Empirical Antibiotic Therapy for Gram-Negative Bacilli Ventilator-Associated Pneumonia: Observational Study and Pharmacodynamic Assessment
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Microbiological Findings
2.3. Antibiotic Therapy
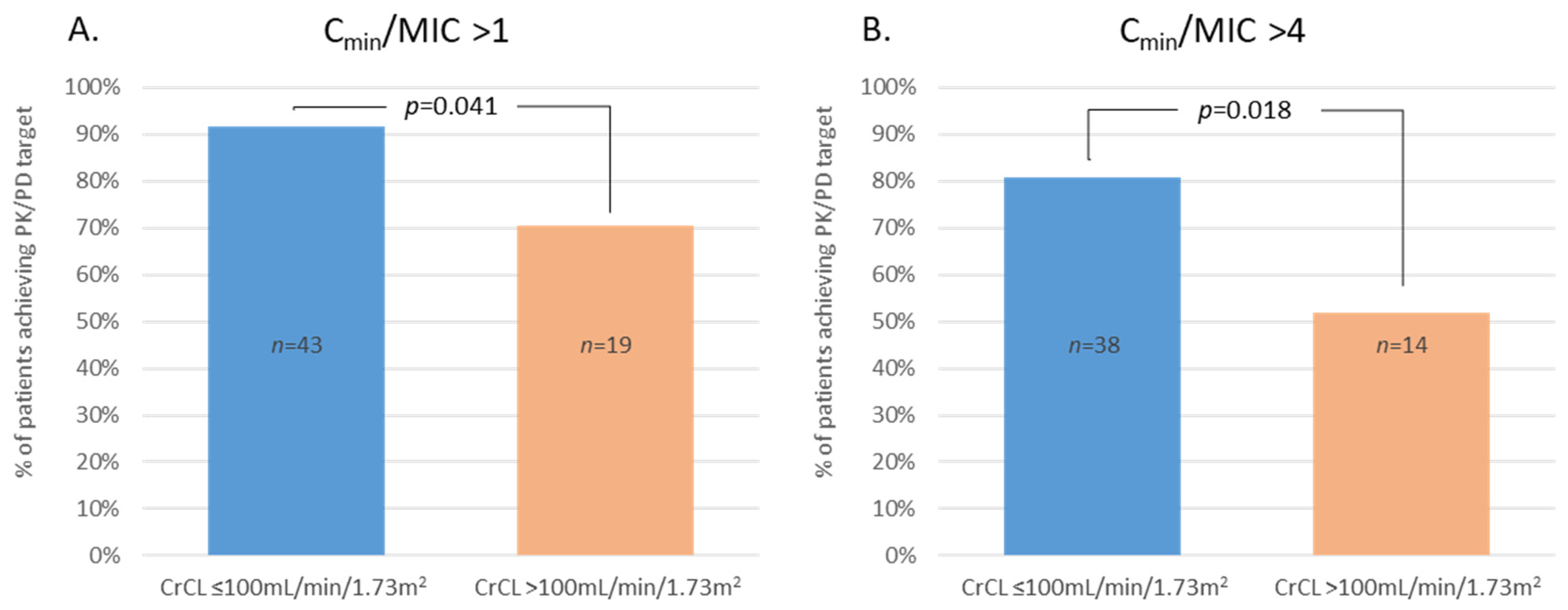
2.4. Pharmacological Parameters
2.5. β-Lactams Cmin Covariables
2.6. Microbiological Outcome
2.7. Clinical Outcome
3. Discussion
4. Materials and Methods
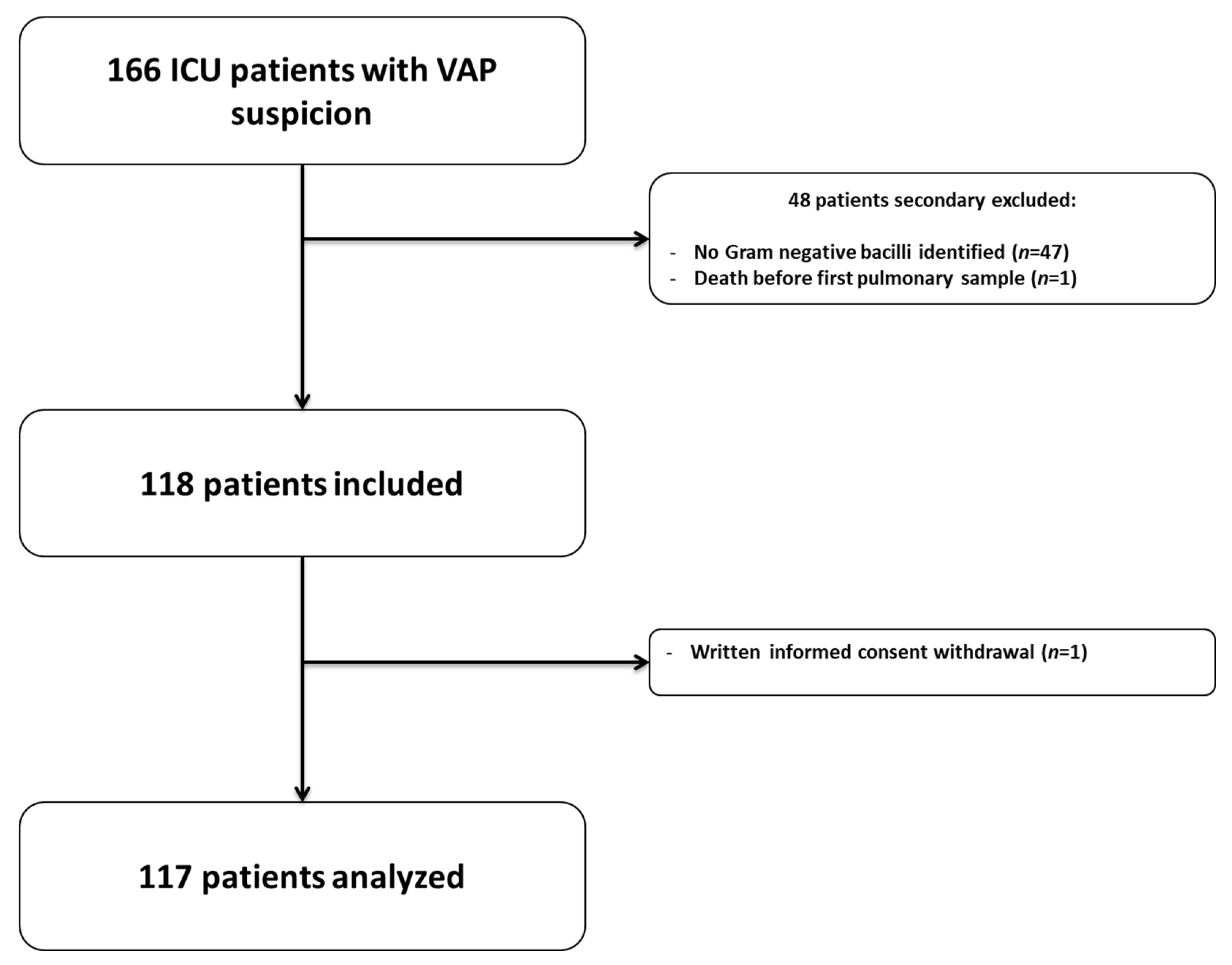
4.1. Patients’ Selection
4.2. Drug Administration
4.3. Data Collected
4.4. Assessment of Renal Function
4.5. Blood Sampling and Analytical Method
4.6. Microbiological Analysis
4.7. Pharmacodynamic Analysis
4.8. Outcome Assessment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 26, 1–10; quiz 11–12. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Brady, K.; Cotta, M.O.; Roberts, J.A. Therapeutic Drug Monitoring of Antibiotics: Defining the Therapeutic Range. Ther. Drug Monit. 2022, 44, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.D.; Lietman, P.S.; Smith, C.R. Clinical Response to Aminoglycoside Therapy: Importance of the Ratio of Peak Concentration to Minimal Inhibitory Concentration. J. Infect. Dis. 1987, 155, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kashuba, A.D.; Nafziger, A.N.; Drusano, G.L.; Bertino, J.S. Optimizing Aminoglycoside Therapy for Nosocomial Pneumonia Caused by Gram-Negative Bacteria. Antimicrob. Agents Chemother. 1999, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.; Nix, D.E.; Ballow, C.H.; Goss, T.F.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of Intravenous Ciprofloxacin in Seriously Ill Patients. Antimicrob. Agents Chemother. 1993, 37, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Cotta, M.O.; Roberts, J.A. Pharmacokinetics/Pharmacodynamics of β-Lactams and Therapeutic Drug Monitoring: From Theory to Practical Issues in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2019, 40, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.; Abdul-Aziz, M.H.; Roberts, J.A. Pharmacokinetic/Pharmacodynamics-Optimized Antimicrobial Therapy in Patients with Hospital-Acquired Pneumonia/Ventilator-Associated Pneumonia. Semin. Respir. Crit. Care Med. 2017, 38, 271–286. [Google Scholar] [CrossRef] [PubMed]
- de Montmollin, E.; Bouadma, L.; Gault, N.; Mourvillier, B.; Mariotte, E.; Chemam, S.; Massias, L.; Papy, E.; Tubach, F.; Wolff, M.; et al. Predictors of Insufficient Amikacin Peak Concentration in Critically Ill Patients Receiving a 25 Mg/Kg Total Body Weight Regimen. Intensive Care Med. 2014, 40, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current β-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-Lactam Concentrations in the Early Phase of Severe Sepsis and Septic Shock. Crit. Care Lond. Engl. 2010, 14, R126. [Google Scholar] [CrossRef]
