susceptible Staphylococcus aureus from food products in Denmark. Front. Microbiol. 2019, 10, 2681. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Elhadidy, M.; Tamura, T. Molecular characterization and antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus in retail chicken. J. Food Prot. 2015, 78, 1879–1884. [Google Scholar] [CrossRef]
- Hasanpour Dehkordi, A.; Khaji, L.; Sakhaei Shahreza, M.H.; Mashak, Z.; Safarpoor Dehkordi, F.; Safaee, Y.; Hosseinzadeh, A.; Alavi, I.; Ghasemi, E.; Rabiei-Faradonbeh, M. One-year prevalence of antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus recovered from raw meat. Trop. Biomed. 2017, 34, 396–404. [Google Scholar] [PubMed]
- Zhang, P.; Liu, X.; Zhang, J.; Fu, X.; Wan, Y.; Pan, H.; Wu, C.; Wang, X. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from retail yak butter in Tibet, China. J. Dairy Sci. 2021, 104, 9596–9606. [Google Scholar] [CrossRef] [PubMed]
- Akrami-Mohajeri, F.; Derakhshan, Z.; Ferrante, M.; Hamidiyan, N.; Soleymani, M.; Conti, G.O.; Tafti, R.D. The prevalence and antimicrobial resistance of Listeria spp in raw milk and traditional dairy products delivered in Yazd, central Iran (2016). Food Chem. Toxicol. 2018, 114, 141–144. [Google Scholar] [CrossRef]
- Escolar, C.; Gómez, D.; García, M.D.C.R.; Conchello, P.; Herrera, A. Antimicrobial resistance profiles of Listeria monocytogenes and Listeria innocua isolated from ready-to-eat products of animal origin in Spain. Foodborne Pathog. Dis. 2017, 14, 357–363. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, G.; Yang, J.; Zhao, L.; Jiang, Y.; Guo, D.; Wang, X.; Zhi, S.; Xu, X.; Dong, Q.; et al. Prevalence, antibiotic resistance, and molecular epidemiology of Listeria monocytogenes isolated from imported foods in China during 2018 to 2020. Int. J. Food Microbiol. 2022, 382, 109916. [Google Scholar] [CrossRef]
- Sugiri, Y.D.; Gölz, G.; Meeyam, T.; Baumann, M.P.; Kleer, J.; Chaisowwong, W.; Alter, T. Prevalence and antimicrobial susceptibility of Listeria monocytogenes on chicken carcasses in Bandung, Indonesia. J. Food Prot. 2014, 77, 1407–1410. [Google Scholar] [CrossRef]
- Maung, A.T.; Mohammadi, T.N.; Nakashima, S.; Liu, P.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Antimicrobial resistance profiles of Listeria monocytogenes isolated from chicken meat in Fukuoka, Japan. Int. J. Food Microbiol. 2019, 304, 49–57. [Google Scholar] [CrossRef]
- Sosnowski, M.; Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. Int. J. Food Microbiol. 2019, 289, 1–6. [Google Scholar] [CrossRef]
- Tîrziu, E.; Herman, V.; Nichita, I.; Morar, A.; Imre, M.; Ban-Cucerzan, A.; Bucur, I.; Tîrziu, A.; Mateiu-Petrec, O.C.; Imre, K. Diversity and antibiotic resistance profiles of Listeria monocytogenes serogroups in different food products from the Transylvania region of central Romania. J. Food Prot. 2022, 85, 54–59. [Google Scholar] [CrossRef]
- Arslan, S.; Baytur, S. Prevalence and antimicrobial resistance of Listeria species and subtyping and virulence factors of Listeria monocytogenes from retail meat. J. Food Saf. 2019, 39, e12578. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils--a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Bisht, D.S.; Menon, K.R.K.; Singhal, M.K. Comparative antimicrobial activity of essential oils of Cuminum cyminum L. and Foeniculum vulgare Mill. seeds against Salmonella typhimurium and Escherichia coli. J. Essent. Oil Bear. Plants 2014, 17, 617–622. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Goswami, P.; Singh, V.R.; Chauhan, A.; Verma, S.K.; Iqbal, H.; Verma, R.K.; Chanda, D.; et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind. Crop. Prod. 2017, 104, 144–155. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Pióro-Jabrucka, E.; Przybył, J.L.; Gniewosz, M.; Kraśniewska, K.; Szyndel, M.S.; Costa, R.; Bączek, K.B. Antioxidant and antibacterial activity of Helichrysum italicum (Roth) G. Don. from central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Marchetti, M.; Campagna, P.; Usai, M. Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso collected in South-West Sardinia. Saudi J. Biol. Sci. 2019, 26, 897–905. [Google Scholar]
- De Falco, E.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crop. Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Al Maqtari, M.A.A.; Alghalibi, S.M.; Alhamzy, E.H. Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk. J. Biochem. 2011, 36, 342–349. [Google Scholar]
- Abdelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016, 94, 197–205. [Google Scholar] [CrossRef]
- Silva, V.A.; da Sousa, J.P.; de Luna Freire Pessôa, H.; de Freitas, A.F.R.; Coutinho, H.D.M.; Alves, L.B.N.; Lima, E.O. Ocimum basilicum: Antibacterial activity and association study with antibiotics against bacteria of clinical importance. Pharm. Biol. 2016, 54, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Masci, V.L.; Tiezzi, A.; Trillia, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.; da Costa, R.; Pereira, P.S.; Lima, L.F.; de Matos, Y.; et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar] [CrossRef]
