Design of a Bacteriophage Cocktail Active against Shigella Species and Testing of Its Therapeutic Potential in Galleria mellonella
Abstract
:1. Introduction
2. Results
2.1. Phage Isolation
2.2. Phage Genome Analysis
| # | Strain | Serotype | Origin | # | Strain | Serotype | Origin |
|---|---|---|---|---|---|---|---|
| 1 | S. flexneri 27 | 1 | Vietnam | 50 | S. flexneri 83 | 3a | Kenya |
| 2 | S. flexneri 46 | 1 | Thailand | 51 | S. flexneri 84 | 3a | Kenya |
| 3 | S. flexneri 13 | 1a | Bhutan | 52 | S. flexneri J17B * | 3a | Japan |
| 4 | S. flexneri 28 | 1a | Vietnam | 53 | S. flexneri 38 | 3b | Vietnam |
| 5 | S. flexneri 61 | 1a | Nepal | 54 | S. flexneri 9 | 4 | Cambodia |
| 6 | S. flexneri 2 | 1b | Cambodia | 55 | S. flexneri 22 | 4 | Bhutan |
| 7 | S. flexneri 3 | 1b | Cambodia | 56 | S. flexneri 39 | 4 | Vietnam |
| 8 | S. flexneri 14 | 1b | Bhutan | 57 | S. flexneri 54 | 4 | Thailand |
| 9 | S. flexneri 15 | 1b | Bhutan | 58 | S. flexneri 70 | 4 | Nepal |
| 10 | S. flexneri 19 | 1b | Bhutan | 59 | S. flexneri 40 | 4a | Vietnam |
| 11 | S. flexneri 29 | 1b | Vietnam | 60 | S. flexneri 41 | 4a | Vietnam |
| 12 | S. flexneri 30 | 1b | Vietnam | 61 | S. flexneri 55 | 5 | Thailand |
| 13 | S. flexneri 47 | 1b | Thailand | 62 | S. flexneri M90T | 5a | USA |
| 14 | S. flexneri 62 | 1b | Nepal | 63 | S. flexneri M90T55 b* | 5a | Laboratory |
| 15 | S. flexneri 82 | 1b | Kenya | 64 | S. flexneri 10 | 6 | Cambodia |
| 16 | S. flexneri 63 | 1c | Nepal | 65 | S. flexneri 11 | 6 | Cambodia |
| 17 | S. flexneri 16 | 2 | Bhutan | 66 | S. flexneri 23 | 6 | Bhutan |
| 18 | S. flexneri 31 | 2 | Vietnam | 67 | S. flexneri 24 | 6 | Bhutan |
| 19 | S. flexneri 48 | 2 | Thailand | 68 | S. flexneri 42 | 6 | Vietnam |
| 20 | S. flexneri 4 | 2a | Cambodia | 69 | S. flexneri 43 | 6 | Vietnam |
| 21 | S. flexneri 5 | 2a | Cambodia | 70 | S. flexneri 56 | 6 | Thailand |
| 22 | S. flexneri 17 | 2a | Bhutan | 71 | S. flexneri 57 | 6 | Thailand |
| 23 | S. flexneri 18 | 2a | Bhutan | 72 | S. flexneri 71 | 6 | Nepal |
| 24 | S. flexneri 32 | 2a | Vietnam | 73 | S. flexneri 72 | 6 | Nepal |
| 25 | S. flexneri 33 | 2a | Vietnam | 74 | S. flexneri 85 | 6 | Kenya |
| 26 | S. flexneri 49 | 2a | Thailand | 75 | S. flexneri SSU2415 * | 6 | USA |
| 27 | S. flexneri 50 | 2a | Thailand | 76 | S. flexneri CCH060 * | 6 | Unknown |
| 28 | S. flexneri 64 | 2a | Nepal | 77 | S. flexneri 58 | var. X | Thailand |
| 29 | S. flexneri 65 | 2a | Nepal | 78 | S. flexneri 44 | var. Y | Vietnam |