- Gao, W.; Passarell, J.; Patel, Y.T.; Zhang, Z.; Lin, G.; Fiedler-Kelly, J.; Bruno, C.J.; Rhee, E.G.; De Anda, C.S.; Feng, H.-P. Exposure-Efficacy Analyses Support Optimal Dosing Regimens of Ceftolozane/Tazobactam in Participants with Hospital-Acquired/Ventilator-Associated Bacterial Pneumonia in ASPECT-NP. Antimicrob. Agents Chemother. 2022, 66, e01399-21. [Google Scholar] [CrossRef] [PubMed]
- Razzazzadeh, S.; Darazam, I.A.; Hajiesmaeili, M.; Salamzadeh, J.; Mahboubi, A.; Sadeghnezhad, E.; Sahraei, Z. Investigation of Pharmacokinetic and Clinical Outcomes of Various Meropenem Regimens in Patients with Ventilator-Associated Pneumonia and Augmented Renal Clearance. Eur. J. Clin. Pharmacol. 2022, 78, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J.; Chen, Y.; Wu, J.; Guo, B.; Wu, X.; Zhang, Y.; Wang, M.; Ya, R.; Huang, H. Combined PK/PD Index May Be a More Appropriate PK/PD Index for Cefoperazone/Sulbactam against Acinetobacter Baumannii in Patients with Hospital-Acquired Pneumonia. Antibiotics 2022, 11, 703. [Google Scholar] [CrossRef]
- Crandon, J.L.; Luyt, C.-E.; Aubry, A.; Chastre, J.; Nicolau, D.P. Pharmacodynamics of Carbapenems for the Treatment of Pseudomonas Aeruginosa Ventilator-Associated Pneumonia: Associations with Clinical Outcome and Recurrence. J. Antimicrob. Chemother. 2016, 71, 2534–2537. [Google Scholar] [CrossRef] [PubMed]
- Pajot, O.; Burdet, C.; Couffignal, C.; Massias, L.; Armand-Lefevre, L.; Foucrier, A.; Da Silva, D.; Lasocki, S.; Laouénan, C.; Mentec, H.; et al. Impact of Imipenem and Amikacin Pharmacokinetic/Pharmacodynamic Parameters on Microbiological Outcome of Gram-Negative Bacilli Ventilator-Associated Pneumonia. J. Antimicrob. Chemother. 2015, 70, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Touzw, D.J.; Horrevorts, A.M.; Vinks, A.A. Comparative Pharmacokinetics of the Carbapenems: Clinical Implications. Clin. Pharmacokinet. 2000, 39, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Carrette, S.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Does Consistent Piperacillin Dosing Result in Consistent Therapeutic Concentrations in Critically Ill Patients? A Longitudinal Study over an Entire Antibiotic Course. Int. J. Antimicrob. Agents 2014, 43, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early Therapeutic Monitoring of β-Lactams and Associated Therapy Outcomes in Critically Ill Patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients with Sepsis: A Randomized Controlled Trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Lluch, O.; Gerónimo-Pardo, M.; Peyró-García, R.; Lizán-García, M. Glomerular