- Ooi, L.S.; Li, Y.; Kam, S.L.; Wang, H.; Wong, E.Y.; Ooi, V.E. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Li, C.Z.; Dai, J.M.; Cui, H.Y.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crop. Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Valková, V.; Ďúranová, H.; Kowalczewski, P.Ł.; Said-Al Ahl, H.; Hikal, W.M.; Vukic, M.; Savitskaya, T.; et al. Chemical composition, in vitro and in situ antimicrobial and antibiofilm activities of Syzygium aromaticum (Clove) essential oil. Plants 2021, 10, 2185. [Google Scholar] [CrossRef]
- Pontes, E.; Melo, H.M.; Nogueira, J.; Firmino, N.; de Carvalho, M.G.; Júnior, F.C.; Cavalcante, T. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2018, 28, 633–639. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef]
- Awang, K.; Ibrahim, H.; Syamsir, D.R.; Mohtar, M.; Ali, R.M.; Ali, N.A.M. Chemical constituents and antimicrobial activity of the leaf and rhizome oils of Alpinia pahangensis Ridl., an endemic wild ginger from peninsular Malaysia. Chem. Biodivers. 2011, 8, 668–673. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 471. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.H.; Hsia, S.M.; Wu, C.H.; Ko, S.Y.; Chen, M.Y.; Shih, Y.H.; Shieh, T.M.; Chuang, L.C.; Wu, C.Y. Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE 2016, 11, e0163147. [Google Scholar] [CrossRef] [Green Version]
- Gómez-García, M.; Sol, C.; de Nova, P.; Puyalto, M.; Mesas, L.; Puente, H.; Mencía-Ares, Ó.; Miranda, R.; Argüello, H.; Rubio, P.; et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porcine Health Manag. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rúa, J.; Del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of carvacrol and thymol: Antimicrobial activity against Staphylococcus aureus and antioxidant activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.X.; Wang, Y.W.; Wu, C.S.; Lin, Y.H.; Hung, C.H.; Huang, H.H.; Kuo, S.M. Therapeutic efficacy of sesquiterpene farnesol in treatment of Cutibacterium acnes-induced dermal disorders. Molecules 2021, 26, 5723. [Google Scholar] [CrossRef] [PubMed]
- Wiatkowski, P.; Sienkiewicz, M.; Pruss, A.; Łopusiewicz, Ł.; Arszyńska, N.; Wojciechowska-Koszko, I.; Kilanowicz, A.; Kot, B.; Dołęgowska, B. Antibacterial and anti-biofilm activities of essential oil compounds against new delhi metallo-β-lactamase-1-producing uropathogenic Klebsiella pneumoniae strains. Antibiotics 2022, 11, 147. [Google Scholar] [CrossRef]
- de Moura, D.F.; Rocha, T.A.; Barros, D.D.M.; da Silva, M.M.; Santana, M.D.S.; Neta, B.M.; Cavalcanti, I.M.F.; Martins, R.D.; da Silva, M.V. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch. Microbiol. 2021, 203, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.H.; Abdel-Ghany, A.E.; Afifi, S.I.; Sedik, S.H. Genotypic characterization of Campylobacter species isolated from livestock and poultry and evaluation of some herbal oil antimicrobial effect against selected Campylobacter species. Adv. Anim. Vet. Sci. 2019, 7, 1083–1092. [Google Scholar] [CrossRef]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Zhang, J.R.; Farha, A.K.; Li, H.B.; Zhu, F.; Wang, X.H.; Corke, H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1018–1055. [Google Scholar] [CrossRef] [Green Version]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Elmi, A.; Prosperi, A.; Zannoni, A.; Bertocchi, M.; Scorpio, D.G.; Forni, M.; Foni, E.; Bacci, M.L.; Ventrella, D. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res. Vet. Sci. 2019, 127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Tassou, C.; Koutsoumanis, K.; Nychas, G.J.E. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res. Int. 2000, 33, 273–280. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Ta, C.A.; Arnason, J.T. Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules 2015, 21, E29. [Google Scholar] [CrossRef]
- Soumya, E.A.; Saad, I.K.; Hassan, L.; Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Venkitanarayanan, K. Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Prot. 2011, 74, 200–208. [Google Scholar] [CrossRef]
- Sharma, G.; Raturi, K.; Dang, S.; Gupta, S.; Gabrani, R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis. J. Asian Nat. Prod. Res. 2014, 16, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef] [Green Version]
- Amaral, S.C.; Pruski, B.B.; de Freitas, S.B.; Allend, S.O.; Ferreira, M.; Moreira, C., Jr.; Pereira, D.; Junior, A.; Hartwig, D.D. Origanum vulgare essential oil: Antibacterial activities and synergistic effect with polymyxin B against multidrug-resistant Acinetobacter baumannii. Mol. Biol. Rep. 2020, 47, 9615–9625. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis essential oil: Phytochemical characterization, bioactivity evaluation and synergistic effect with antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef]
- Özel, Y.; Yılmaz, U.; Ünlü, M.; Ünlü, G.V. Antibacterial activity and synergistic interaction of various essential oil components and antibiotics. Mikrobiyol. Bul. 2022, 56, 95–102. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Visvalingam, J.; Palaniappan, K.; Holley, R.A. In vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control 2017, 79, 288–291. [Google Scholar] [CrossRef]
- Rodrigues, I.D.A.; Ferrari, R.G.; Panzenhagen, P.H.N.; Mano, S.B.; Conte-Junior, C.A. Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 2020, 112, 143–183. [Google Scholar]
