Comparison of Fitness Cost, Stability, and Conjugation Frequencies of tet(X4)-Positive Plasmids in Chicken and Pig Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Fitness Cost of the Tet(X4)-Bearing Plasmids in Engineered Bacteria TOP10, Pig E. coli, and Chicken E. coli
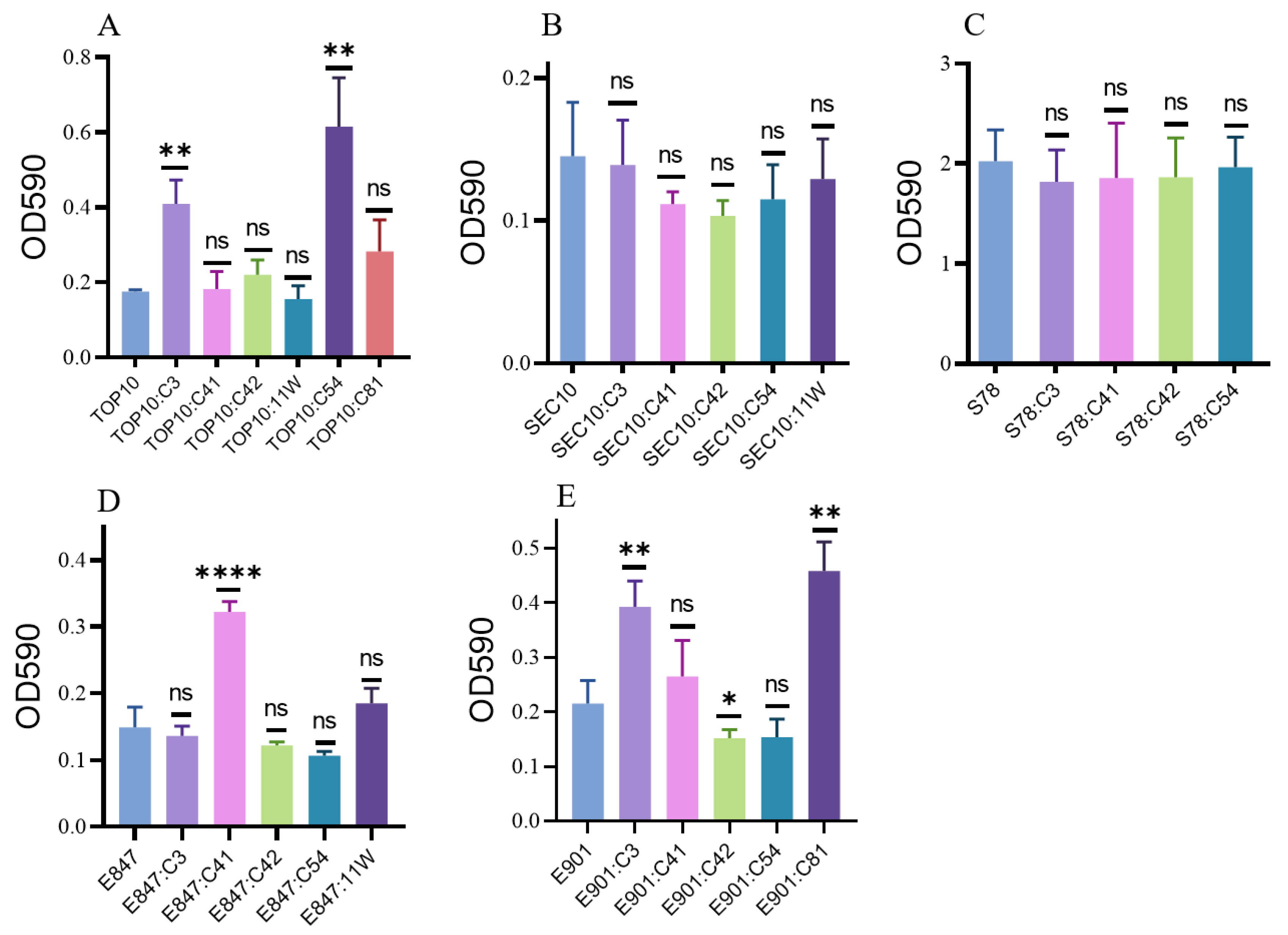
2.2. Biofilm Formation of the Tested Strains after Acquiring Various Tet(X4)-Bearing Plasmids
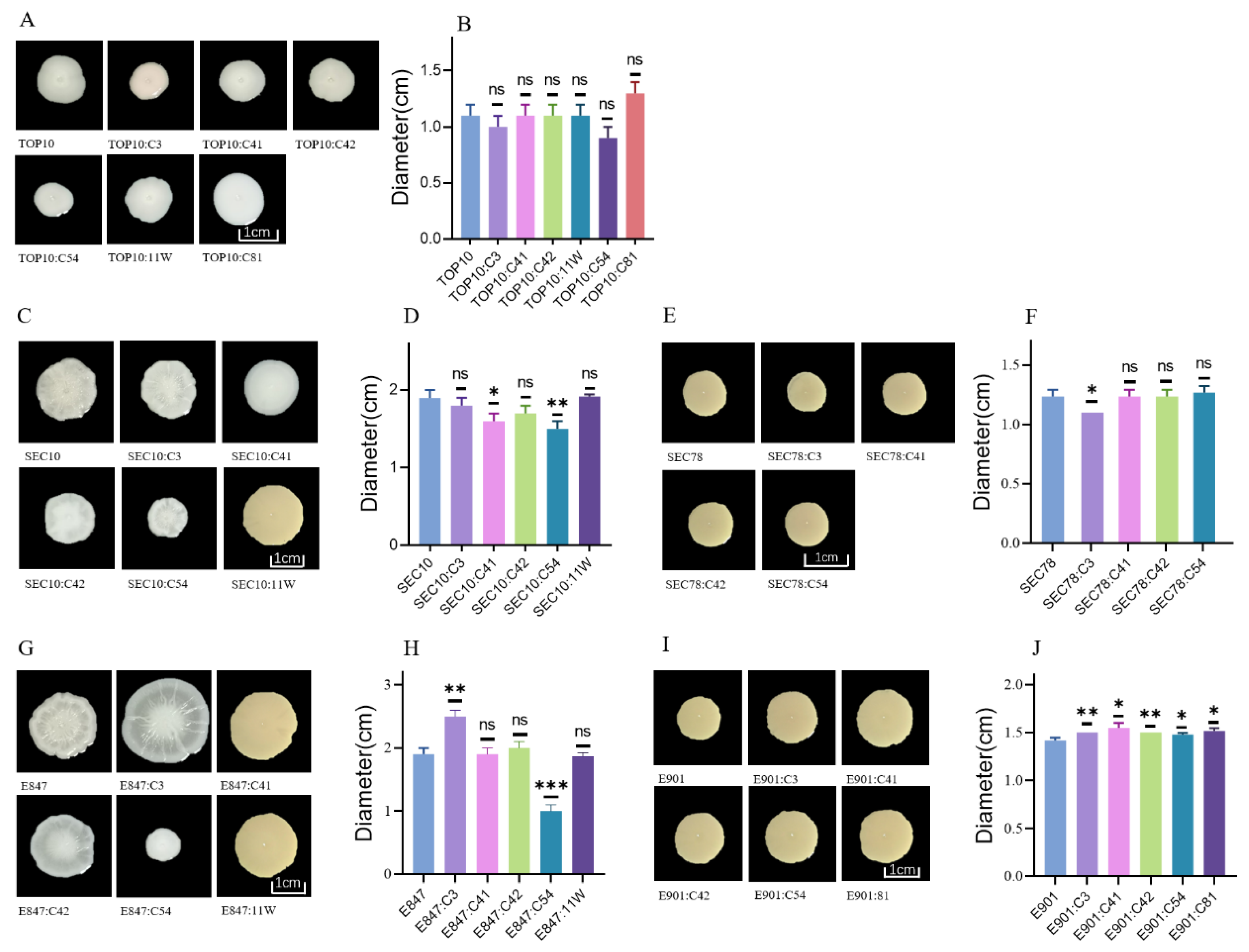
2.3. Swimming Motility of the Tested Strains after Acquiring Various Tet(X4)-Bearing Plasmids
2.4. Effect of Tetracycline on the Stability of Tet(X4)-Bearing Plasmids
2.5. Effect of Temperature on Conjugation Frequencies of the Tet(X4)-Bearing Plasmids
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Plasmids
4.2. Construction of Transformants
4.3. Pairwise Competition Assay
4.4. Biofilm Formation Assay
4.5. Motility Test
4.6. Stability of Tet(X4)-Bearing Plasmids during Serial Passaging
4.7. Conjugation Frequencies of Tet(X4)-Bearing Plasmids at 37 °C and 42 °C
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, T.; Wang, R.; Liu, D.; Walsh, T.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wen, J.; Wang, Y.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; Zhao, X.; Wu, Y.; et al. Dissemination and prevalence of plasmid-mediated high-level tigecycline resistance gene tet (X4). Front. Microbiol. 2022, 13, 969769. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, C.; Cui, C.-Y.; Zhang, Y.; Liu, X.; Cui, Z.-H.; Ma, X.-Y.; Feng, Y.-J.; Fang, L.-X.; Lian, X.-L.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
- Li, R.; Lu, X.; Peng, K.; Liu, Z.; Li, Y.; Liu, Y.; Xiao, X.; Wang, Z. Deciphering the Structural Diversity and Classification of the Mobile Tigecycline Resistance Gene tet (X)-Bearing Plasmidome among Bacteria. mSystems 2020, 5, e00134-20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Peng, K.; Liu, Y.; Xiao, X.; Mohsin, M.; Li, R.; Wang, Z. Distribution and genomic characterization of tigecycline-resistant tet(X4)-positive Escherichia coli of swine farm origin. Microb. Genom. 2021, 7, 000667. