Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups?
Abstract
:1. Introduction
2. Results
2.1. OCT’s Antimicrobial Activity Is Not Altered in E. coli Mutants Deficient in LPS, PG and CL
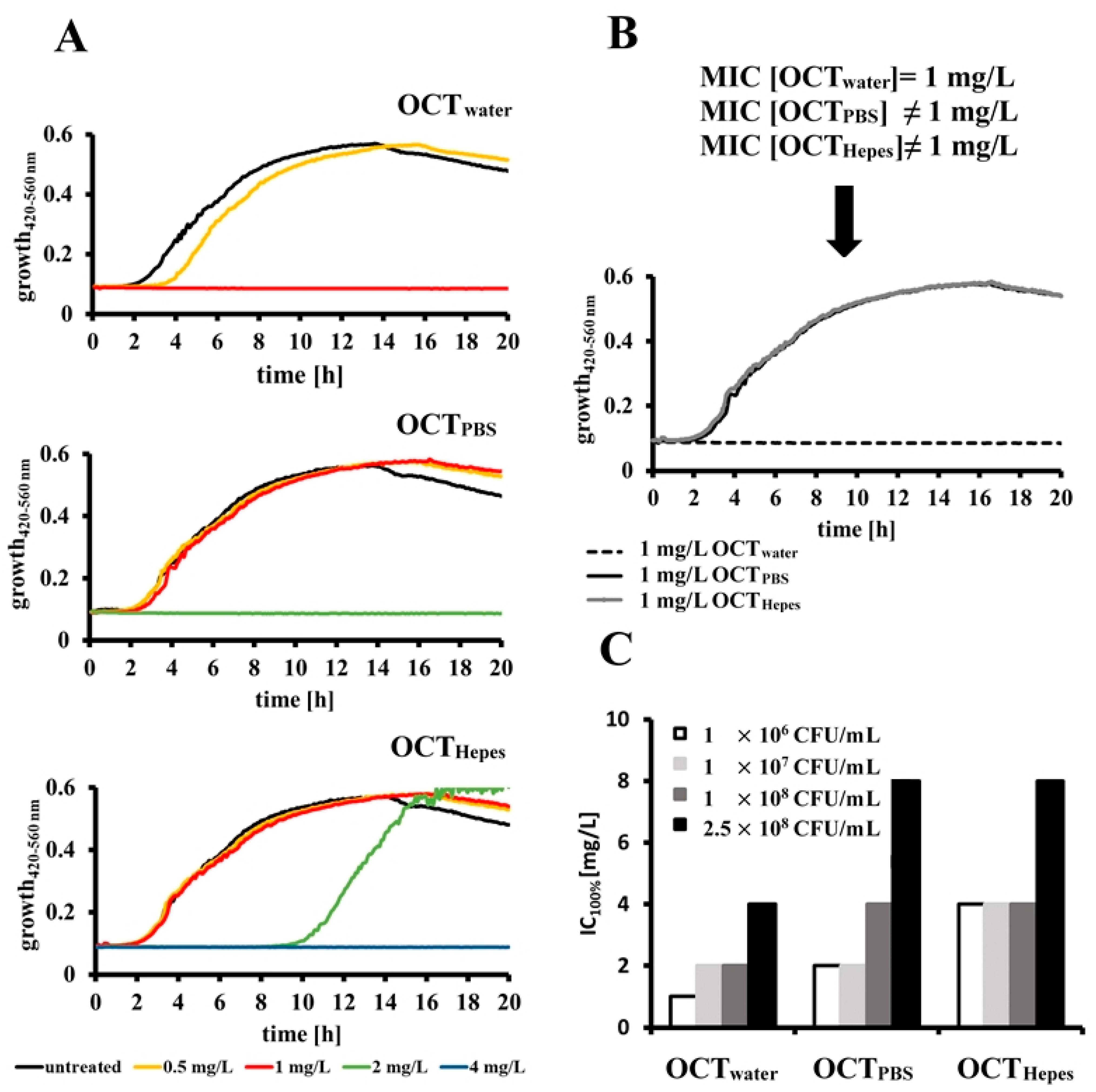
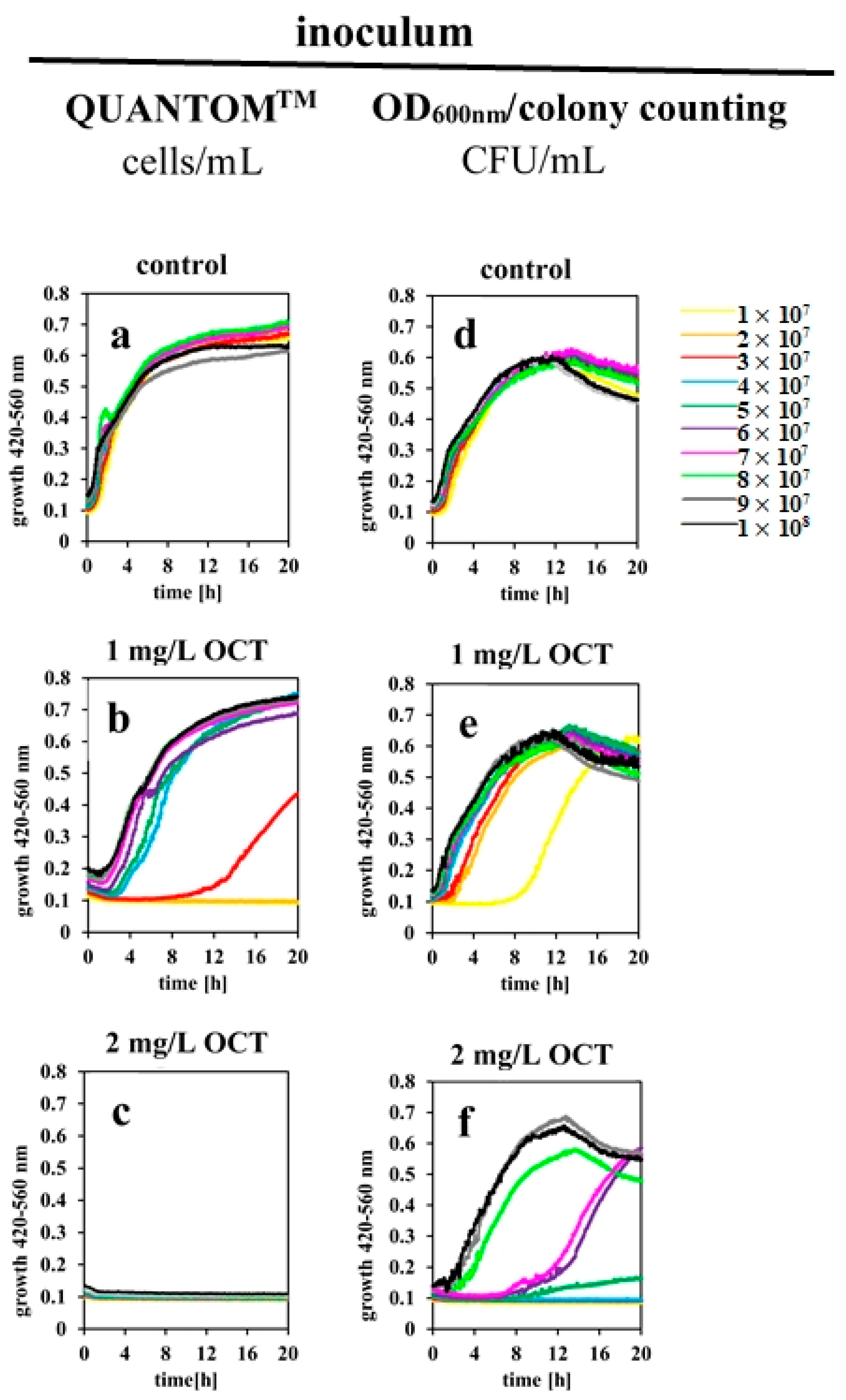
2.2. OCT’s Antimicrobial Activity Is Influenced by the Buffer System and Bacterial Concentration in Relation to a Given Dose of Antiseptic
2.3. Determined IC100% Values Are Influenced by Different Methods for Estimating Bacterial Cell Counts
2.4. Low Numbers of OCT Molecules Are Taken Up by Bacteria
3. Discussion
4. Materials and Methods
4.1. Preparation of Reagents
4.1.1. Preparation of OCT Solutions
4.1.2. Preparation of Bacterial Cultures
4.2. Estimation of Bacterial Cell Concentration
4.2.1. CFU/mL by Literature Obtained Values
4.2.2. CFU/mL by Counting Colonies
4.2.3. Cells/mL Using QUANTOM™ Total Cell Staining
4.3. Assessment of Bacterial Survival in the Presence of OCT by Micro Dilution Assay
4.4. Assessment of OCT’s Adsorption on the Bacterial Surface
4.4.1. Calculation of OCT Molecules Bound on Cells as Measured by Optical Density of Bacterial Culture at 420–560 nm after OCT Treatment
4.4.2. Calculation of OCT Molecules Bound on Cells as Measured by Absorption of OCT at 281 nm after Incubation of OCT with Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assadian, O. Octenidine dihydrochloride: Chemical characteristics and antimicrobial properties. J. Wound Care 2016, 25, S3–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koburger, T.; Hubner, N.O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conceição, T.; de Lencastre, H.; Aires-De-Sousa, M. Efficacy of octenidine against antibiotic-resistant Staphylococcus aureuse epidemic clones. J. Antimicrob. Chemother. 2016, 71, 2991–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Marin, R.; Aires-de-Sousa, M.; Nordmann, P.; Kieffer, N.; Poirel, L. Antimicrobial activity of octenidine against multidrug-resistant Gram-negative pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2379–2383. [Google Scholar] [CrossRef] [Green Version]
- Conceição, T.; de Lencastre, H.; Aires-De-Sousa, M. Bactericidal activity of octenidine against Staphylococcus aureus harbouring genes encoding multidrug resistance efflux pumps. J. Glob. Antimicrob. Resist. 2019, 16, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Pitten, F.-A.; Werner, H.-P.; Kramer, A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J. Hosp. Infect. 2003, 55, 108–115. [Google Scholar] [CrossRef]