- Rozwandowicz, M.; Brouwer, M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Sung, K.; Kweon, O.; Khan, S.; Nawaz, S.; Steele, R. Characterisation of novel mutations involved in quinolone resistance in Escherichia coli isolated from imported shrimp. Int. J. Antimicrob. Agents 2015, 45, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Porrero, M.C.; Téllez, S.; Palomo, G.; García, M.; Domínguez, L. Polymorphism of genes encoding PmrAB in colistin-resistant strains of Escherichia coli and Salmonella enterica isolated from poultry and swine. J. Antimicrob. Chemother. 2015, 70, 71–74. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Hofy, F.I.; Shafik, S.M.; Abdelrahman, M.A.; Elsaid, G.A. Characterization of virulence-associated genes, antimicrobial resistance genes, and class 1 integrons in Salmonella enterica serovar Typhimurium isolates from chicken meat and humans in Egypt. Foodborne Pathog. Dis. 2016, 13, 281–288. [Google Scholar] [CrossRef]
- Neuert, S.; Nair, S.; Day, M.R.; Doumith, M.; Ashton, P.M.; Mellor, K.C.; Jenkins, C.; Hopkins, K.L.; Woodford, N.; de Pinna, E.; et al. Prediction of phenotypic antimicrobial resistance profiles from whole genome sequences of non-typhoidal Salmonella enterica. Front. Microbiol. 2018, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- McMillan, E.A.; Gupta, S.K.; Williams, L.E.; Jové, T.; Hiott, L.M.; Woodley, T.A.; Barrett, J.B.; Jackson, C.R.; Wasilenko, J.L.; Simmons, M.; et al. Antimicrobial resistance genes, cassettes, and plasmids present in Salmonella enterica associated with United States food animals. Front. Microbiol. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauteri, C.; Maggio, F.; Serio, A.; Festino, A.R.; Paparella, A.; Vergara, A. Overcoming multidrug resistance in Salmonella spp. isolates obtained from the swine food chain by using essential oils: An in vitro study. Front. Microbiol. 2022, 12, 808286. [Google Scholar] [CrossRef]
- Nobrega, D.B.; Naushad, S.; Naqvi, S.A.; Condas, L.; Saini, V.; Kastelic, J.P.; Luby, C.; De Buck, J.; Barkema, H.W. Prevalence and genetic basis of antimicrobial resistance in non-aureus Staphylococci isolated from Canadian Dairy herds. Front. Microbiol. 2018, 9, 256. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zeng, H.; Chen, M.; Ding, Y.; et al. Staphylococcus aureus isolated from retail meat and meat products in China: Incidence, antibiotic resistance and genetic diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, H.; Nobrega, D.B.; Cobo, E.R.; Han, B.; Zhao, Z.; Li, S.; Li, M.; Barkema, H.W.; Gao, J. Molecular epidemiology and distribution of antimicrobial resistance genes of Staphylococcus species isolated from Chinese dairy cows with clinical mastitis. J. Dairy Sci. 2019, 102, 1571–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic resistance in bacteria-A review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Nam, H.M.; Nguyen, L.T.; Tamilselvam, B.; Murinda, S.E.; Oliver, S.P. Prevalence of antimicrobial resistance genes in Listeria monocytogenes isolated from dairy farms. Foodborne Pathog. Dis. 2005, 2, 201–211. [Google Scholar] [CrossRef]
- Kayode, A.; Semerjian, L.; Osaili, T.; Olapade, O.; Okoh, A. Occurrence of multidrug-resistant Listeria monocytogenes in environmental waters: A menace of environmental and public health concern. Front. Environ. Sci. 2021, 12, 373. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, X.; Kang, J.; Pei, F.; Du, R.; Ye, Z.; Ding, H.; Ping, W.; Ge, J. Intraspecific and interspecific quorum sensing of bacterial community affects the fate of antibiotic resistance genes during chicken manure composting under penicillin G stress. Bioresour. Technol. 2022, 347, 126372. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Moo, C.L.; Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef]
- Mikulášová, M.; Chovanová, R.; Vaverková, Š. Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrug-resistant Staphylococci. Phytochem. Rev. 2016, 15, 651–662. [Google Scholar] [CrossRef]
- Ghafari, O.; Sharifi, A.; Ahmadi, A.; Fasaei, B.N. Antibacterial and anti-PmrA activity of plant essential oils against fluoroquinolone-resistant Streptococcus pneumoniae clinical isolates. Lett. Appl. Microbiol. 2018, 67, 564–569. [Google Scholar] [CrossRef]
- Islamieh, D.I.; Goudarzi, H.; Khaledi, A.; Afshar, D.; Esmaeili, D. Reduced efflux pumps expression of Pseudomonas eeruginosa with Satureja khuzistanica essential oil. Iran. J. Med. Sci. 2020, 45, 463–468. [Google Scholar]
- Saviuc, C.; Gheorghe, I.; Coban, S.; Drumea, V.; Chifiriuc, M.C.; Otilia, B.; Banu, O.; Bezirtzoglou, E.; Laz, V. Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR Strains. Rom. Biotech. Lett. 2016, 21, 11796–11804. [Google Scholar]
- Limaverde, P.W.; Campina, F.F.; da Cunha, F.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Oliveira-Tintino, C.D.D.M.; de Matos, Y.; Morais-Braga, M.; Menezes, I.; et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Chovanová, R.; Mezovská, J.; Vaverková, Š.; Mikulášová, M. The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three Salvia species. Lett. Appl. Microbiol. 2015, 61, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.; Bezerra, C.F.; Confortin, C.; da Silva, L.E.; Marinho, E.M.; Marinho, M.M.; Vasconcelos, M.A.; da Silva, T.G.; Marinho, E.S.; Teixeira, A.; et al. Chemical composition and potentiating action of Norfloxacin mediated by the essential oil of Piper caldense C.D.C. against Staphylococcus aureus strains overexpressing efflux pump genes. Arch. Microbiol. 2021, 203, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Mohammadzadeh, A.; Salehi, T.Z.; Mahmoodi, P.; Nourian, A. Cuminum cyminum L. essential oil: A promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 667833. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017, 104, 56–63. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef] [Green Version]