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Hassan, B.; Martins, W.M.; Li, R.; Abdullah, S.; Sands, K.; Walsh, T.R. Emergence of plasmid-mediated tigecycline resistance tet(X4) gene in Escherichia coli isolated from poultry, food and the environment in South Asia. Sci. Total Environ. 2021, 787, 147613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, K.; Yin, Y.; Sun, X.; Zhang, W.; Li, R.; Wang, Z. Occurrence and Molecular Characterization of Abundant tet(X) Variants Among Diverse Bacterial Species of Chicken Origin in Jiangsu, China. Front. Microbiol. 2021, 12, 751006. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhan, Z.; Shi, C. International Spread of Tet(X4)-Producing Escherichia coli Isolates. Foods 2022, 11, 2010. [Google Scholar] [CrossRef]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef]
- Bosák, J.; Hrala, M.; Pirková, V.; Micenková, L.; Čížek, A.; Smola, J.; Kučerová, D.; Vacková, Z.; Budinská, E.; Koláčková, I.; et al. Porcine pathogenic Escherichia coli strains differ from human fecal strains in occurrence of bacteriocin types. Veter Microbiol. 2019, 232, 121–127. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Alonso, M.P.; Blanco, J.E.; Blanco, J.; Mora, A. Clones of enterotoxigenic and Shiga toxin-producing Escherichia coli implicated in swine enteric colibacillosis in Spain and rates of antibiotic resistance. Veter Microbiol. 2020, 252, 108924. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Yassin, A.K.; Zhang, J.; Gong, J.; Qi, K.; Ganta, R.R.; Zhang, Y.; Yang, Y.; Han, X.; et al. Genetic characterization of extraintestinal Escherichia coli isolates from chicken, cow and swine. AMB Express 2018, 8, 117. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Veter Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, B.W.; Bearson, B.L.; Bearson, S.M.D.; Casey, T.A. Multidrug-Resistant Salmonella enterica Serovar Typhimurium Isolates Are Resistant to Antibiotics That Influence Their Swimming and Swarming Motility. mSphere 2017, 2, e00306-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Tang, F.; Jiang, L.; Li, R.; Wang, Z.; Liu, Y. Histone-Like Nucleoid Structuring Protein Modulates the Fitness of tet(X4)-Bearing IncX1 Plasmids in Gram-Negative Bacteria. Front. Microbiol. 2021, 12, 763288. [Google Scholar] [CrossRef]