Hyperfiltration and Albuminuria in Critically Ill Patients. Anaesth. Intensive Care 2008, 36, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between Augmented Renal Clearance and Clinical Outcomes in Patients Receiving β-Lactam Antibiotic Therapy by Continuous or Intermittent Infusion: A Nested Cohort Study of the BLING-II Randomised, Placebo-Controlled, Clinical Trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Taccone, F.S.; Roberts, J.A.; Jacobs, F.; Cotton, F.; Wolff, F.; Creteur, J.; Vincent, J.-L.; Hites, M. β-Lactam Dosage Regimens in Septic Patients with Augmented Renal Clearance. Antimicrob. Agents Chemother. 2018, 62, e02534-17. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients: Association between Augmented Renal Clearance and Low Trough Drug Concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Besnard, T.; Carrié, C.; Petit, L.; Biais, M. Increased Dosing Regimens of Piperacillin-Tazobactam Are Needed to Avoid Subtherapeutic Exposure in Critically Ill Patients with Augmented Renal Clearance. Crit. Care 2019, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between Augmented Renal Clearance, Antibiotic Exposure and Clinical Outcome in Critically Ill Septic Patients Receiving High Doses of β-Lactams Administered by Continuous Infusion: A Prospective Observational Study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Casu, G.S.; Hites, M.; Jacobs, F.; Cotton, F.; Wolff, F.; Beumier, M.; De Backer, D.; Vincent, J.-L.; Taccone, F.S. Can Changes in Renal Function Predict Variations in β-Lactam Concentrations in Septic Patients? Int. J. Antimicrob. Agents 2013, 42, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Lipman, J.; Wallis, S.C.; Boots, R.J. Cefepime Versus Cefpirome: The Importance of Creatinine Clearance. Anesth. Analg. 2003, 97, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.; Kruger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are Standard Doses of Piperacillin Sufficient for Critically Ill Patients with Augmented Creatinine Clearance? Crit. Care 2015, 19, 28. [Google Scholar] [CrossRef]
- Falcone, M.; Menichetti, F.; Cattaneo, D.; Tiseo, G.; Baldelli, S.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Di Paolo, A.; Pai, M.P. Pragmatic Options for Dose Optimization of Ceftazidime/Avibactam with Aztreonam in Complex Patients. J. Antimicrob. Chemother. 2021, 76, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; McKinnon, P.S.; Akins, R.L.; Drusano, G.L.; Rybak, M.J. Pharmacokinetics and Pharmacodynamics of Cefepime in Patients with Various Degrees of Renal Function. Antimicrob. Agents Chemother. 