- Hadjilouka, A.; Mavrogiannis, G.; Mallouchos, A.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Effect of lemongrass essential oil on Listeria monocytogenes gene expression. LWT 2017, 77, 510–516. [Google Scholar] [CrossRef]
- Das, S.; Chourashi, R.; Mukherjee, P.; Kundu, S.; Koley, H.; Dutta, M.; Mukhopadhyay, A.K.; Okamoto, K.; Chatterjee, N.S. Inhibition of growth and virulence of Vibrio cholerae by carvacrol, an essential oil component of Origanum spp. J. Appl. Microbiol. 2021, 131, 1147–1161. [Google Scholar] [CrossRef]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive effect of eugenol and its nanoemulsion on quorum sensing-mediated virulence factors and biofilm formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Walasek, M.; Aljarba, T.M.; Stapleton, P.; Gibbons, S.; Xiao, J.; Łuszczki, J.J. The anticonvulsant and anti-plasmid conjugation potential of Thymus vulgaris chemistry: An in vivo murine and in vitro study. Food Chem. Toxicol. 2018, 120, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, M. Physical stress and bacterial colonization. FEMS Microbiol. Rev. 2014, 38, 1250–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tytgat, H.L.P.; Nobrega, F.L.; van der Oost, J.; de Vos, W.M. Bowel biofilms: Tipping points between a healthy and compromised gut? Trends Microbiol. 2019, 27, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef] [Green Version]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Front. Cell. Infect. Microbiol. 2021, 10, 624622. [Google Scholar] [CrossRef]
- Corsini, P.M.; Wang, S.; Rehman, S.; Fenn, K.; Sagar, A.; Sirovica, S.; Cleaver, L.; Edwards-Gayle, C.; Mastroianni, G.; Dorgan, B.; et al. Molecular and cellular insight into Escherichia coli SslE and its role during biofilm maturation. NPJ Biofilms Microbiomes 2022, 8, 9. [Google Scholar] [CrossRef]
- Zhang, B.; Shao, Y.; Liu, D.; Yin, P.; Guo, Y.; Yuan, J. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian. Pathol. 2012, 41, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yu, C.; Chen, Y.; Liu, S.; Azevedo, P.; Gong, J.; O, K.; Yang, C. Citral alleviates peptidoglycan-induced inflammation and disruption of barrier functions in porcine intestinal epithelial cells. J. Cell Physiol. 2022, 237, 1768–1779. [Google Scholar] [CrossRef]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe 2018, 23, 470–484.e7. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial crohn’s disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaure, P.C.; Boarca, B.; Popescu, R.C.; Savu, D.; Trusca, R.; Vasile, B.Ș.; Grumezescu, A.M.; Holban, A.M.; Bolocan, A.; Andronescu, E. Bioactive mesoporous silica nanostructures with anti-microbial and anti-biofilm properties. Int. J. Pharm. 2017, 531, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dohare, S.; Dubey, S.D.; Kalia, M.; Verma, P.; Pandey, H.; Singh, N.K.; Agarwal, V. Anti-biofilm activity of Eucalyptus globulus oil encapsulated silica nanoparticles against E. coli biofilm. Int. J. Pharm. Sci. Res. 2014, 5, 5013–5018. [Google Scholar]
- Ju, X.; Li, J.; Zhu, M.; Lu, Z.; Lv, F.; Zhu, X.; Bie, X. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res. Int. 2018, 107, 385–393. [Google Scholar] [CrossRef]
- Reichling, J. Anti-biofilm and virulence factor-reducing activities of essential oils and oil components as a possible option for bacterial infection control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Vidallon, M.L.P.; Teo, B.M. Recent developments in biomolecule-based nanoencapsulation systems for antimicrobial delivery and biofilm disruption. Chem. Commun. 2020, 56, 13907–13917. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [Green Version]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Oz, Y.; Nabawy, A.; Fedeli, S.; Gupta, A.; Huang, R.; Sanyal, A.; Rotello, V.M. Biodegradable poly (lactic acid) stabilized nanoemulsions for the treatment of multidrug-resistant bacterial biofilms. ACS Appl. Mater. Interfaces 2021, 13, 40325–40331. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Vadivel, V. Citral and linalool nanoemulsions: Impact of synergism and ripening inhibitors on the stability and antibacterial activity against Listeria monocytogenes. J. Food Sci. Technol. 2020, 57, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Marinković, J.; Nikolić, B.; Marković, T.; Radunović, M.; Ilić, J.; Bošković, M.; Ćirić, A.; Marković, D. Cymbopogon citratus essential oil: An active principle of nanoemulsion against Enterococcus faecalis root canal biofilm. Future Microbiol. 2021, 16, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Mneimneh, A.T.; El Jalil, K.A. Levofloxacin-loaded naturally occurring monoterpene-based nanoemulgel: A feasible efficient system to circumvent MRSA ocular infections. Drug Dev. Ind. Pharm. 2020, 46, 1787–1799. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.; Duran, N.; Nakazato, G.; Kobayashi, R.K. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef]
