Bioactive Peptides against Human Apicomplexan Parasites
Abstract
1. Introduction
1.1. Toxoplasmosis
1.2. Cryptosporidiosis
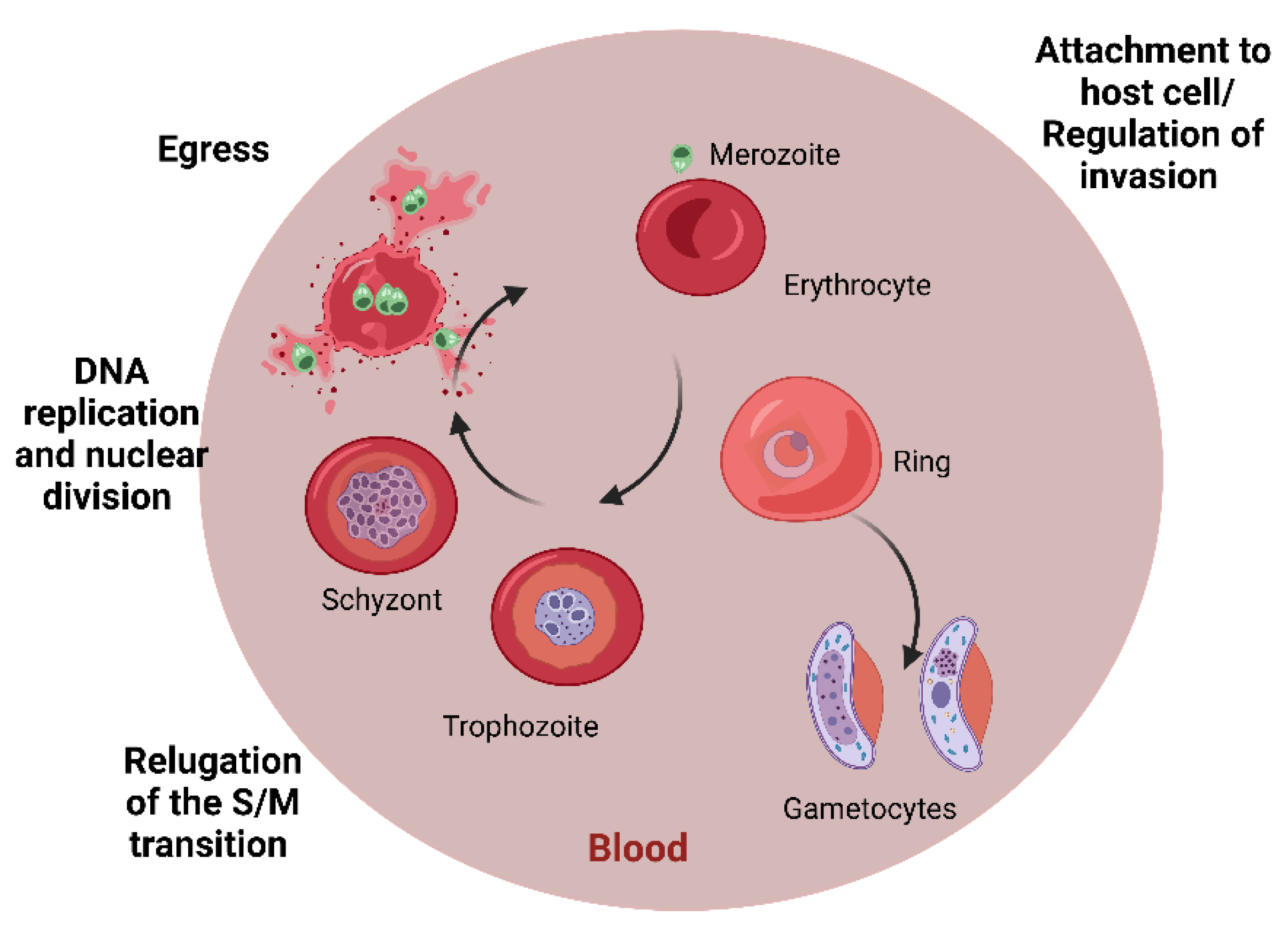
1.3. Malaria
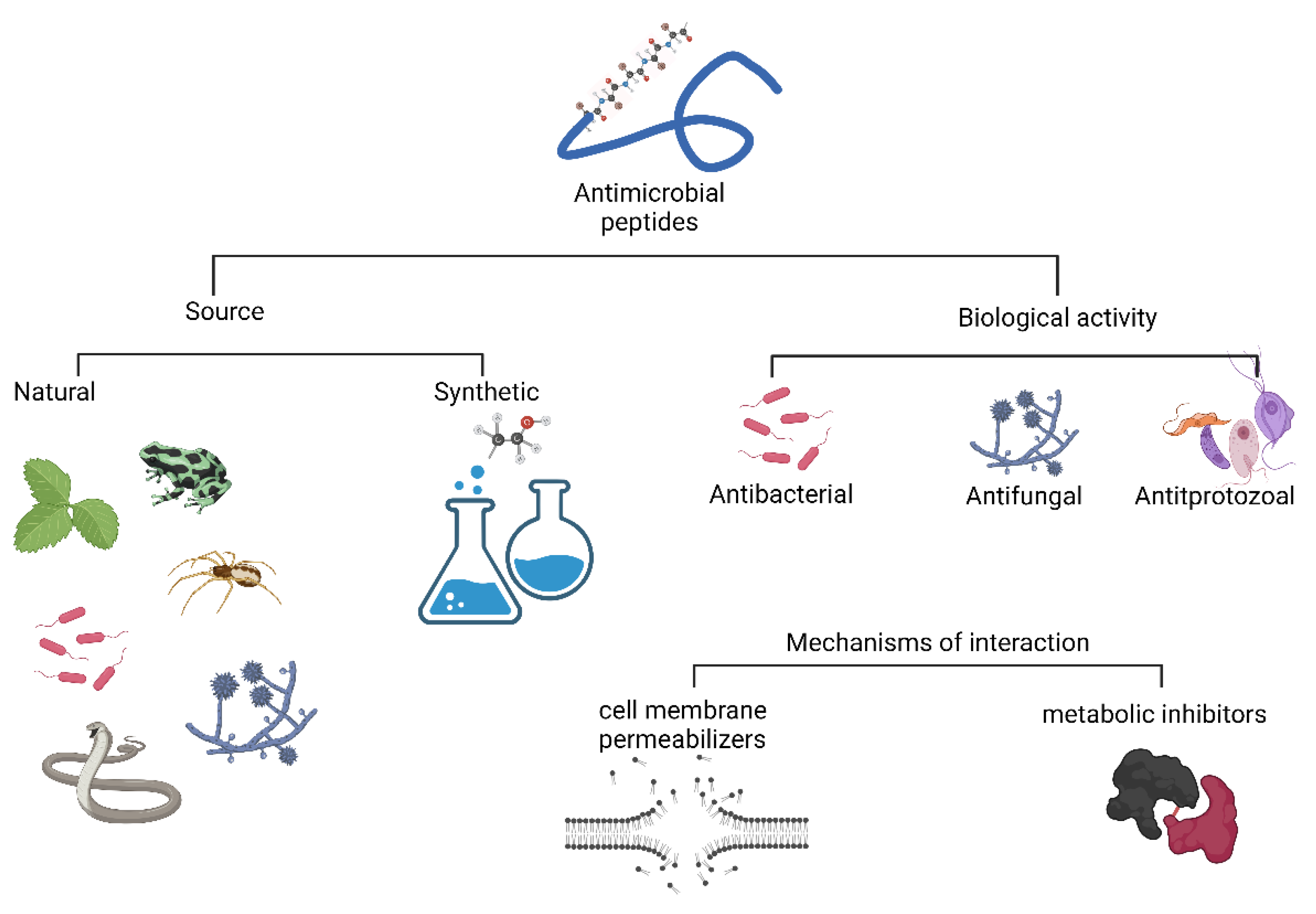
2. Antimicrobial Peptide Classification
3. Mechanisms of Interaction by AMPs
4. Peptides Active against Apicomplexan Parasites
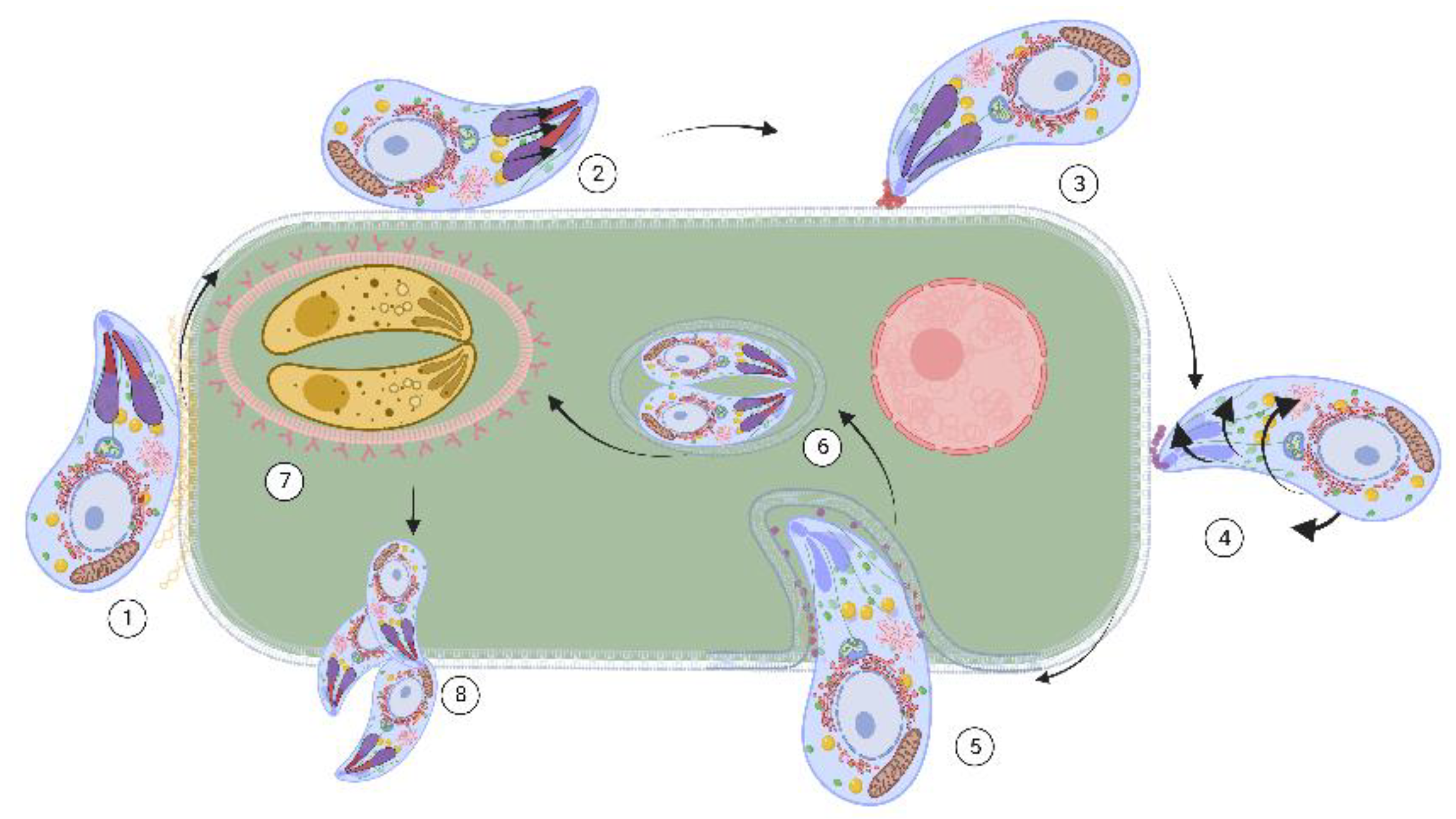
4.1. Toxoplasma gondii
4.2. Cryptosporidum spp.
| AMP Name | Type | Evaluated Concentrations | Cytotoxicity | Activity and Possible Mechanisms of Action | |
|---|---|---|---|---|---|
| Buforin II [85] | α-Helical | 20 μM | None in A549 cells. | Reduces sporozoites viability. Cell membrane Disruption | |
| Ranalexin [84] | Cationic | 64 µg/mL | Non in A549 cells. | Sporozoites growth suppression. Cell membrane Disruption | |
| Ranalexin, Magainin II. Indolicidin [82] | Cationic, helix and tridecapeptide | 50 mM | Non in A549 cells | Sporozoites growth suppression. Cell membrane damage by synergic effect between peptide and lipophilic antibiotics | |
| Shiva-10 [89] | Lytic peptide | 10 µM | ND | Reduces sporozoite viability. Membrane lytic effect | |
| Cecropin P1, magainin II, ranalexin, and indolicidin [83] | Cationic peptides | 50 μM | ND | Reduction in the proliferation of schizonts. Inhibition of Na/H and Na/Ca2 exchanges in the cell membrane | |
| KFFKFFKFF and IKFLKFLKFL [81] | Cationic peptides | 100 µg/mL | ND | Reduction in the viability of sporozoites. Cell membrane disruption | |
| SMAP-29, BMAP-28, PG-1, Bac-7 [80] | Helical peptides | 100 μg/mL | ND | Strong cytotoxic effect on sporozoites. Alterations in the glycoprotein of the apical complex | |
| Indolicidin, Magainin II, Ranalexin [79] | Cathionic peptides | 50 μM | ND | Reduction in merozoites proliferation | |
| Octaarginine-6-FAM-Nitazoanide combination [86] | Cathionic peptides | 197 nM | No cytotoxic effects in human ileocecal adenocarcinoma cells | Reduction in trophozoites and meronts replication | |
| Lactoferrin B, cathelicidin LL3, indolicidin, βdefens1in, ß defensin 2. [90] | Cathionic peptides | 10 µg/mL | Low cytotoxic effect in human colorectal adenocarcinoma cells | Inhibition of sporozoites attachment and invasion. Transmembrane pore formation | |
| Buforin II, Magainin II, Lasalocid. [78] | Cathionic peptides | 10 µg/mL | ND | Reduction in oocysts infectivity. Membrane disruption | |
4.3. Peptides Active against Plasmodium spp.