2003, 47, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Guidi, M.; Calpini, V.; Lamoth, F.; Decosterd, L.; Robatel, C.; Buclin, T.; Csajka, C.; Marchetti, O. Effect of Renal Clearance and Continuous Renal Replacement Therapy on Appropriateness of Recommended Meropenem Dosing Regimens in Critically Ill Patients with Susceptible Life-Threatening Infections. J. Antimicrob. Chemother. 2018, 73, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.A.M.; Roberts, J.A.; Carlier, M.; Verstraete, A.G.; Stove, V.; De Waele, J.J. Population Pharmacokinetics of Continuous Infusion of Piperacillin in Critically Ill Patients. Int. J. Antimicrob. Agents 2018, 51, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Fagon, J.Y.; Chastre, J.; Lecso, M.; Dombret, M.C.; Trouillet, J.L.; Gibert, C. Follow-up Protected Specimen Brushes to Assess Treatment in Nosocomial Pneumonia. Am. Rev. Respir. Dis. 1993, 147, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute Renal Failure in Critically Ill PatientsA Multinational, Multicenter Study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Craig, W.A.; Ebert, S.C. Killing and Regrowth of Bacteria in Vitro: A Review. Scand. J. Infect. Dis. Suppl. 1990, 74, 63–70. [Google Scholar]
- Craig, W.A.; Andes, D. Pharmacokinetics and Pharmacodynamics of Antibiotics in Otitis Media. Pediatr. Infect. Dis. J. 1996, 15, 255–259. [Google Scholar] [CrossRef]
- Eagle, H.; Fleischman, R.; Levy, M. “Continuous” vs. “Discontinuous” Therapy with Penicillin; the Effect of the Interval between Injections on Therapeutic Efficacy. N. Engl. J. Med. 1953, 248, 481–488. [Google Scholar] [CrossRef]
- Tamma, P.D.; Putcha, N.; Suh, Y.D.; Van Arendonk, K.J.; Rinke, M.L. Does Prolonged β-Lactam Infusions Improve Clinical Outcomes Compared to Intermittent Infusions? A Meta-Analysis and Systematic Review of Randomized, Controlled Trials. BMC Infect. Dis. 2011, 11, 181. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A Prospective, Two-Centre, Open-Labelled Randomised Controlled Trial of Continuous versus Intermittent Beta-Lactam Infusion in Critically Ill Patients with Severe Sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical Pharmacodynamics of Meropenem in Patients with Lower Respiratory Tract Infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of Area under the Inhibitory Curve (AUIC) and Time above the Minimum Inhibitory Concentration (T > MIC) as Predictors of Outcome for Cefepime and Ceftazidime in Serious Bacterial Infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.-A.; Abdul-Aziz, M.-H.; Lipman, J.; Roberts, J.A. Defining Antibiotic Dosing in Lung Infections. Clin. Pulm. Med. 2013, 20, 121–128. [Google Scholar] [CrossRef]