| 30 | S. flexneri 81 | 2a | Kenya | 79 | S. sonnei 1 | NA | Cambodia |
| 31 | S. flexneri 2457T * | 2a | Japan | 80 | S. sonnei 12 | NA | Bhutan |
| 32 | S. flexneri BS103 a* | 2a | Laboratory | 81 | S. sonnei 26 | NA | Vietnam |
| 33 | S. flexneri 6 | 2b | Cambodia | 82 | S. sonnei 45 | NA | Thailand |
| 34 | S. flexneri 34 | 2b | Vietnam | 83 | S. sonnei 60 | NA | Nepal |
| 35 | S. flexneri 66 | 2b | Nepal | 84 | S. sonnei Moseley * | NA | USA |
| 36 | S. flexneri ATCC 12022 | 2b | Unknown | 85 | S. sonnei ATCC 25931 | NA | Panama |
| 37 | S. flexneri 35 | 2ab | Vietnam | 86 | S. dysenteriae 59 | 1 | Thailand |
| 38 | S. flexneri 51 | 3 | Thailand | 87 | S. dysenteriae 73 | 1 | Nepal |
| 39 | S. flexneri 7 | 3a | Cambodia | 88 | S. dysenteriae 1617 * | 1 | Guatemala |
| 40 | S. flexneri 8 | 3a | Cambodia | 89 | S. dysenteriae 74 | 2 | Nepal |
| 41 | S. flexneri 20 | 3a | Bhutan | 90 | S. dysenteriae 75 | 9 | Nepal |
| 42 | S. flexneri 21 | 3a | Bhutan | 91 | S. dysenteriae 76 | 12 | Nepal |
| 43 | S. flexneri 36 | 3a | Vietnam | 92 | S. dysenteriae 87 | 12 | Kenya |
| 44 | S. flexneri 37 | 3a | Vietnam | 93 | S. boydii 77 | 1 | Nepal |
| 45 | S. flexneri 52 | 3a | Thailand | 94 | S. boydii 25 | 2 | Bhutan |
| 46 | S. flexneri 53 | 3a | Thailand | 95 | S. boydii 78 | 2 | Nepal |
| 47 | S. flexneri 67 | 3a | Nepal | 96 | S. boydii 86 | 2 | Kenya |
| 48 | S. flexneri 68 | 3a | Nepal | 97 | S. boydii 79 | 10 | Nepal |
| 49 | S. flexneri 69 | 3a | Nepal | 98 | S. boydii 80 | 12 | Nepal |
| Phage ID | Genome Size, bp | Accession No. | Phage taxonomy a | Closest Relative in NCBI Database b | |||
|---|---|---|---|---|---|---|---|
| Family | Subfamily | Genus | Definition | Accession No. | |||
| ESh1 | 39,034 | ON528715 | Autographiviridae | Studiervirinae | Teseptimavirus | 64795_ec1 | KU927499 |
| ESh2 | 39,818 | ON528716 | Autographiviridae | Studiervirinae | Teseptimavirus | JeanTinguely Bas64 | MZ501081 |
| ESh3 | 39,180 | ON528717 | Autographiviridae | Studiervirinae | Teseptimavirus | 64795_ec1 | KU927499 |
| ESh4 | 51,077 | ON528718 | Drexlerviridae | Tempevirinae | Hanrivervirus | herni | NC_049823 |
| ESh6 | 39,381 | ON528719 | Autographiviridae | Studiervirinae | Teseptimavirus | JeanTinguely Bas64 | MZ501081 |
| ESh7 | 39,724 | ON528720 | Autographiviridae | Studiervirinae | Teetrevirus | vB_KpnP_IME305 | OK149215 |
| ESh8 | 38,701 | ON528721 | Autographiviridae | Studiervirinae | Teetrevirus | phiYe-F10 | NC_047755 |
| ESh9 | 39,308 | ON528722 | Autographiviridae | Studiervirinae | Teetrevirus | 2050H2 | NC_047844 |