5. Concluding Remarks and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, R.D.; Hokke, C.H.; Haslam, S.M. Parasitic infections. In Essentials of Glycobiology, 4th ed.; Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Memariani, H.; Memariani, M. Melittin as a promising anti-protozoan peptide: Current knowledge and future prospects. AMB Express 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R.; Frischknecht, F. The Riveting Cellular Structures of Apicomplexan Parasites. Trends Parasitol. 2020, 36, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Tusting, L.S.; Bottomley, C.; Saito, K.; Djouaka, R.; Lines, J. Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: A systematic review and meta-analysis. Lancet Planet. Health 2022, 6, e257–e269. [Google Scholar] [CrossRef]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Gargala, G. Drug treatment and novel drug target against Cryptosporidium. Parasite 2008, 15, 275–281. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B. Combination Therapy Strategies for the Treatment of Malaria. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. 2016, 6, 194. [Google Scholar] [CrossRef]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.-M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and allergenic properties of antimicrobial peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef]
- Nogrado, K.; Adisakwattana, P.; Reamtong, O. Antimicrobial peptides: On future antiprotozoal and anthelminthic applications. Acta. Trop. 2022, 235, 106665. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E. Antimicrobial peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Preti, A.; Gyppaz, D.; Gureje, O.; Carta, M.G. Association between toxoplasmosis and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2022, 153, 284–291. [Google Scholar] [CrossRef]
- Hajimohammadi, B.; Ahmadian, S.; Firoozi, Z.; Askari, M.; Mohammadi, M.; Eslami, G.; Askari, V.; Loni, E.; Barzegar-Bafrouei, R.; Boozhmehrani, M.J. A Meta-Analysis of the Prevalence of Toxoplasmosis in Livestock and Poultry Worldwide. EcoHealth 2022, 19, 55–74. [Google Scholar] [CrossRef]
- De Barros, R.A.M.; Torrecilhas, A.C.; Marciano, M.A.M.; Mazuz, M.L.; Pereira-Chioccola, V.L.; Fux, B. Toxoplasmosis in Human and Animals Around the World. Diagnosis and Perspectives in the One Health Approach. Acta Trop. 2022, 231, 106432. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar] [PubMed]
- Robinson, E.; de Valk, H.; Villena, I.; Le Strat, Y.; Tourdjman, M. National perinatal survey demonstrates a decreasing seroprevalence of Toxoplasma gondii infection among pregnant women in France, 1995 to 2016: Impact for screening policy. Eurosurveillance 2021, 26, 1900710. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Riahi, S.M.; Contopoulos-Ioannidis, D.G.; Gamble, H.R.; Fakhri, Y.; Shiadeh, M.N.; Foroutan, M.; Behniafar, H.; Taghipour, A.; Maldonado, Y.A.; et al. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007807. [Google Scholar] [CrossRef] [PubMed]
- López-Fabal, F.; Gómez-Garcés, J.L. Marcadores serológicos de gestantes españolas e inmigrantes en un área del sur de Madrid durante el periodo 2007–2010. Rev. Esp. Quimioter. 2013, 26, 108–111. [Google Scholar] [PubMed]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263. [Google Scholar] [CrossRef]