- Tang, F.; Cai, W.; Jiang, L.; Wang, Z.; Liu, Y. Large-Scale Analysis of Fitness Cost of tet(X4)-Positive Plasmids in Escherichia coli. Front. Cell. Infect. Microbiol. 2022, 12, 798802. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chen, C.; Cui, C.; Li, X.; Zhang, Y.; Liao, X.; Sun, J.; Liu, Y. Emerging High-Level Tigecycline Resistance: Novel Tetracycline Destructases Spread via the Mobile Tet(X). BioEssays 2020, 42, e2000014. [Google Scholar] [CrossRef]
- Sun, C.; Cui, M.; Zhang, S.; Wang, H.; Song, L.; Zhang, C.; Zhao, Q.; Lui, D.; Wang, Y.; Shen, J.; et al. Plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from food-producing animals, China, 2008–2018. Emerg. Microbes. Infect. 2019, 8, 1524–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Liu, F.; Cao, J.; Lv, N.; Zhu, B.; Zhang, G.; Gao, G.F. Integrated metagenomic and metatranscriptomic profiling reveals differentially expressed resistomes in human, chicken, and pig gut microbiomes. Environ. Int. 2020, 138, 105649. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, L.; Fang, Y.; Jia, X.; Chen, J. High temperatures can effectively degrade residual tetracyclines in chicken manure through composting. J. Hazard. Mater. 2019, 380, 120862. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2020, 753, 141975. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X.; et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: An epidemiological comparative study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef]
- Gilmour, M.W.; Lawley, T.D.; Rooker, M.M.; Newnham, P.J.; Taylor, D.E. Cellular location and temperature-dependent assembly of IncHI1 plasmid R27-encoded TrhC-associated conjugative transfer protein complexes. Mol. Microbiol. 2001, 42, 705–715. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; A Wagenaar, J.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [Green Version]