- Kakar, A.; Sastré-Velásquez, L.E.; Hess, M.; Galgóczy, L.; Papp, C.; Holzknecht, J.; Romanelli, A.; Váradi, G.; Malanovic, N.; Marx, F. The Membrane Activity of the Amphibian Temporin B Peptide Analog TB_KKG6K Sheds Light on the Mechanism That Kills Candida albicans. mSphere 2022, 16, e0029022. [Google Scholar] [CrossRef] [PubMed]
- Spettel, K.; Bumberger, D.; Camp, I.; Kriz, R.; Willinger, B. Efficacy of octenidine against emerging echinocandin-, azole- and multidrug-resistant Candida albicans and Candida glabrata. J. Glob. Antimicrob. Resist. 2022, 29, 23–28. [Google Scholar] [CrossRef]
- Malanovic, N.; Buttress, J.A.; Vejzovic, D.; Ön, A.; Piller, P.; Kolb, D.; Lohner, K.; Strahl, H. Disruption of the Cytoplasmic Membrane Structure and Barrier Function Underlies the Potent Antiseptic Activity of Octenidine in Gram-Positive Bacteria. Appl. Environ. Microbiol. 2022, 88, e0018022. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel insights into the detailed killing mechanism of Gram-negative bacteria at a cellular and molecular level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef]
- Shepherd, M.; Moore, G.; Wand, M.; Sutton, J.M.; Bock, L. Pseudomonas aeruginosa adapts to octenidine in the laboratory and a simulated clinical setting, leading to increased tolerance to chlorhexidine and other biocides. J. Hosp. Infect. 2018, 100, e23–e29. [Google Scholar] [CrossRef]
- Wand, M.E.; Jamshidi, S.; Bock, L.; Rahman, K.M.; Sutton, J.M. SmvA is an important efflux pump for cationic biocides in Klebsiella pneumoniae and other Enterobacteriaceae. Sci. Rep. 2019, 9, 1344. [Google Scholar] [CrossRef] [Green Version]
- Bock, L.J.; Ferguson, P.M.; Clarke, M.; Pumpitakkul, V.; Wand, M.E.; Fady, P.-E.; Allison, L.; Fleck, R.A.; Shepherd, M.J.; Mason, A.J.; et al. Pseudomonas aeruginosa adapts to octenidine via a combination of efflux and membrane remodelling. Commun. Biol. 2021, 4, 1058. [Google Scholar] [CrossRef] [PubMed]