- McLeod, R.; Cohen, W.; Dovgin, S.; Finkelstein, L.; Boyer, K.M. Human toxoplasma infection. In Toxoplasma Gondii; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–227. [Google Scholar]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Dubey, J.P. The history and life cycle of Toxoplasma gondii. In Toxoplasma Gondii; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Dunay Ildiko, R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya Jose, G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Adkins, P.R.F. Cryptosporidiosis. Vet. Clin. N. Am. Food Anim. 2022, 38, 121–131. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Korpe, P.S.; Valencia, C.; Haque, R.; Mahfuz, M.; McGrath, M.; Houpt, E.; Kosek, M.; McCormick, B.J.J.; Penataro Yori, P.; Babji, S.; et al. Epidemiology and Risk Factors for Cryptosporidiosis in Children From 8 Low-income Sites: Results From the MAL-ED Study. Clin. Infect. Dis. 2018, 67, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, E.; Safarpour, H.; Xiao, L.; Zarean, M.; Hatam-Nahavandi, K.; Barac, A.; Picot, S.; Rahimi, M.T.; Rubino, S.; Mahami-Oskouei, M.; et al. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite 2020, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Hill, K.; Deere, D. Review of generic screening level assumptions for quantitative microbial risk assessment (QMRA) for estimating public health risks from Australian drinking water sources contaminated with Cryptosporidium by recreational activities. Water. Res. 2022, 220, 118659. [Google Scholar] [CrossRef] [PubMed]
- Urrea-Quezada, A.; González-Díaz, M.; Villegas-Gómez, I.; Durazo, M.; Hernández, J.; Xiao, L.; Valenzuela, O. Clinical manifestations of cryptosporidiosis and identification of a new Cryptosporidium subtype in patients from Sonora, Mexico. J. Pediatr. Infect. Dis. 2018, 37, e136–e138. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.; Striepen, B. The Biology of the Intestinal Intracellular Parasite Cryptosporidium. Cell Host Microbe 2020, 28, 509–515. [Google Scholar] [CrossRef]
- English, E.D.; Guérin, A.; Tandel, J.; Striepen, B. Live imaging of the Cryptosporidium parvum life cycle reveals direct development of male and female gametes from type I meronts. PLoS Biol. 2022, 20, e3001604. [Google Scholar] [CrossRef]
- Tandel, J.; English, E.D.; Sateriale, A.; Gullicksrud, J.A.; Beiting, D.P.; Sullivan, M.C.; Pinkston, B.; Striepen, B. Life cycle progression and sexual development of the Apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019, 4, 2226–2236. [Google Scholar] [CrossRef]
- Borowski, H.; Thompson, R.C.A.; Armstrong, T.; Clode, P.L. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 2010, 137, 13–26. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 26 September 2022).