- Rozwandowicz, M.; Brouwer, M.S.M.; Mughini-Gras, L.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Mevius, D.J.; Hordijk, J. Successful Host Adaptation of IncK2 Plasmids. Front. Microbiol. 2019, 10, 2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas-Ferrando, E.; Guirado, P.; Miró, E.; Iglesias-Torrens, Y.; Navarro, F.; Alioto, T.; Gómez-Garrido, J.; Madrid, C.; Balsalobre, C. Tetracycline resistance transmission in Campylobacter is promoted at temperatures resembling the avian reservoir. Veter Microbiol. 2020, 244, 108652. [Google Scholar] [CrossRef]
- Maluta, R.P.; Logue, C.M.; Casas, M.R.T.; Meng, T.; Guastalli, E.A.L.; Rojas, T.C.G.; Montelli, A.C.; Sadatsune, T.; de Carvalho Ramos, M.; Nolan, L.K.; et al. Overlapped Sequence Types (STs) and Serogroups of Avian Pathogenic (APEC) and Human Extra-Intestinal Pathogenic (ExPEC) Escherichia coli Isolated in Brazil. PLoS ONE 2014, 9, e105016. [Google Scholar] [CrossRef]
- Ievy, S.; Hoque, M.N.; Islam, S.; Sobur, A.; Ballah, F.M.; Rahman, M.S.; Rahman, B.; Hassan, J.; Khan, M.F.R.; Rahman, T. Genomic characteristics, virulence, and antimicrobial resistance in avian pathogenic Escherichia coli MTR_BAU02 strain isolated from layer farm in Bangladesh. J. Glob. Antimicrob. Resist. 2022, 30, 155–162. [Google Scholar] [CrossRef]
- Hayer, S.S.; Lim, S.; Hong, S.; Elnekave, E.; Johnson, T.; Rovira, A.; Vannucci, F.; Clayton, J.B.; Perez, A.; Alvarez, J. Genetic Determinants of Resistance to Extended-Spectrum Cephalosporin and Fluoroquinolone in Escherichia coli Isolated from Diseased Pigs in the United States. mSphere 2020, 5, e00990-20. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Hikoda, Y.; Fujii, Y.; Murata, M.; Miyoshi, H.; Ogura, Y.; Gotoh, Y.; Iwata, T.; Hayashi, T.; Akiba, M. Emergence of a Multidrug-Resistant Shiga Toxin-Producing Enterotoxigenic Escherichia coli Lineage in Diseased Swine in Japan. J. Clin. Microbiol. 2016, 54, 1074–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, Q.; Peng, K.; Liu, Y.; Li, R.; Wang, Z. Emergence of Carbapenem- and Tigecycline-Resistant Proteus cibarius of Animal Origin. Front. Microbiol. 2020, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Lu, X.; Peng, K.; Chen, S.; He, S.; Li, R. Structural Diversity, Fitness Cost, and Stability of a BlaNDM-1-Bearing Cointegrate Plasmid in Klebsiella pneumoniae and Escherichia coli. Microorganisms 2021, 9, 2435. [Google Scholar] [CrossRef]