- Htun, H.L.; Hon, P.Y.; Holden, M.T.G.; Ang, B.; Chow, A. Chlorhexidine and octenidine use, carriage of qac genes, and reduced antiseptic susceptibility in methicillin-resistant Staphylococcus aureus isolates from a healthcare network. Clin. Microbiol. Infect. 2019, 25, 1154.e1–1154.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescat, M.; Magnan, M.; Kenmoe, S.; Nordmann, P.; Poirel, L. Co-Lateral Effect of Octenidine, Chlorhexidine and Colistin Selective Pressures on Four Enterobacterial Species: A Comparative Genomic Analysis. Antibiotics 2021, 11, 50. [Google Scholar] [CrossRef]
- Malanovic, N.; Marx, L.; Blondelle, S.E.; Pabst, G.; Semeraro, E.F. Experimental concepts for linking the biological activities of antimicrobial peptides to their molecular modes of action. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183275. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.J.; Hind, C.K.; Sutton, J.M.; Wand, M.E. Growth media and assay plate material can impact on the effectiveness of cationic biocides and antibiotics against different bacterial species. Lett. Appl. Microbiol. 2018, 66, 368–377. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G.; Monner, D.A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J. Bacteriol. 1975, 121, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G.; Eriksson-Grennberg, K.G.; Normark, S.; Matsson, E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet. Res. 1968, 12, 169–185. [Google Scholar] [CrossRef]
- Grundström, T.; Jaurin, B.; Edlund, T.; Normark, S. Physical mapping and expression of hybrid plasmids carrying chromosomal beta-lactamase genes of Escherichia coli K-12. J. Bacteriol. 1980, 143, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnaud, S.; Spiller, C.; Moriarty, L.C.; Patel, A.; Gant, V.; Odell, E.W.; Evans, R.W. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. Fems Microbiol. Lett. 2004, 233, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Gunne, H.; Oderberg, J.; Kogner, P.; Bomann, H.G.; Gudmundsson, G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 2005, 92, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havekes, L.; Tommassen, J.; Hoekstra, W.; Lugtenberg, B. Isolation and characterization of Escherichia coli K-12 F- mutants defective in conjugation with an I-type donor. J. Bacteriol. 1977, 129, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [Green Version]