- Su, X.-z.; Lane, K.D.; Xia, L.; Sá, J.M.; Wellems, T.E. Plasmodium Genomics and Genetics: New Insights into Malaria Pathogenesis, Drug Resistance, Epidemiology, and Evolution. Clin. Microbiol. Rev. 2019, 32, e00019-19. [Google Scholar] [CrossRef]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Sinnis, P.; Zavala, F. The skin: Where malaria infection and the host immune response begin. Semin. Immunopathol. 2012, 34, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Drahansky, M. Liveness Detection in Biometrics. Available online: https://www.intechopen.com/chapters/17746 (accessed on 26 September 2022).
- Abugri, J.; Ayariga, J.; Sunwiale, S.S.; Wezena, C.A.; Gyamfi, J.A.; Adu-Frimpong, M.; Agongo, G.; Dongdem, J.T.; Abugri, D.; Dinko, B. Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates. Heliyon 2022, 8, e10390. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Jereb, M.; Muzlovic, I.; Prabhu, R.M. Clinical review: Severe malaria. Crit. Care Med. 2003, 7, 315. [Google Scholar] [CrossRef]
- Schofield, L.; Grau, G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Liang, X.; Cui, L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 102–118. [Google Scholar] [CrossRef]
- Hernández-Aristizábal, Antimicrobial Peptides with Antibacterial Activity against Vancomycin-Resistant Staphylococcus aureus Strains: Classification, Structures, and Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 7927. [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef]
- Böhmová, E.; Machová, D.; Pechar, M.; Pola, R.; Venclíková, K.; Janoušková, O.; Etrych, T. Cell-Penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiol. Res. 2018, 67, S267–S279. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 2559. [Google Scholar] [CrossRef]
- Lee, H.-T.; Lee, C.-C.; Yang, J.-R.; Lai, J.Z.C.; Chang, K.Y. A large-scale structural classification of antimicrobial peptides. Biomed. Res. Int. 2015, 2015, 475062. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Jiant, X.; Bergen, P.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef] [PubMed]
- Straub, K.W.; Cheng, S.J.; Sohn, C.S.; Bradley, P.J. Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell. Microbiol. 2009, 11, 590–603. [Google Scholar] [CrossRef]
- Sabiá Júnior, E.F.; Menezes, L.F.S.; de Araújo, I.F.S.; Schwartz, E.F. Natural occurrence in venomous arthropods of antimicrobial peptides active against protozoan parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Liu, R.; Ni, Y.; Song, J.; Xu, Z.; Qiu, J.; Wang, L.; Zhu, Y.; Huang, Y.; Ji, M.; Chen, Y. Research on the effect and mechanism of antimicrobial peptides HPRP-A1/A2 work against Toxoplasma gondii infection. Parasite Immunol. 2019, 41, e12619. [Google Scholar] [CrossRef]
- Seeber, F. An enzyme-release assay for the assessment of the lytic activities of complement or antimicrobial peptides on extracellular Toxoplasma gondii. J. Microbiol. Methods 2000, 39, 189–196. [Google Scholar] [CrossRef]
- Shin, I.S.; Seo, C.S.; Lee, M.Y.; Ha, H.K.; Huh, J.I.; Shin, H.K. In vitro and in vivo evaluation of the genotoxicity of Gumiganghwal-tang, a traditional herbal prescription. J. Ethnopharmacol. 2012, 141, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Tang, X.; Wu, M.; Hou, S.; Liu, X.; Li, J.; Deng, M.; Huang, S.; Jiang, L. Anti-Toxoplasma gondii Effects of a Novel Spider Peptide XYP1 In Vitro and In Vivo. Biomedicines 2021, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hou, S.; Li, X.; Wu, M.; Ma, B.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; Tang, X.; et al. Anti-Parasitic effect on Toxoplasma gondii induced by a spider peptide lycosin-I. Exp. Parasitol. 2019, 198, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Maeda, H.; Galay, R.L.; Boldbattar, D.; Umemiya-Shirafuji, R.; Suzuki, H.; Xuan, X.; Tsuji, N.; Fujisaki, K. Tick longicin implicated in the arthropod transmission of Toxoplasma gondii. J. Vet. Sci. Technol. 2012, 3, 3633–3640. [Google Scholar] [CrossRef]
- Tanaka, T.; Maeda, H.; Matsuo, T.; Boldbattar, D.; Umemiya-Shirafuji, R.; Kume, A.; Suzuki, H.; Xuan, X.; Tsuji, N.; Fujisaki, K. Parasiticidal activity of Haemaphysalis longicornis longicin P4 peptide against Toxoplasma gondii. Peptides 2012, 34, 242–250. [Google Scholar] [CrossRef]