- Liu, B.; Shui, L.; Zhou, K.; Jiang, Y.; Li, X.; Guan, J.; Li, Q.; Zhuo, C. Impact of Plasmid-Encoded H-NS–like Protein on blaNDM-1-Bearing IncX3 Plasmid in Escherichia coli. J. Infect. Dis. 2020, 221, S229–S236. [Google Scholar] [CrossRef] [PubMed]



| Strains | C3 (Pig) | C41 (Pig) | C42 (Pig) | C54 (Pig) | C81 (Pig) | 11W (Chicken) |
|---|---|---|---|---|---|---|
| TOP10 | TOP10:C3 | TOP10:C41 | TOP10:C42 | TOP10:C54 | TOP10:C81 | TOP10:11W |
| SEC10 (pig) | SEC10:C3 | SEC10:C41 | SEC10:C42 | SEC10:C54 | F | SEC10:11W |
| SEC78 (pig) | SEC78:C3 | SEC78:C41 | SEC78:C42 | SEC78:C54 | F | F |
| E847 (chicken) | E847:C3 | E847:C41 | E847:C42 | E847:C54 | F | E847:11W |
| E901 (chicken) | E901:C3 | E901:C41 | E901:C42 | E901:C54 | E901:C81 | F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, H.; Xiao, X.; Liu, Y.; Li, R.; Wang, Z. Comparison of Fitness Cost, Stability, and Conjugation Frequencies of tet(X4)-Positive Plasmids in Chicken and Pig Escherichia coli. Antibiotics 2022, 11, 1657. https://doi.org/10.3390/antibiotics11111657
Liu Z, Zhang H, Xiao X, Liu Y, Li R, Wang Z. Comparison of Fitness Cost, Stability, and Conjugation Frequencies of tet(X4)-Positive Plasmids in Chicken and Pig Escherichia coli. Antibiotics. 2022; 11(11):1657. https://doi.org/10.3390/antibiotics11111657
Chicago/Turabian StyleLiu, Ziyi, Huiru Zhang, Xia Xiao, Yuan Liu, Ruichao Li, and Zhiqiang Wang. 2022. "Comparison of Fitness Cost, Stability, and Conjugation Frequencies of tet(X4)-Positive Plasmids in Chicken and Pig Escherichia coli" Antibiotics 11, no. 11: 1657. https://doi.org/10.3390/antibiotics11111657
APA StyleLiu, Z., Zhang, H., Xiao, X., Liu, Y., Li, R., & Wang, Z. (2022). Comparison of Fitness Cost, Stability, and Conjugation Frequencies of tet(X4)-Positive Plasmids in Chicken and Pig Escherichia coli. Antibiotics, 11(11), 1657. https://doi.org/10.3390/antibiotics11111657