- Rathmann, C.; Schlösser, A.S.; Schiller, J.; Bogdanov, M.; Brüser, T. Tat transport in Escherichia coli requires zwitterionic phosphatidylethanolamine but no specific negatively charged phospholipid. FEBS Lett. 2017, 591, 2848–2858. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Rangarajan, N.; Weisshaar, J.C. Resistance of early stationary phase E. coli to membrane permeabilization by the antimicrobial peptide Cecropin A. Biochim. Biophys. Acta Biomembr. 2019, 1861, 182990. [Google Scholar] [CrossRef]
- Oliver, P.M.; Crooks, J.A.; Leidl, M.; Yoon, E.J.; Saghatelian, A.; Weibel, D.B. Localization of anionic phospholipids in Escherichia coli cells. J. Bacteriol. 2014, 196, 3386–3398. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.K.; Bogdanov, M.; Zhao, J.; Dowhan, W.; Raetz, C.R.H.; Guan, Z. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl. Acad. Sci. USA 2012, 109, 16504–16509. [Google Scholar] [CrossRef] [Green Version]
- Brook, I. Inoculum effect. Rev. Infect. Dis. 1989, 11, 361–368. [Google Scholar] [CrossRef]
- Loffredo, M.R.; Savini, F.; Bobone, S.; Casciaro, B.; Franzyk, H.; Mangoni, M.L.; Stella, L. Inoculum effect of antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2021, 118, e2014364118. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Luca, V.; Bocedi, A.; Massoud, R.; Park, Y.; Mangoni, M.L.; Stella, L. Cell-Density Dependence of Host-Defense Peptide Activity and Selectivity in the Presence of Host Cells. ACS Chem. Biol. 2017, 12, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Marx, L.; Semeraro, E.F.; Mandl, J.; Kremser, J.; Frewein, M.P.; Malanovic, N.; Lohner, K.; Pabst, G. Bridging the Antimicrobial Activity of Two Lactoferricin Derivatives in E. coli and Lipid-Only Membranes. Front. Med. Technol. 2021, 3, 778645. [Google Scholar] [CrossRef]
- Cantón, R.; Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. Fems Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183291. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Kantor, G.D.; Nishijima, M.; Newman, K.F. Cardiolipin accumulation in the inner and outer membranes of Escherichia coli mutants defective in phosphatidylserine synthetase. J. Bacteriol. 1979, 139, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef] [Green Version]