- Gustavo Tempone, A.; de Souza Carvalho Melhem, M.; Oliveira Prado, F.; Motoie, G.; Mitsuyoshi Hiramoto, R.; Maria Antoniazzi, M.; Fernando Baptista Haddad, C.; Jared, C. Amphibian secretions for drug discovery studies: A search for new antiparasitic and antifungal compounds. Lett. Drug Des. Discov. 2007, 4, 67–73. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Y.; Tang, Y.; Wu, M.; Guan, J.; Li, X.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z. Anti-Toxoplasma gondii effect of two spider venoms in vitro and in vivo. Toxicon 2019, 166, 9–14. [Google Scholar] [CrossRef]
- Khaleghi Rostamkolaie, L.; Hamidinejat, H.; Razi Jalali, M.H.; Jafari, H.; Najafzadeh Varzi, H.; Seifi Abadshapouri, M.R. In vitro therapeutic effect of Hemiscorpius lepturus venom on tachyzoites of Toxoplasma gondii. J. Parasit. Dis. 2019, 43, 472–478. [Google Scholar] [CrossRef]
- De Assis, D.R.R.; Pimentel, P.M.d.O.; Dos Reis, P.V.M.; Rabelo, R.A.N.; Vitor, R.W.A.; Cordeiro, M.d.N.; Felicori, L.F.; Olórtegui, C.D.C.; Resende, J.M.; Teixeira, M.M. Tityus Serrulatus (Scorpion): From the Crude Venom to the Construction of Synthetic Peptides and Their Possible Therapeutic Application Against Toxoplasma gondii Infection. Front. Cell. Infect. Microbiol. 2021, 11, 706618. [Google Scholar] [CrossRef]
- De León-Nava, M.A.; Romero-Núñez, E.; Luna-Nophal, A.; Bernáldez-Sarabia, J.; Sánchez-Campos, L.N.; Licea-Navarro, A.F.; Morales-Montor, J.; Muñiz-Hernández, S. In vitro effect of the synthetic cal14.1a conotoxin, derived from Conus californicus, on the human parasite Toxoplasma gondii. Mar. Drugs 2016, 14, 66. [Google Scholar] [CrossRef]
- De Oliveira Cardoso, M.F.; Moreli, J.B.; Gomes, A.O.; de Freitas Zanon, C.; Silva, A.E.; Paulesu, L.R.; Ietta, F.; Mineo, J.R.; Ferro, E.A.; Oliani, S.M. Annexin A1 peptide is able to induce an anti-parasitic effect in human placental explants infected by Toxoplasma gondii. Microb. Pathog. 2018, 123, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Giovati, L.; Santinoli, C.; Mangia, C.; Vismarra, A.; Belletti, S.; ’Adda, T.; Fumarola, C.; Ciociola, T.; Bacci, C.; Magliani, W. Novel activity of a synthetic decapeptide against Toxoplasma gondii tachyzoites. Front. Microbiol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Barchiesi, F.; Scalise, G. Short-term exposure to membrane-active antibiotics inhibits Cryptosporidium parvum infection in cell culture. Antimicrob. Agents Chemother. 2000, 44, 3473–3475. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Caselli, F.; Scalise, G. In vitro activity of polycationic peptides against Cryptosporidium parvum, Pneumocystis carinii and yeast clinical isolates. J. Antimicrob. Chemother. 1999, 44, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Skerlavaj, B.; Circo, R.; Zanetti, M.; Scalise, G. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 2003, 51, 843–847. [Google Scholar] [CrossRef][Green Version]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; Kasprzykowski, F.; Barchiesi, F.; Del Prete, M.S.; Maćkiewicz, Z.; Scalise, G. In vitro effect of short-term exposure to two synthetic peptides, alone or in combination with clarithromycin or rifabutin, on Cryptosporidium parvum infectivity. Peptides 2002, 23, 1015–1018. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Fortuna, M.; Scalise, G. In vitro anticryptosporidial activity of ranalexin alone and in combination with other peptides and with hydrophobic antibiotics. Eur. J. Clin. Microbiol. 1999, 18, 827–829. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Ancarani, F.; Scalise, G. In vitro anti-cryptosporidial activity of cationic peptides alone and in combination with inhibitors of ion transport systems. J. Antimicrob. Chemother. 2000, 45, 651–654. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Scalise, G. Anticryptosporidial activity of ranalexin, lasalocid and azithromycin alone and in combination in cell lines. J. Antimicrob. Chemother. 2000, 45, 375–377. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Barchiesi, F.; Fineo, A.; Scalise, G. Activity of buforin II alone and in combination with azithromycin and minocycline against Cryptosporidium parvum in cell culture. J. Antimicrob. Chemother. 2001, 47, 97–99. [Google Scholar] [CrossRef][Green Version]