- Schießer, S.; Hitzenbichler, F.; Kees, M.G.; Kratzer, A.; Lubnow, M.; Salzberger, B.; Kees, F.; Dorn, C. Measurement of Free Plasma Concentrations of Beta-Lactam Antibiotics: An Applicability Study in Intensive Care Unit Patients. Ther. Drug Monit. 2021, 43, 264–270. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: Guidelines for the Management of Hospital-Acquired Pneumonia (HAP)/Ventilator-Associated Pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Berton, D.C.; Kalil, A.C.; Teixeira, P.J.Z. Quantitative versus Qualitative Cultures of Respiratory Secretions for Clinical Outcomes in Patients with Ventilator-Associated Pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD006482. [Google Scholar] [CrossRef]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin. Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef]
- Katsube, T.; Wajima, T.; Ishibashi, T.; Arjona Ferreira, J.C.; Echols, R. Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Cefiderocol, a Parenteral Siderophore Cephalosporin, for Dose Adjustment Based on Renal Function. Antimicrob. Agents Chemother. 2016, 61, e01381-16. [Google Scholar] [CrossRef]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63, e01265-19. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous Infusion of Beta-Lactam Antibiotics in Severe Sepsis: A Multicenter Double-Blind, Randomized Controlled Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults: A Randomized Trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.E.; Kunches, L.M.; Lichtenberg, D.A.; Kollisch, N.R.; Barry, M.A.; Heeren, T.C.; McCabe, W.R. Nosocomial Infection and Fatality in Medical and Surgical Intensive Care Unit Patients. Arch. Intern. Med. 1988, 148, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Luna, C.M.; Blanzaco, D.; Niederman, M.S.; Matarucco, W.; Baredes, N.C.; Desmery, P.; Palizas, F.; Menga, G.; Rios, F.; Apezteguia, C. Resolution of Ventilator-Associated Pneumonia: Prospective Evaluation of the Clinical Pulmonary Infection Score as an Early Clinical Predictor of Outcome. Crit. Care Med. 2003, 31, 676–682. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
| Value | |
|---|---|
| Age (years) | 62 {53–72} |
| Female | 30 (26) |
| Weight (kg) | 75 {61–90} |
| Body Mass Index (kg/m2) | 25.6 {22–29.5} |
| SAPS2 at admission | 54 {43–64} |
| Admission category Medical | 90 (77) |
| Surgical | 27 (23) |
| McCabe classification 1 | 89 (81.5) |
| 2 | 17 (15.5) |
| 3 | 4 (3) |
| Reason for admission | |
| Respiratory failure | 42 (36) |
| Hemodynamic failure | 26 (22) |
| Sepsis | 10 (8.5) |
| Abdominal disease/Hepatic failure | 3 (3) |
| Acute renal failure/Metabolic disease | 2 (2.5) |
| Neurologic failure | 31 (26.5) |
| Other | 2 (2) |