- Comin, V.M.; Lopes, L.Q.; Quatrin, P.M.; de Souza, M.E.; Bonez, P.C.; Pintos, F.G.; Raffin, R.P.; Vaucher, R.; Martinez, D.S.; Santos, R.C. Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microb. Pathog. 2016, 93, 120–125. [Google Scholar] [CrossRef]
- Perez, A.P.; Perez, N.; Lozano, C.; Altube, M.J.; de Farias, M.A.; Portugal, R.V.; Buzzola, F.; Morilla, M.J.; Romero, E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine 2019, 57, 339–351. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Lim, H.S.; Paik, I.K.; Sohn, T.; Kim, W.Y. Effects of supplementary copper chelates in the form of methionine, chitosan and yeast on the performance of broilers. Asian-Australas. J. Anim. Sci. 2006, 19, 1322–1327. [Google Scholar] [CrossRef]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Nanoencapsulation (in vitro and in vivo) as an efficient technology to boost the potential of garlic essential oil as alternatives for antibiotics in broiler nutrition. Animal 2021, 15, 100022. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Meimandipour, A. Feeding broilers with thyme essential oil loaded in chitosan nanoparticles: An efficient strategy for successful delivery. Br. Poult. Sci. 2018, 59, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Effects of nanoencapsulated cumin essential oil as an alternative to the antibiotic growth promoter in broiler diets. J. Appl. Poult. Res. 2020, 29, 875–885. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Abdel-Moneim, A.E.; Shehata, A.M.; Tan, C.; Kharazmi, M.S.; Li, Y.; Assadpour, E.; Castro-Muñoz, R.; et al. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock; purposes, techniques and applications. Adv. Colloid Interface Sci. 2022, 308, 102772. [Google Scholar] [CrossRef]
| Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|
| Australia | Dairy cows | Amoxicillin-clavulanate, ceftiofur, cefoxitin, gentamicin | [33] |
| Brazil | Chicken carcass | Aminoglycosides, colistin, β-lactams, macrolides, quinolones, sulfonamides, tetracyclines, trimethoprim | [34] |
| Cambodia | Broiler carcass and pig carcass | Ampicillin, cefotaxime, ceftazidime, chloramphenicol, ciprofloxacin, streptomycin, tetracyclines, trimethoprim | [35] |
| China | Retail chicken and pork | Ampicillin/sulbactam, aztreonam, cefepime, cefotaxime, ciprofloxacin, colistin, doxycycline, gentamicin, levofloxacin, minocycline, piperacillin/tazobactam constant 4, polymyxin B, tigecycline, trimethoprim/sulfamethoxazole | [36] |
| Denmark | Pig carcass | Ampicillin, chloramphenicol, gentamicin, streptomycin, trimethoprim | [37] |
| Egypt | Raw dromedary camel milk | cefoxitin, erythromycin, novobiocin, piperacillin, rifampicin, rifamycin, streptomycin | [38] |
| Ethiopia | Chicken, goat, and beef meat | Ampicillin, chloramphenicol, erythromycin, gentamycin, streptomycin, tetracyclines, trimethoprim/sulfamethoxazole | [39] |
| Germany | Retail chicken meat | Ampicillin, cephalosporin, ciprofloxacin, nalidixic acid, streptomycin, tetracyclines, trimethoprim | [40] |
| Italy | Pig carcass | Ampicillin, chloramphenicol, gentamicin, streptomycin, tetracyclines, trimethoprim | [37] |
| South Africa | Raw meat | Ampicillin, ceftazidime, streptomycin, sulphafurazole, tetracyclines | [41] |
| Thailand | Broiler carcass and pig carcass | Ampicillin, cefpodoxime, ceftazidime, ciprofloxacin, gentamicin, sulfamethoxazole, tetracyclines, trimethoprim | [35] |
| United States | Dairy cattle | Azithromycin, ciprofloxacin, gamithromycin, tulathromycin | [42] |
| Vietnam | Retail raw foods (chicken, pork, fish, and shrimp) | Chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid, streptomycin, tetracyclines, trimethoprim/sulfamethoxazole | [43] |
| Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|
| Brazil | Fresh tilapia fillets | amoxicillin/clavulanic acid, chloramphenicol, sulfonamide, tetracyclines | [44] |
| Cambodia | Retail poultry | Amoxicillin, cefalotin, chloramphenicol, cotrimoxazole, nalidixic acid, streptomycin, sulfonamide, tetracyclines, ticarcillin | [45] |
| China Egypt Iran | Pork, chicken, duck, and fish | Ampicillin, streptomycin, tetracyclines | [46] |
| Chicken meat | Amoxicillin, ampicillin, erythromycin, nalidixic acid, oxytetracyclines, penicillin, sulfamethoxazole | [47] | |
| Chicken meat | Difloxacin, erythromycin, florfenicol, flumequine, lincomycin/spectinomycin, penicillin, tetracyclines, tiamulin, trimethoprim/sulfamethoxazole, tylosin | [48] | |
| Mexico | Ground beef | amoxicillin-clavulanic acid, carbenicillin, chloramphenicol, tetracyclines, trimethoprim-sulfamethoxazole | [49] |
| Thailand | Retail pork | Ampicillin, streptomycin, tetracyclines | [50] |
| United States | Retail meat | Amoxicillin-clavulanate, ampicillin, cefoxitin, ceftiofur, ceftriaxone, tetracyclines, gentamicin, streptomycin | [51] |
| Species | Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|---|
| Staphylococcus aureus | Brazil | Cow milk | ampicillin, cefoxitin, ceftiofur, clindamycin, erythromycin, oxacillin, penicillin, streptomycin, teicoplanin | [52] |
| China | Raw cow milk | Clindamycin/norfloxacin, erythromycin, gentamicin, tetracyclines, | [53] | |