- Nguyen-Ho-Bao, T.; Ambe, L.A.; Berberich, M.; Hermosilla, C.; Taubert, A.; Daugschies, A.; Kamena, F. Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens 2022, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Tosini, F.; Ludovisi, A.; Tonanzi, D.; Amati, M.; Cherchi, S.; Pozio, E.; Gómez-Morales, M.A. Delivery of SA35 and SA40 peptides in mice enhances humoral and cellular immune responses and confers protection against Cryptosporidium parvum infection. Parasites Vectors 2019, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Connor, E.; Lehnert, M. Volatile organic compounds in the strongly fragrant fern genus Melpomene (Polypodiaceae). Plant. Biol. 2015, 17, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Arrowood, M.J.; Jaynes, J.M.; Healey, M.C. In vitro activities of lytic peptides against the sporozoites of Cryptosporidium parvum. Antimicrob. Agents Chemother. 1991, 35, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Carryn, S.; Schaefer, D.A.; Imboden, M.; Homan, E.J.; Bremel, R.D.; Riggs, M.W. Phospholipases and cationic peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and non-parasiticidal mechanisms. J. Parasitol. 2012, 98, 199–204. [Google Scholar] [CrossRef]
- Martins, G.G.; de Jesus Holanda, R.; Alfonso, J.; Gómez Garay, A.F.; dos Santos, A.P.d.A.; de Lima, A.M.; Francisco, A.F.; Garcia Teles, C.B.; Zanchi, F.B.; Soares, A.M. Identification of a peptide derived from a Bothrops moojeni metalloprotease with in vitro inhibitory action on the Plasmodium falciparum purine nucleoside phosphorylase enzyme (PfPNP). Biochimie 2019, 162, 97–106. [Google Scholar] [CrossRef]
- Chaianantakul, N.; Sungkapong, T.; Supatip, J.; Kingsang, P.; Kamlaithong, S.; Suwanakitti, N. Antimalarial effect of cell penetrating peptides derived from the junctional region of Plasmodium falciparum dihydrofolate reductase-thymidylate synthase. Peptides 2020, 131, 170372. [Google Scholar] [CrossRef]
- Teixeira, C.; Gomes, J.R.; Gomes, P. Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011, 18, 1555–1572. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, V.; Tellis, M.B.; Joshi, R.S.; Pandey, K.C.; Singh, S. Cyclic peptide engineered from phytocystatin inhibitory hairpin loop as an effective modulator of falcipains and potent antimalarial. J. Biomol. Struct. Dyn. 2022, 40, 3642–3654. [Google Scholar] [CrossRef]
- Sweeney-Jones, A.M.; Gagaring, K.; Antonova-Koch, J.; Zhou, H.; Mojib, N.; Soapi, K.; Skolnick, J.; McNamara, C.W.; Kubanek, J. Antimalarial Peptide and Polyketide Natural Products from the Fijian Marine Cyanobacterium Moorea producens. Mar. Drugs 2020, 18, 167. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Silva, A.F.; Andrade, G.P.; Pedron, C.N.; Cerchiaro, G.; Ribeiro, A.O.; Oliveira, V.X., Jr.; de la Fuente-Nunez, C. The wasp venom antimicrobial peptide polybia-CP and its synthetic derivatives display antiplasmodial and anticancer properties. Bioeng. Transl. Med. 2020, 5, e10167. [Google Scholar] [CrossRef] [PubMed]
- El Chamy Maluf, S.; Hayashi, M.A.F.; Campeiro, J.D.; Oliveira, E.B.; Gazarini, M.L.; Carmona, A.K. South American rattlesnake cationic polypeptide crotamine trafficking dynamic in Plasmodium falciparum-infected erythrocytes: Pharmacological inhibitors, parasite cycle and incubation time influences in uptake. Toxicon 2022, 208, 47–52. [Google Scholar] [CrossRef] [PubMed]
- El Chamy Maluf, S.; Dal Mas, C.; Oliveira, E.B.; Melo, P.M.; Carmona, A.K.; Gazarini, M.L.; Hayashi, M.A.F. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides 2016, 78, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Somsri, S.; Mungthin, M.; Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Aunpad, R. A Mitochondria-Penetrating Peptide Exerts Potent Anti-Plasmodium Activity and Localizes at Parasites’ Mitochondria. Antibiotics 2021, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; He, X.; Zhang, P.; Shen, C.; Mwangi, J.; Xu, C.; Mo, G.; Lai, R.; Zhang, Z. In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins 2019, 11, 379. [Google Scholar] [CrossRef]