- van Rogers Katwyk, S.; Hoffman, S.J.; Mendelson, M.; Taljaard, M.; Grimshaw, J.M. Strengthening the science of addressing antimicrobial resistance: A framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res. Policy Syst. 2020, 18, 60. [Google Scholar] [CrossRef]
- Brill, F.; Goroncy-Bermes, P.; Sand, W. Influence of growth media on the sensitivity of Staphylococcus aureus and Pseudomonas aeruginosa to cationic biocides. Int. J. Hyg. Environ. Health 2006, 209, 89–95. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y.; Hatton, B.D. Drug self-assembly for synthesis of highly-loaded antimicrobial drug-silica particles. Sci. Rep. 2018, 8, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzycki, M.; Drabik, D.; Szostak-Paluch, K.; Hanus-Lorenz, B.; Kraszewski, S. Unraveling the mechanism of octenidine and chlorhexidine on membranes: Does electrostatics matter? Biophys. J. 2021, 120, 3392–3408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mohapatra, S.; Weisshaar, J.C. Rigidification of the Escherichia coli cytoplasm by the human antimicrobial peptide LL-37 revealed by superresolution fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Kaji, T.; Yano, Y.; Matsuzaki, K. In-Cell FRET Indicates Magainin Peptide Induced Permeabilization of Bacterial Cell Membranes at Lower Peptide-to-Lipid Ratios Relevant to Liposomal Studies. ACS Infect. Dis. 2021, 7, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, E.F.; Marx, L.; Mandl, J.; Letofsky-Papst, I.; Mayrhofer, C.; Frewein, M.P.K.; Scott, H.L.; Prévost, S.; Bergler, H.; Lohner, K.; et al. Lactoferricins impair the cytosolic membrane of Escherichia coli within a few seconds and accumulate inside the cell. Elife 2022, 11, e72850. [Google Scholar] [CrossRef]
- Roversi, D.; Luca, V.; Aureli, S.; Park, Y.; Mangoni, M.L.; Stella, L. How many antimicrobial peptide molecules kill a bacterium? The case of PMAP-23. ACS Chem. Biol. 2014, 9, 2003–2007. [Google Scholar] [CrossRef] [Green Version]
- Jomehzadeh, N.; Jahangirimehr, F.; Chegeni, S.A. Virulence-associated genes analysis of carbapenemase-producing Escherichia coli isolates. PLoS ONE 2022, 17, e0266787. [Google Scholar] [CrossRef]
- Jomehzadeh, N.; Ahmadi, K.; Bahmanshiri, M.A. Investigation of plasmid-mediated quinolone resistance genes among clinical isolates of Klebsiella pneumoniae in southwest Iran. J. Clin. Lab. Anal. 2022, 36, e24342. [Google Scholar] [CrossRef]