| Clinical presentation at VAP diagnosis or D1 | |
| Reason for mechanical ventilation | |
| Cardiac arrest | 18 (15) |
| Surgery | 12 (10) |
| Septic shock | 4 (3) |
| Hemorrhagic shock | 1 (1) |
| Cardiogenic shock | 4 (3.5) |
| Acute respiratory failure | 43 (37) |
| Toxic or metabolic coma | 4 (3.5) |
| Neurologic failure | 24 (20) |
| Other | 7 (6) |
| Time intubation—VAP (days) | 9 {6–11} |
| Temperature (°C) | 38.5 {37.8–39.1} |
| mCPIS | 6 {5–7} |
| SAPS2 | 47 {38–61} |
| SOFA | 8 {6–10} |
| Vasopressor | 45 (38.5) |
| PaO2/FiO2 ratio (mmHg) | 167 {108–240} |
| Leukocytes (G/L) | 15 {11–19} |
| Serum creatinine at D1 (µmol/L) | 68.5 {48–114} |
| Creatinine clearance at D1 (mL/min/1.73 m2) | 94 {54–112} |
| Serum albumin at D1 (g/L) | 19 {15–24} |
| Weight (kg) | 78.5 {65–92} |
| RRT (D-2 to D0) | 1 (1) |
| Fluid intake during D1 (mL) | 2477 {1485–3450} |
| Value | |
|---|---|
| Respiratory sample | |
| Broncho Alveolar Lavage | 17 (14.5) |
| Plugged Telescopic Catheter | 41 (35) |
| Tracheal Aspirate | 59 (50.5) |
| Positive blood culture | 9 (8) |
| Polymicrobial VAP | 43 (37) |
| All GNB identified (n = 167) | |
| P. aeruginosa | 48 (40) |
| Enterobacter spp. | 29 (23) |
| E. coli | 24 (20) |
| Klebsiella spp. | 19 (16) |
| Haemophilus influenzae | 10 (8) |
| Stenotrophomonas maltophila | 6 (5) |
| Citrobacter spp. | 5 (4) |
| Morganella spp. | 5 (4) |
| Acinetobacter spp. | 4 (4) |
| Serratia spp. | 4 (3) |
| Proteus spp. | 4 (3) |
| Others | 9 (8) |
| Value | ||
|---|---|---|
| n | MIC | |
| β-lactams | ||
| Piperacillin/tazobactam | 41 | 2 {0.85–4} (0.2;256) |
| Cefepime | 22 | 0.1 {0.0395–1} (0.023;4) |
| Ceftazidime | 9 | 1.5 {0.8–13} (0.1;256) |
| Imipenem | 9 | 0.35 {0.2–2.62} (0.1;32) |
| Meropenem | 8 | 0.2 {0.043–1.05} (0.023;32) |
| Piperacillin | NA | |
| Cefotaxime | NA | |
| Aminoglycosides | ||
| Amikacin | 52 | 2 {1.5–3} (0.4;256) |
| Tobramycin | 1 | 0.9 |
| Quinolones | ||
| Ciprofloxacin | 2 | 0.094 (0.032;6.4) |
| Value | |||
|---|---|---|---|
| n (%) | Daily Dose Regimen | ||
| Number of antibiotics per patient | |||
| 1 | 45 (38.5) | ||
| 2 | 70 (60) | ||
| 3 | 2 (1.5) | ||
| Antibiotics and dose regimen | |||
| β-lactams | 117 (100) | (dose in g/day) | CI * |
| Piperacillin/tazobactam | 53 (45.5) | 16/2 {12/1.5–16/2} (8/1;20/2.5) | |
| Cefepime | 28 (24) | 6 {6–6} (4;8) | |
| Ceftazidime | 12 (10) | 6 {6–6} (6;6) | 6 {6-6} (4;8) |
| Imipenem | 13 (11) | 3 {3–3} (2;4) | |
| Meropenem | 8 (7) | 5 {3–6} (1;6) | 3 |
| Piperacillin | 2 (2) | 16 (16;16) | |
| Cefotaxime | 1 (0.5) | 8 | |
| Aminoglycosides | 68 (58) | (dose in mg/kg/day) | |
| Amikacin | 67 (57) | 26 {23–29} (16;35.5) | |
| Tobramycin | 1 (0.5) | 9.5 | |