| Japan | Raw cow milk | Ampicillin, oxacillin, cefazolin, enrofloxacin, gentamicin, kanamycin | [54] | |
| South Africa | Raw meat | Erythromycin, oxacillin/cefoxitin, penicillin, tetracyclines | [41] | |
| Thailand | Fresh pork | Ampicillin, tetracyclines, vancomycin | [55] | |
| United States | Pork, beef, turkey, and chicken | Clindamycin, dalfopristin/quinupristin, erythromycin, gentamicin, levofloxacin, mupirocin, oxacillin, penicillin, tetracyclines | [56] | |
| Methicillin-resistant S. aureus (MRSA) | Brazil | Cow milk | Ampicillin, erythromycin, oxacillin, penicillin, tetracyclines | [57] |
| China | Bovine milk | Amoxicillin, ampicillin, cefoxitin, ceftiofur, cefuroxime, ciprofloxacin, clarithromycin, clindamycin, penicillin, sulfadiazine sodium | [58] | |
| Denmark | Retail food products (chicken, turkey, and pork) | Macrolides, penicillin, tetracyclines | [59] | |
| Egypt | Retail chicken | Amikacin, amoxicillin, ampicillin, chloramphenicol, ciprofloxacin, cloxacillin, erythromycin, gentamicin, netilmicin, penicillin, rifampicin, streptomycin, sulfamethoxazole-trimethoprim, tetracyclines, vancomycin | [60] | |
| Iran | Raw meat (beef, sheep, and goat) | Amoxicillin-clavulanic acid, ampicillin, azithromycin, ceftriaxone, clindamycin, cotrimoxazole, erythromycin, gatifloxacin, lincomycin, minocycline, oxacillin, penicillin G, tetracyclines | [61] | |
| United States | Pork, beef, turkey, and chicken | Cefoxitin, clindamycin, dalfopristin/quinupristin, erythromycin, gentamicin, levofloxacin, oxacillin, penicillin, tetracyclines | [56] | |
| S. aureus and MRSA | China | Retail yak butter | amoxicillin/clavulanic acid, ampicillin, cefoperazone, cefoxitin, erythromycin, gentamicin, oxacillin, penicillin, sulfamethoxazole, tetracycliness, trimethoprim | [62] |
| Species | Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|---|
| Listeria spp. | Iran | Raw milk and traditional dairy products | Amoxicillin/clavulanic acid, chloramphenicol, penicillin, tetracyclines | [63] |
| Spain | Meat and dairy products | Ciprofloxacin, clindamycin, tetracyclines | [64] | |
| Listeria monocytogenes | China | Pork, fish, sheep casing, chicken, and beef | Chloramphenicol, clindamycin, oxacillin, tetracyclines | [65] |
| Indonesia | Chicken carcass | Ampicillin, erythromycin, penicillin | [66] | |
| Japan | Chicken meat | Cefoxitin, clindamycin, flomoxef, fosfomycin, linezolid, oxacillin | [67] | |
| Poland | Ready-to-eat food (heat-treated sausages and delicatessen), raw meat, raw sausages, and seafood (Fish and shrimp). | Ceftriaxone, ciprofloxacin, clindamycin, gatifloxacin, gentamycin, linezolid, oxacillin, tetracyclines | [68] | |
| Romania | Ready-to-eat food (sausages and ham), minced pork, and cheeses | Benzylpenicillin, ciprofloxacin, clindamycin, fosfomycin, fusidic acid, imipenem, oxacillin, rifampin, tetracyclines, trimethoprim-sulfamethoxazole | [69] | |
| Turkey | Chicken meat and beef | Ampicillin, ceftriaxone, clindamycin, fusidic acid, penicillin | [70] |
| Family | Latin Name | Part Used | Extraction Method | Location | Main Constituents | Target Bacteria | Doses | MIC | References |
|---|---|---|---|---|---|---|---|---|---|
| Apiaceae | Carum carvi L. | Seeds | Hydro-distillation | Kelibia | γ-terpinene (31.03%), β-pinene (18.77%), p-cymene (17.16) | E. coli, S. aureus, S. Typhimurium, Listeria monocytogenes | - | 0.469 mg/mL (E. coli, L. monocytogenes), 0.117 mg/mL (S. aureus), 0.234 mg/mL (S. Typhimurium) | [73] |
| Coriandrum sativum L. | Seeds | Hydro-distillation | Kelibia | Linalool (76.41), γ-terpinene (5.35%), α-pinene (4.44%) | E. coli, S. aureus, S. Typhimurium, L. monocytogenes | - | 0.938 mg/mL (E. coli, L. monocytogenes, S. Typhimurium), 0.234 mg/mL (S. aureus) | [73] | |
| Foeniculum vulgare Mill. | Seeds | Hydro-distillation | India | trans-anethole (50.4%), methyl chavicol (22.4%), limonene (11.4%) | E. coli, S. Typhimurium | 0.0075–2.0% (v/v) | 0.062% (E. coli), 0.031% (S. Typhimurium) (v/v) | [74] | |
| Asteraceae | Achillea millefolium L. | Inflorescence, leaves, whole aerial parts | Hydro-distillation | India | Borneol (4.7–24.9%), sabinene (4.0–38.9%), germacrene D (1.1–46.6%) | S. aureus, S. epidermidis, Klebsiella pneumoniae | - | 125–500 μg/mL | [75] |
| Helichrysum italicum (Roth) G. Don | Inflo-rescence | Hydro-distillation | Central Europe | Neryl acetate (16.38%), nerol (15.73%), geraniol (6.32%) | E. coli, S. aureus, Pseudomonas aeruginosa | - | 64 mg/mL (E. coli, P. aeruginosa), 1 mg/mL (S. aureus) | [76] | |
| Helichrysum microphyllum subsp. tyrrhenicum | - | Hydro-distillation | Iglesias | γ-curcumene (28.94%), linalool (14.21%), 5-eudesmen-11-ol (9.81%) | E. coli, S. aureus, P. aeruginosa | 0.063–4 mg/mL | >4 mg/mL (E. coli, P. aeruginosa), 2 mg/mL (S. aureus) | [77] | |
| Lamiaceae | Origanum vulgare L. spp. | O. vulgare L. ssp. virens | n-Hexane hydro-distillation | Southern Italy | Carvacrol (63.8%), γ-terpinene (7.4%), p-cymene (6.7%) | E. coli, S. aureus, S. Typhi | 0.8–100 μg/mL | 50 μg/mL (E. coli, S. aureus), 100 μg/mL (S. Typhi) | [78] |
| Rosmarinus officinalis L. | Air-dried leaves | Steam distillation | Taizhou, Zhejiang | 1,8-Cineole (26.54%), α-pinene (20.14%), camphor (12.88%), camphene (11.88%) | S. aureus, S. epidermidis, Bacillus subtilis | 0.2–4% (v/v) | 0.03–1.0% (v/v) | [79] | |