- Gao, Y.; Lecker, S.; Post, M.J.; Hietaranta, A.J.; Li, J.; Volk, R.; Li, M.; Sato, K.; Saluja, A.K.; Steer, M.L.; et al. Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha degradation by a naturally occurring antibacterial peptide. J. Clin. Investig. 2005, 106, 439–448. [Google Scholar] [CrossRef]

| E. coli Strain | Relevant Genotype | Defect | MIC [mg/L] | Source/Reference |
|---|---|---|---|---|
| ATCC 25992 | wt | 1 | LGC Standards GmbH, Germany | |
| BW25113 (K-12) | F- DE(araD-araB)567 lacZ4787(del)::rrnB-3 LAM- rph-1 DE(rhaD-rhaB)568 hsdR514 | LPS O-Antigen | 1 | [18] |
| d12 | F-, proA23, lac-28, tsx-81, trp-30, his-51, rpsL173(strR), ampCp-1 | LPS O-Antigen | 1 | [19,20,21] |
| d12f2 | F-, proA23, lac-28, tsx-81, trp-30, his-51, rpsL173(strR), rfa-31, rfa-1, ampCp-1 | LPS O-Antigen Outer core Inner core | 1 | [19,22,23,24] |
| MG1655 (K-12) | F-, λ-, ilvG, rfb-50, rph-1 | LPS O-Antigen | 1 | [25] |
| BKT12 | F-, λ-, ilvG, rfb-50, ΔclsB861, ΔclsC788::kan, ΔclsA856, IN(rrnD-rrnE)1, rph-1 | LPS O-Antigen CL | 1 | [26,27,28,29] |
| BKT29 | F-, λ-, ilvG, rfb-50, ΔaraBAD-714, rcsF1386::mini-Tn10cam, λ-, ΔclsB861, ΔymdB787::kan, ΔclsC888, ΔclsA856, lpp-2, Δ pgsA792, rph-1 | LPS O-Antigen CL, PG | 1 | [26,27,28,29] |
| Literature | Manual Colony Counting | QUANTOMTM | |
|---|---|---|---|
| OD600 nm | 1 | 1 | 1 |
| CFU/mL | 8 × 108 | 6.0 ± 1.6 × 108 | - |
| cells/mL | 8 × 108 | - | 3.9 ± 0.2 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejzovic, D.; Iftic, A.; Ön, A.; Semeraro, E.F.; Malanovic, N. Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups? Antibiotics 2022, 11, 1665. https://doi.org/10.3390/antibiotics11111665
Vejzovic D, Iftic A, Ön A, Semeraro EF, Malanovic N. Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups? Antibiotics. 2022; 11(11):1665. https://doi.org/10.3390/antibiotics11111665
Chicago/Turabian StyleVejzovic, Djenana, Azra Iftic, Ayse Ön, Enrico F. Semeraro, and Nermina Malanovic. 2022. "Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups?" Antibiotics 11, no. 11: 1665. https://doi.org/10.3390/antibiotics11111665
APA StyleVejzovic, D., Iftic, A., Ön, A., Semeraro, E. F., & Malanovic, N. (2022). Octenidine’s Efficacy: A Matter of Interpretation or the Influence of Experimental Setups? Antibiotics, 11(11), 1665. https://doi.org/10.3390/antibiotics11111665