- Tonk, M.; Pierrot, C.; Cabezas-Cruz, A.; Rahnamaeian, M.; Khalife, J.; Vilcinskas, A. The Drosophila melanogaster antimicrobial peptides Mtk-1 and Mtk-2 are active against the malarial parasite Plasmodium falciparum. Parasitol. Res. 2019, 118, 1993–1998. [Google Scholar] [CrossRef]
- Pedron, C.N.; Silva, A.F.; Torres, M.D.T.; de Oliveira, C.S.; Andrade, G.P.; Cerchiaro, G.; Pinhal, M.A.S.; de la Fuente-Nunez, C.; Oliveira, V.X., Jr. Net charge tuning modulates the antiplasmodial and anticancer properties of peptides derived from scorpion venom. J. Pept. Sci. 2021, 27, e3296. [Google Scholar] [CrossRef]
- Rangel, G.W.; Llinás, M. Re-Envisioning Anti-Apicomplexan Parasite Drug Discovery Approaches. Front. Cell. Infect. Microbiol. 2021, 11, 691121. [Google Scholar] [CrossRef]
- Kloehn, J.; Harding, C.R.; Soldati-Favre, D. Supply and demand—Heme synthesis, salvage and utilization by Apicomplexa. FEBS Lett. 2021, 288, 382–404. [Google Scholar] [CrossRef]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Teixeira, C.; Alves, C.; Gomes, P.; Almeida, J.R. Peptides to Tackle Leishmaniasis: Current Status and Future Directions. Int. J. Mol. Sci. 2021, 22, 4400. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Juretić, D. Designed Multifunctional Peptides for Intracellular Targets. Antibiotics 2022, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
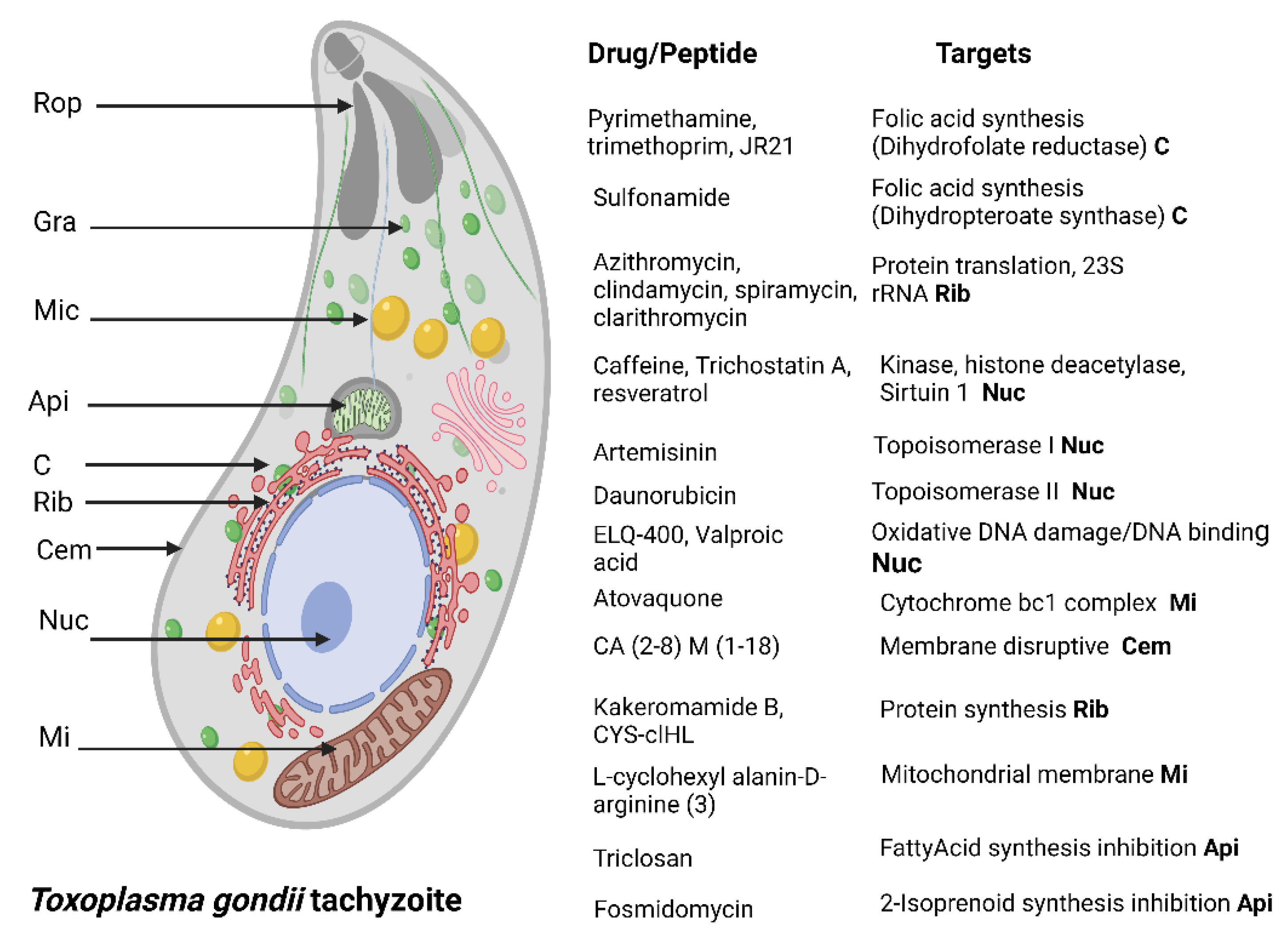

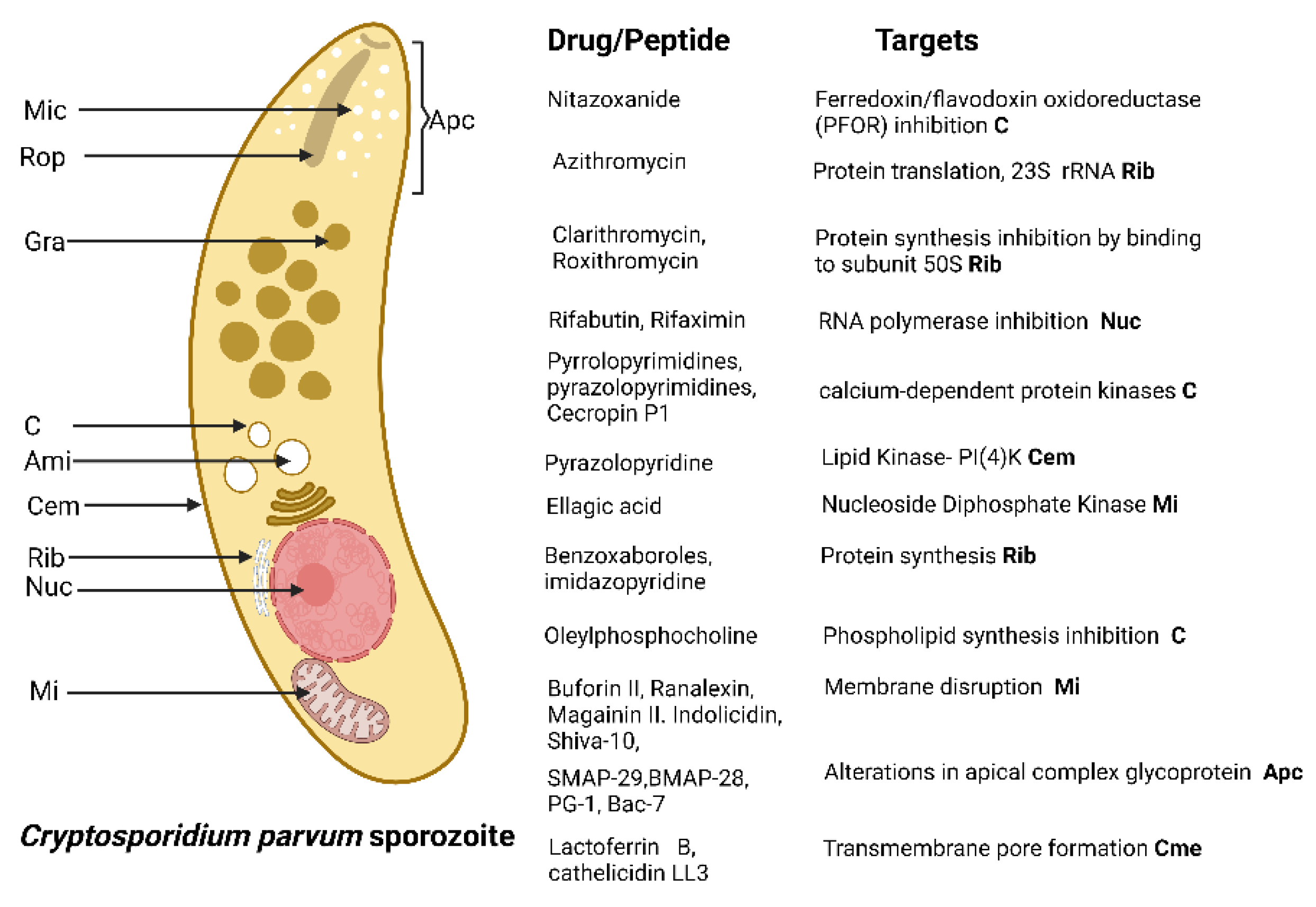

| AMP Name | Type | Source | Evaluated Concentrations | Cytotoxicity | Activity and Possible Mechanism of Action | IC50 |
|---|---|---|---|---|---|---|
| Frog skin secretion [71] | ND | Phyllomedusa distincta [Amphibia] Corythomanti greening [Amphibia] | 25 µg/mL and 22 µg/mL respectively | None in human Fibroblasts | Inhibits invasion | ND |
| CA (2–8) M(1–18) [65] | Cecropin/ melittin hybrid peptide | Synthetic | 5 µM | None in human fibroblasts | Reduces viability Membrane lytic activity | ND |
| Ac2-26 peptide mimetic of Annexin A1 [76] | Human peptide | Synthetic | 5 μM | ND | Decreases proliferation rate | ND |
| Lycosin-Ι [68] | Linear peptide | Lycosa singoriensis [Arachnida] | 20 µM | Cytotoxic at 34.69 µM in human fibroblasts | Invasion and proliferation inhibition. Cell membrane alteration | 28 and 10.08 μM for intracellular and extracellular tachyzoites, respectively |
| Longicin [69] | Cationic | Haemaphysalis longicornis [Arachnida] | 50 µM | ND | Reduces proliferation. Cell membrane disruption | ND |
| ND [72] | Venoms | Ornitoctonus huwena Chilobrachys jingzhao [Arachnida] | 12.5 µg/mL | Cytotoxic to Hella cells | Proliferation and invasion reduction | ND |
| XYP1 [67] | Cationic | synthesized | 2.5–40 µM | Low cytotoxicity at 20 µM in human fibroblasts | Inhibition of viability, invasion, and proliferation. Damage to membrane associated proteins (HSP29) | 38.79 µM |
| cal14.1a [75] | Conotoxin | Conus californicus [Gastropoda] | 10–50 µM | Not detected up to 50 µM in Hep-2 cells | Affects viability and replication by disrupting cell membrane | ND |
| ND [73] | Venom | Hemiscorpius Lepturus [Arachnida] | 50 µg/mL | CC50 72.46 µg/mL (Vero cells) | Reduces viability and invasion. Probably damaging ion channels and enzymatic activity | 39.06 µg/mL |
| Killer peptide (KP) [77] | Decapeptide | Synthetic | 25–200 µg/mL | Nontoxic to Vero cells. Genotoxic effects were reported | Reduces invasion and proliferation. Maybe triggers an apoptosis like cell death | ND |
| Longicin P4 [70] | ND | Haemaphysalis Longicornis [Arachnida] | 50 µM | Nontoxic up to 25 µM | Reduces proliferation. Induces aggregation and affects membrane integrity | ND |
| HPRP-A1/A2 [64] | Cationic peptide | Synthetic | 10–40 µg/mL | Nontoxic in peritoneal macrophages. | Reduces viability, adhesion, and invasion | ND |
| Sub6-B, Pep1, Pep2a and Pep2b [74] | Venom fractions | Tityus serrulatus [Arachnida] | 100 µg/mL | Nontoxic in peritoneal macrophages | Reduces invasion and replication. Disruption of cell membrane | ND |
| AMP Name | Type | Source | Evaluated Concentration | Cytotoxicity | Activity and Possible Mechanisms of Action | IC50 |