| Thymus vulgaris | Dried leaves | Hydro-distillation | North Yemen | Thymol (51.34%), p-cymene (18.35%), caryophyllene (4.26%), α-pinene (2.95%) | E. coli, S. aureus, B. subtilis, Mycobacterium smegmatis | 0.01–30 mg/mL | 0.075–1.1 mg/mL | [80] | |
| Mentha pulegium L. | Air-dried leaves | Steam distillation | Algerian | Pulegone (70.66%), neo-menthol (11.21%), menthone (2.63%) | E. coli, S. aureus, B. subtilis | 0.3–20 μL/mL | 1.25–10 μL/mL | [81] | |
| Ocimum basilicum L. | Leaves | Steam distillation | Ponta Grossa, Brazil | Linalool (55.2%), 1,8-cineole (8.8%), α-trans-bergamotene (7.0%), eugenol (3.2%) | S. aureus | 2–1024 μg/mL | 1024 μg/mL | [82] | |
| Lavandula x intermedia (lavandin) ‘Grosso’ | Flowers, stems | Steam distillation | Lazio Region, Italy | Linalool (35.8%), 1,8-cineole (19.8%), α-pinene (8.7%) | E. coli, B. cereus | - | 1.87% (E. coli), 0.94% (B. cereus) (v/v) | [83] | |
| Chenopodium ambrosioides L. | Leaves | Hydro-distillation | Crato, Brazil | α-terpinene (40.73%), p-cymene (21.81%), trans-ascaridol (12.48%) | S. aureus | 0.5–1024 μg/mL | ≥1024 μg/mL | [84] | |
| Lauraceae | Cinnamomum cassia Blume | - | Hydro-distillation | China | Cinnamaldehyde (85.06%) | E. coli, S. aureus, P. aeruginosa, Proteus vulgaris, Enterobacter aerogenes, Vibrio parahaemolyticus, V. cholerae | - | 75–600 μg/mL | [85] |
| Cinnamomum camphora var. linaloofera Fujita | - | - | Guangzhou | Linalool (69.94%), camphor (10.90%), nerolidol (10.92%), safrole (8.24%) | E. coli | - | 0.2 μL/mL | [86] | |
| Litsea cubeba (Lour.) Pers. | - | - | Caussols plateau, France | β-Citral (39.25%), α-citral (30.90%), limonene (8.28%), trans-verbenol (4.18%) | MRSA | 0.125–4 mg/mL | 0.5 mg/mL | [87] | |
| Myrtaceae | Eucalyptus globulus L. | Aerial parts | Hydro-distillation | Takelsa | p-cymene (12.58–37.82%), α-pinene (10.41–13.39%), 1,8-cineole (7.71–13.23%), γ-terpinene (2.94–10.57%) | S. aureus, MRSA, B. cereus | - | 1–4 mg/mL | [88] |
| Syzygium aromaticum | Fresh leaves | Steam distillation | Nitra, Slovakia | Eugenol (82.4%), (E)-caryophyllene (14.0%), α-humulene (1.8%) | S. aureus | 0.2–400 μL/mL | 93.35 μL/mL | [89] | |
| Poaceae | Cymbopogon nardus | Leaves | Cleavenge hydro-distillation | Ceara’, Brazil | Geraniol (33.88%), citronellal (27.55%), citronellol (14.40%), carvone (10.06%) | S. aureus, E. coli | 0.125–8 mg/mL | 0.5 mg/mL (S. aureus), >8 mg/mL (E. coli) | [90] |
| Rutaceae | Citrus limon L. Burm. | Peels | - | Sichuan Province | Limonene (48.48%), β-terpinene (17.08%), 4-carene (8.46%) | S. mutans | 2.25–9 mg/mL | 4.5 mg/mL | [91] |
| Zingiberaceae | Alpinia pahangensis Ridl. | Rhizomes | Hydro-distillation | Pahang, Peninsular Malaysia | γ-selinene (11.60%), β-pinene (10.87%), (E,E)-farnesyl acetate (8.65%), α-terpineol (6.38%) | S. aureus | 0.039–5 mg/mL | <0.31 mg/mL | [92] |
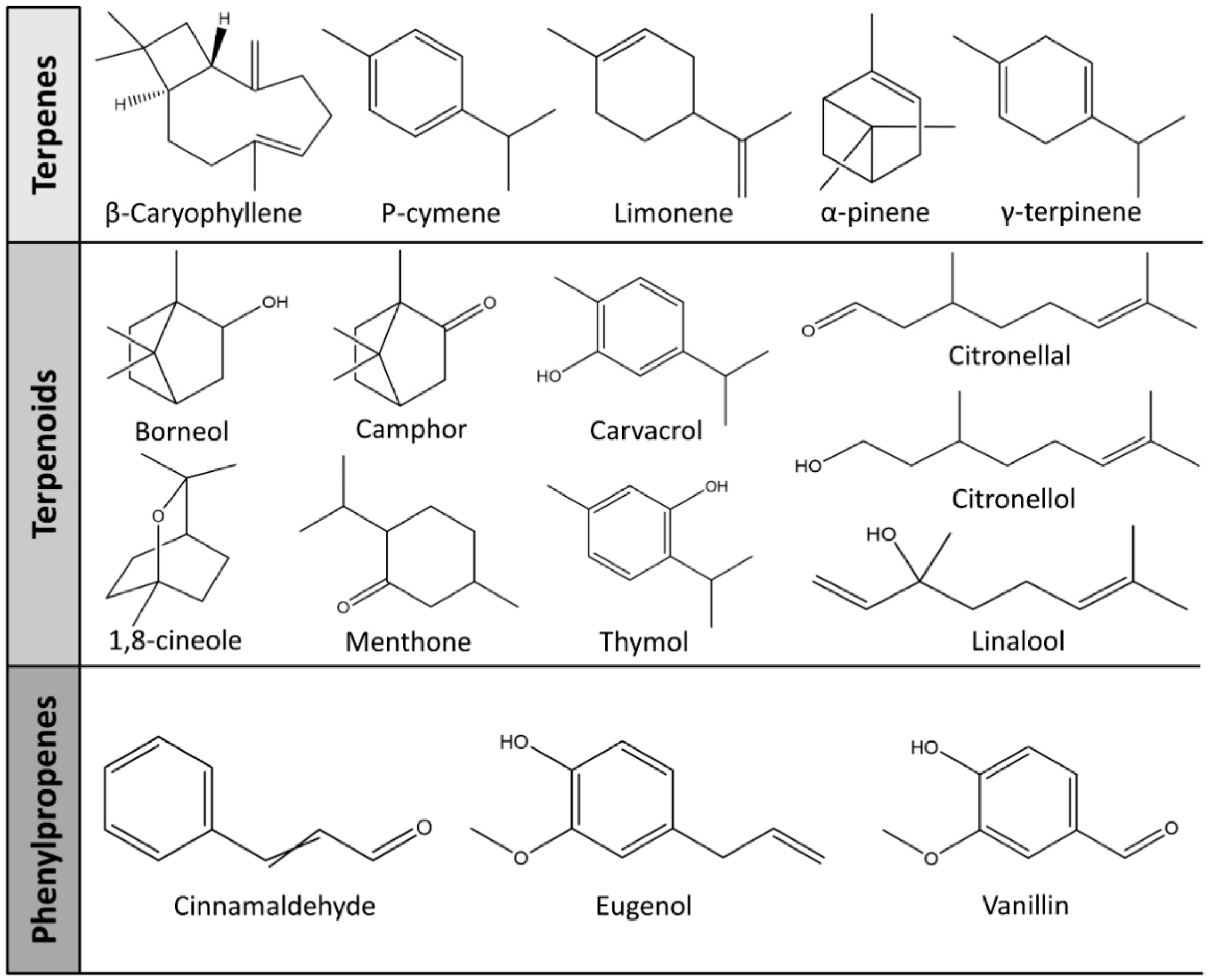
| Item | Individual Compounds | Chemical Structures | Target Bacteria | Doses | MIC | References |
|---|---|---|---|---|---|---|
| Terpenes | β-Caryophyllene |  | E. coli, S. aureus | 0.1–4 mg/mL | >4 mg/mL | [93] |
| Limonene |  | E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli, S. aureus, B. cereus), 0.06 mg/mL (S. Typhimurium) | [94] | |
| Terpenoids | Borneol | 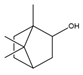 | E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli), 0.03 mg/mL (S. aureus), 0.12 mg/mL (B. cereus, S. Typhimurium) | [94] |
| Camphor |  | E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli, S. Typhimurium, B. cereus), 0.015 mg/mL (S. aureus) | [94] | |
| Carvacrol | 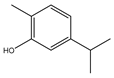 | E. coli, MRSA, S. mutans, Aggregatibacter actinomycetemcomitans | - | 0.4 mg/mL (E. coli, MRSA, S. mutans), 0.2 mg/mL (A. actinomycetemcomitans) | [95] | |
| E. coli, Salmonella spp., Clostridium perfringens | 0.075–2 mg/mL | >0.6 mg/mL | [96] | |||
| S. aureus | 0.05–3.2 mg/mL | >0.4 mg/mL | [97] | |||
| Citral |  | E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.06 mg/mL (E. coli, S. aureus, B. cereus), 0.07 mg/mL (S. Typhimurium) | [94] | |
| Citronellal | 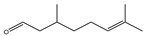 | E. coli, S. aureus | - | 0.3 mg/mL (E. coli), 0.4 mg/mL (S. aureus) | [98] | |
| Citronellol | 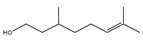 | E. coli, S. aureus | - | 0.005 mg/mL (E. coli), 0.375 mg/mL (S. aureus) | [98] | |
| Farnesol | 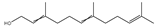 | Cutibacterium acnes | 0.004–0.576 μmol/mL | 0.14 μmol/mL | [99] | |
| trans-Geraniol | 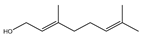 | E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.06 mg/mL (E. coli), 0.03 mg/mL (S. aureus, S. Typhimurium), 0.07 mg/mL (B. cereus) | [94] | |
| Linalool | 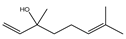 | E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL | [94] | |
| Menthone |  | K. pneumoniae | - | 224 mg/mL | [100] | |
| Nerolidol |  | S. aureus, K. pneumonia, P. aeruginosa | - | 2 mg/mL (S. aureus), 0.5 mg/mL (K. pneumonia, P. aeruginosa) | [101] | |
| Thymol |  | E. coli, MRSA, A. actinomycetemcomitans, S. mutans | - | 0.2 mg/mL (E. coli, MRSA, S. mutans), 0.1 mg/mL (A. actinomycetemcomitans) | [95] | |
| E. coli, C. perfringens, Salmonella spp. | 0.075–2 mg/mL | >1.2 μL/mL | [96] | |||