| Quinolones | 2 (1.5) | (dose in mg/day) | |
| Ciprofloxacin | 2 (1.5) | 1200 (1200;1200) | |
| Overall Population | Alive at D8 | Death at D8 | p | |
|---|---|---|---|---|
| β-lactams | n = 77 | n = 68 | n = 9 | |
| Cmin/MIC | 12.6 {2.5–47.2} (0.001;637) | 11.6 {1.9–60} (0.001;637) | 20.8 {7.5–46} (3.2;116.5) | 0.21 |
| Cmin/MIC > 1 | 64 (83%) | 55 (81) | 9 (100) | 0.34 |
| Cmin/MIC > 4 | 54 (70%) | 46 (68) | 8 (89) | 0.27 |
| Aminoglycosides | n = 47 | n = 40 | n = 7 | |
| Cmax/MIC | 32.5 {19.3–45.5} (0.17;165) | 32.4 {18.2–44.5} (0.17;165) | 33.4 {21.4–44} (0.26;126.9) | 0.63 |
| Cmax/MIC > 8 | 44 (94) | 38 (95) | 6 (86) | 0.39 |
| Cmax/MIC > 10 | 41 (87) | 35 (87.5) | 6 (86) | 1.00 |
| Microbiological Failure | Microbiological Success | p | |
|---|---|---|---|
| β-lactams | n = 26 | n = 28 | |
| Cmin/MIC | 6.2 {1.1–46} (0.001;513) | 25.7 {6.9–119} (0.4;637) | 0.041 |
| Cmin/MIC > 1 | 19 (73) | 26 (93) | 0.11 |
| Cmin/MIC > 4 | 17 (65) | 23 (82) | 0.27 |
| Aminoglycosides | n = 17 | n = 20 | |
| Cmax/MIC | 24.4 {19.6–37} (0.26;61.8) | 35 {21–51} (0.18;165) | 0.91 |
| Cmax/MIC > 8 | 16 (94) | 18 (90) | 1.00 |
| Cmax/MIC > 10 | 16 (94) | 16 (80) | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajot, O.; Lakhal, K.; Lambert, J.; Gros, A.; Bruel, C.; Boulain, T.; Garot, D.; Das, V.; Timsit, J.F.; Cerf, C.; et al. Empirical Antibiotic Therapy for Gram-Negative Bacilli Ventilator-Associated Pneumonia: Observational Study and Pharmacodynamic Assessment. Antibiotics 2022, 11, 1664. https://doi.org/10.3390/antibiotics11111664
Pajot O, Lakhal K, Lambert J, Gros A, Bruel C, Boulain T, Garot D, Das V, Timsit JF, Cerf C, et al. Empirical Antibiotic Therapy for Gram-Negative Bacilli Ventilator-Associated Pneumonia: Observational Study and Pharmacodynamic Assessment. Antibiotics. 2022; 11(11):1664. https://doi.org/10.3390/antibiotics11111664
Chicago/Turabian StylePajot, Olivier, Karim Lakhal, Jérome Lambert, Antoine Gros, Cédric Bruel, Thierry Boulain, Denis Garot, Vincent Das, Jean François Timsit, Charles Cerf, and et al. 2022. "Empirical Antibiotic Therapy for Gram-Negative Bacilli Ventilator-Associated Pneumonia: Observational Study and Pharmacodynamic Assessment" Antibiotics 11, no. 11: 1664. https://doi.org/10.3390/antibiotics11111664
APA StylePajot, O., Lakhal, K., Lambert, J., Gros, A., Bruel, C., Boulain, T., Garot, D., Das, V., Timsit, J. F., Cerf, C., Souweine, B., Chaffaut, C., Mentec, H., Zahar, J. R., Mira, J. P., & Jullien, V. (2022). Empirical Antibiotic Therapy for Gram-Negative Bacilli Ventilator-Associated Pneumonia: Observational Study and Pharmacodynamic Assessment. Antibiotics, 11(11), 1664. https://doi.org/10.3390/antibiotics11111664