|---|---|---|---|---|---|---|
| Pep1 BM [91] | ND | Synthetic | 20 µL | ND | Inhibition of purine nucleoside phosphorylase in P. falciparum rings | 16.14 μg/mL |
| JR21 [92] | ND | Synthetic | 10 µM | ND | Dihydrofolate reductase- thymidylate synthase inhibition in P. falciparum rings | 3.87 µM |
| CYS-IHL [94] | Linear | Synthetic | 69.91 µM | Noncytotoxic in human liver carcinoma cell. | Hemoglobinase activity inhibition in late P. falciparum Trophozoites | 27.55 µM |
| Kakeromamide B [95] | Cyclic | Moorea producens [Cyanobacteria] | 11 µM | Noncytotoxic in HEK293T and HepG2 cells | Reduction in proliferation of P. falciparum sexual blood-stages and P. berghei liver-stage. High affinity to actin, sortilin and subunit A of glutamyl-tRNA amide transferase | 8.9 µM |
| [Gly]1-Pol-CP-NH2 [96] | ND | Synthetic derived from Pol-CP-NH2 | 6.25 µM | Cytotoxic in human mammary adenocarcinoma, Hep G2, SHSY-5Y, and SK-mel-147 | Cell membrane disruption in P. falciparum sporozoites | ND |
| Crotamine [97,98] | Cationic | Crotalusdurissusterrificus [Lepidosauria] | 20 µM | No hemolytic activity in human erythrocytes | Peptide–membrane interactions and H+ homeostasis disruption in P. falciparum asexual blood stages | 1.87 µM |
| (L-cyclohexyl alanin-D- arginine) 3 [99] | ND | Synthetic | 59.16 ng/mL | No cytotoxic effects in human erythrocytes and leukocytes | Chromatin compaction and mitochondrial membrane disruption in P. falciparum asexual blood stages | 8.94 ng/mL |
| rR8-JR21 [92] | ND | Synthetic | 13.22 | ND | Dihydrofolate reductase-thymidylate synthase inhibition in P. falciparum ring stages | 1.53 µM |
| LZ1 [100] | Linear peptide | Synthetic derived fromcathelicidin-BF | 25 µM and 4 mg/kg | ND | Blockade of ATP production by selective inhibition of pyruvate kinase activity in P. falciparum blood stages. | 3.045 µM |
| Mtk-1 y Mtk-2 [101] | Rich in proline | Drosophila melanogaster [Insecta] | 50 µM | Hemolytic activity in pig and mouse (CD1) erythrocytes | Cell membrane disruption in P. falciparum asexual blood stages | ND |
| Stomoxyn [101] | ND | Lucilia sericata [Insecta] | 50 µM | Hemolytic activity in highest concentrations in pig and mouse (CD1) erythrocytes | Cell membrane disruption in P. falciparum asexual blood stages | ND |
| CecA y CecB [101] | Linear cations | Galleria mellonella [Insecta] | 50 µM | Hemolytic activity in highest concentrations in pig and mouse (CD1) erythrocytes | Cell membrane disruption in P. falciparum asexual blood stages. | ND |
| [Arg]3-VmCT1-NH2, [Arg]7-VmCT1-NH2 [102] | Synthetic | 5 µM/L | Lower Cytotoxic effects in MCF-7 human breast epithelial cells, CC50 20 and 18 µM/L | Cell membrane disruption in P. gallinaceum sporozoites | 0.57, 0.51 µM/L | |
| VmCT1-NH2 [102] | Vaejovis mexicanus [Arachnida] | 5 µM/L | CC50 8.3 µM/L in MCF-7 human breast epithelial cells | Cell membrane disruption in P. gallinaceum sporozoites | 0.49 µM/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Fernández, N.; Anacleto-Santos, J.; Casarrubias-Tabarez, B.; López-Pérez, T.d.J.; Rojas-Lemus, M.; López-Valdez, N.; Fortoul, T.I. Bioactive Peptides against Human Apicomplexan Parasites. Antibiotics 2022, 11, 1658. https://doi.org/10.3390/antibiotics11111658
Rivera-Fernández N, Anacleto-Santos J, Casarrubias-Tabarez B, López-Pérez TdJ, Rojas-Lemus M, López-Valdez N, Fortoul TI. Bioactive Peptides against Human Apicomplexan Parasites. Antibiotics. 2022; 11(11):1658. https://doi.org/10.3390/antibiotics11111658
Chicago/Turabian StyleRivera-Fernández, Norma, Jhony Anacleto-Santos, Brenda Casarrubias-Tabarez, Teresa de Jesús López-Pérez, Marcela Rojas-Lemus, Nelly López-Valdez, and Teresa I. Fortoul. 2022. "Bioactive Peptides against Human Apicomplexan Parasites" Antibiotics 11, no. 11: 1658. https://doi.org/10.3390/antibiotics11111658
APA StyleRivera-Fernández, N., Anacleto-Santos, J., Casarrubias-Tabarez, B., López-Pérez, T. d. J., Rojas-Lemus, M., López-Valdez, N., & Fortoul, T. I. (2022). Bioactive Peptides against Human Apicomplexan Parasites. Antibiotics, 11(11), 1658. https://doi.org/10.3390/antibiotics11111658