| S. aureus | 0.05–3.2 mg/mL | >0.8 mg/mL | [97] | |||
| Phenylpropanoids | Cinnamaldehyde |  | E. coli, S. aureus, B. cereus, Yersinia enterocolitica | - | 5 mg/mL (E. coli), 1.875 mg/mL (S. aureus), 2 mg/mL (B. cereus), 5 mg/mL (Yersinia enterocolitica) | [102] |
| E. coli, C. perfringens, Salmonella spp. | 0.075–2 mg/mL | >0.6 μL/mL (E. coli, Salmonella spp.), >0.3 μL/mL (C. perfringens) | [96] | |||
| Eugenol |  | E. coli, S. aureus | 0.1–4 mg/mL | 0.4 mg/mL (E. coli), 1.3 mg/mL (S. aureus) | [93] | |
| Campylobacter spp. | - | 0.5 mg/mL | [103] |
| Species | Resistance Genes and Types of Antibiotics or Antimicrobial Groups | References |
|---|---|---|
| E. coli | blaTEM, blaOXA-1: ampicillin, cefotaxime; cat1, cat2, cmlA: chloramphenicol; sul1, sul2, folP: sulfonamides; tet(A), tet(B): tetracyclines; aphA1, aphA2: kanamycin; aadA1: streptomycin; aac(3)-IV: gentamicin; gyrA (Ser83Leu, Asp87Asn), gyrB (Asp426Asn), parC (Ser80Ile), parE (Leu445His): quinolone; pmrA (Arg81His, Glu106Ala), pmrB (Gly206Arg, Tyr222His): colistin; rpoB (Ile572Phe): rifamycin; 23S: macrolides; 16S rrsB: gentamicin, spectinomycin, tetracyclines; 16S rrsH: spectinomycin | [31,35,38,133,134] |
| Salmonella spp. | blaTEM-1, blaTEM-117, blaTEM-135, blaCTX-M-9, blaCTX-M55, blaCYM-2: β-lactams; gyrA (Ser83Tyr, Asp87Asn), gyrB (Tyr420Cys), parC (Ser80Arg), parE (Ser458Pro): quinolone; pmrB (Val164Met, Arg92Pro): colistin; sul1, sul2, sul3: sulfonamides; tet(A), tet(B), tet(C), tet(G), tet(M), tet(R): tetracyclines; dfrA1, dfrA12, dfrA14: trimethoprim; floR, cmlA1: chloramphenicol; aac(6′)-I, strA, strB, aadA (ant(3”)Ia), aac3-VIa, aph(3′)Ib, aphA (aph(3′)IIa), aac3-IId, aadB (ant(2”)Ia), aac-IVa, aph(4)Ia: aminoglycosides; fosA2: fosfomycin; mph(A), ere(A), mef(B): macrolides; arr2: rifampicin | [135,136,137,138] |
| Staphylococcus spp. | blaZ, mecA, mecC: β-lactams; erm(A), erm(C): erythromycin, clindamycin; mphC, msrA: erythromycin; aacA-aphD: gentamicin; aadD: tobramycin; fusB, fusC: fusidic acid; tet(K), tet(L), tet(M): tetracyclines; fexA: chloramphenicol; fosB: fosfomycin; inuA: lincomycin; vanA: vancomycin; msr(A), mph(C): macrolides, lincosamides, streptogramins | [60,139,140,141] |
| Listeria spp. | blaTEM, blaCTX-M-9: β-lactams; tet(A), tet(B), tet(C), tet(M), tet(O), tet(S): tetracyclines; strA, aadA, aadB, ant6: aminoglycosides; dfrD: trimethoprim; sul1, sul2: sulfonamides; erm(B): macrolides; fos(X), vga(D): lincosamides | [142,143,144] |
| Essential Oils or Components | Emulsifier/Carrier System | Target Bacteria | Antibacterial Effects | References |
|---|---|---|---|---|
| Carvacrol | Polylactic acid nanoemulsions | E. coli, MRSA, Acinetobacter baumannii | Biofilm eradication | [180] |
| Cinnamon oil | Liposomes (average particle size: 144.3 nm) | MRSA | Biofilm eradication | [181] |
| Cinnamon oil, eucalyptus oil, orange oil | Mesoporous silica nanoparticles | S. aureus, E. coli | Inhibition of biofilm formation | [175] |
| Citral | Nanoemulsions (tween 80) | L. monocytogenes | Inhibition of biofilm formation | [182] |
| Eucalyptus oil | Silica nanoparticles | E. coli | Biofilm eradication | [164] |
| Eugenol | Nanoemulsions (tween 80, medium-chain triglyceride) | P. aeruginosa | Inhibition of biofilm formation | [45] |
| Lemongrass oil | Nanoemulsions (tween 80) | Enterococcus faecalis | Inhibition of biofilm formation | [183] |
| Limonene | Nanoemulsions (tween 80, propylene glycol) | MRSA | Reduction in biofilm persistence | [184] |
| Mandarin oil | Chitosan nanoparticles | S. aureus, E. coli | Inhibition of biofilm formation | [185] |
| Oregano oil | Biological silver nanoparticles | S. aureus | Decrease in cell density and exopolysaccharide matrix | [186] |
| Peppermint oil, cinnamaldehyde | Silica nanocapsules | E. coli, S. aureus, P. aeruginosa | Biofilm eradication | [187] |
| Tea tree oil | Nanostructured lipid carriers (average particle size: 166 nm) | P. aeruginosa | Decrease in adhesion and inhibition of biofilm formation | [188] |
| Thyme oil | Nanoarchaeosomes (made by soybean phosphatidylcholine, total polar archaeolipids and polysorbate 80), nanoliposomes (made by soybean phosphatidylcholine and polysorbate 80) | S. aureus | Biofilm eradication, inhibition of biofilm formation | [189] |
| Thyme oil, thymol | Chitosan nanoemulsions | S. aureus, E. coli | Inhibition of biofilm formation | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Gao, F.; Ge, J.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Shi, L. Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review. Antibiotics 2022, 11, 1673. https://doi.org/10.3390/antibiotics11111673
Zhang L, Gao F, Ge J, Li H, Xia F, Bai H, Piao X, Shi L. Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review. Antibiotics. 2022; 11(11):1673. https://doi.org/10.3390/antibiotics11111673
Chicago/Turabian StyleZhang, Lianhua, Fei Gao, Junwei Ge, Hui Li, Fei Xia, Hongtong Bai, Xiangshu Piao, and Lei Shi. 2022. "Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review" Antibiotics 11, no. 11: 1673. https://doi.org/10.3390/antibiotics11111673
APA StyleZhang, L., Gao, F., Ge, J., Li, H., Xia, F., Bai, H., Piao, X., & Shi, L. (2022). Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review. Antibiotics, 11(11), 1673. https://doi.org/10.3390/antibiotics11111673