| ESh10 | 38,729 | ON528723 | Autographiviridae | Studiervirinae | Teetrevirus | vB_YenP_AP5 | KM253764 |
| ESh12 | 39,704 | ON528724 | Autographiviridae | Studiervirinae | Teetrevirus | 2050H2 | NC_047844 |
| ESh15 | 168,076 | ON528725 | Straboviridae | Tevenvirinae | Mosigvirus | SHSML-52-1 | KX130865 |
| ESh16 | 165,784 | ON528726 | Straboviridae | Tevenvirinae | Tequatrovirus | Sfk20 | MW341595 |
| ESh17 | 166,355 | ON528727 | Straboviridae | Tevenvirinae | Tequatrovirus | slur07 | LN881732 |
| ESh18 | 165,470 | ON528728 | Straboviridae | Tevenvirinae | Tequatrovirus | Kha5h | NC_054905 |
| ESh19 | 87,867 | ON528729 | Myoviridae c | Ounavirinae | Mooglevirus | vB_EcoM_3HA14 | MN342151 |
| ESh20 | 89,515 | ON528730 | Myoviridae c | Ounavirinae | Mooglevirus | vB_EcoM_3HA14 | MN342151 |
| ESh21 | 86,414 | ON528731 | Myoviridae c | Ounavirinae | Mooglevirus | KPS64 | MK562502 |
| ESh22 | 88,154 | ON528732 | Myoviridae c | Ounavirinae | Mooglevirus | vB_EcoM_3HA14 | MN342151 |
| ESh23 | 40,156 | ON528733 | Autographiviridae | Studiervirinae | Kayfunavirus | SFPH2 | NC_048025 |
| ESh24 | 167,086 | ON528734 | Straboviridae | Tevenvirinae | Tequatrovirus | vB_EcoM_F1 | NC_054912 |
| ESh25 | 166,499 | ON528735 | Straboviridae | Tevenvirinae | Tequatrovirus | Aplg8 | NC_054902 |
| ESh26 | 167,539 | ON528736 | Straboviridae | Tevenvirinae | Tequatrovirus | UGKSEcP2 | OV876900 |
| ESh27 | 168,955 | ON528737 | Straboviridae | Tevenvirinae | Mosigvirus | phiC120 | NC_055718 |
| ESh28 | 164,289 | ON528738 | Straboviridae | Tevenvirinae | Tequatrovirus | JK23 | MK962752 |
| ESh29 | 166,160 | ON528739 | Straboviridae | Tevenvirinae | Tequatrovirus | vB_EcoM_Shinka | MZ502379 |
| ESh30 | 170,189 | ON528740 | Straboviridae | Tevenvirinae | Tequatrovirus | fPS-2 | NC_054943 |
| ESh31 | 167,224 | ON528741 | Straboviridae | Tevenvirinae | Tequatrovirus | PhiZZ30 | NC_054938 |
| ESh32 | 169,173 | ON528742 | Straboviridae | Tevenvirinae | Tequatrovirus | vB_EcoM_G2133 | MK327928 |
| ESh33 | 166,484 | ON528743 | Straboviridae | Tevenvirinae | Tequatrovirus | vB_SboM_Phaginator | OL615012 |
| ESh34 | 167,055 | ON528744 | Straboviridae | Tevenvirinae | Tequatrovirus | Sfk20 | MW341595 |
| ESh35 | 166,919 | ON528745 | Straboviridae | Tevenvirinae | Tequatrovirus | KIT03 | NC_054923 |
| ESh36 | 170,646 | ON528746 | Straboviridae | Tevenvirinae | Tequatrovirus | T4_ev151 | LR597660 |
2.3. Phage Morphology
2.4. Prototype Phage Cocktails
2.5. Host Range Testing
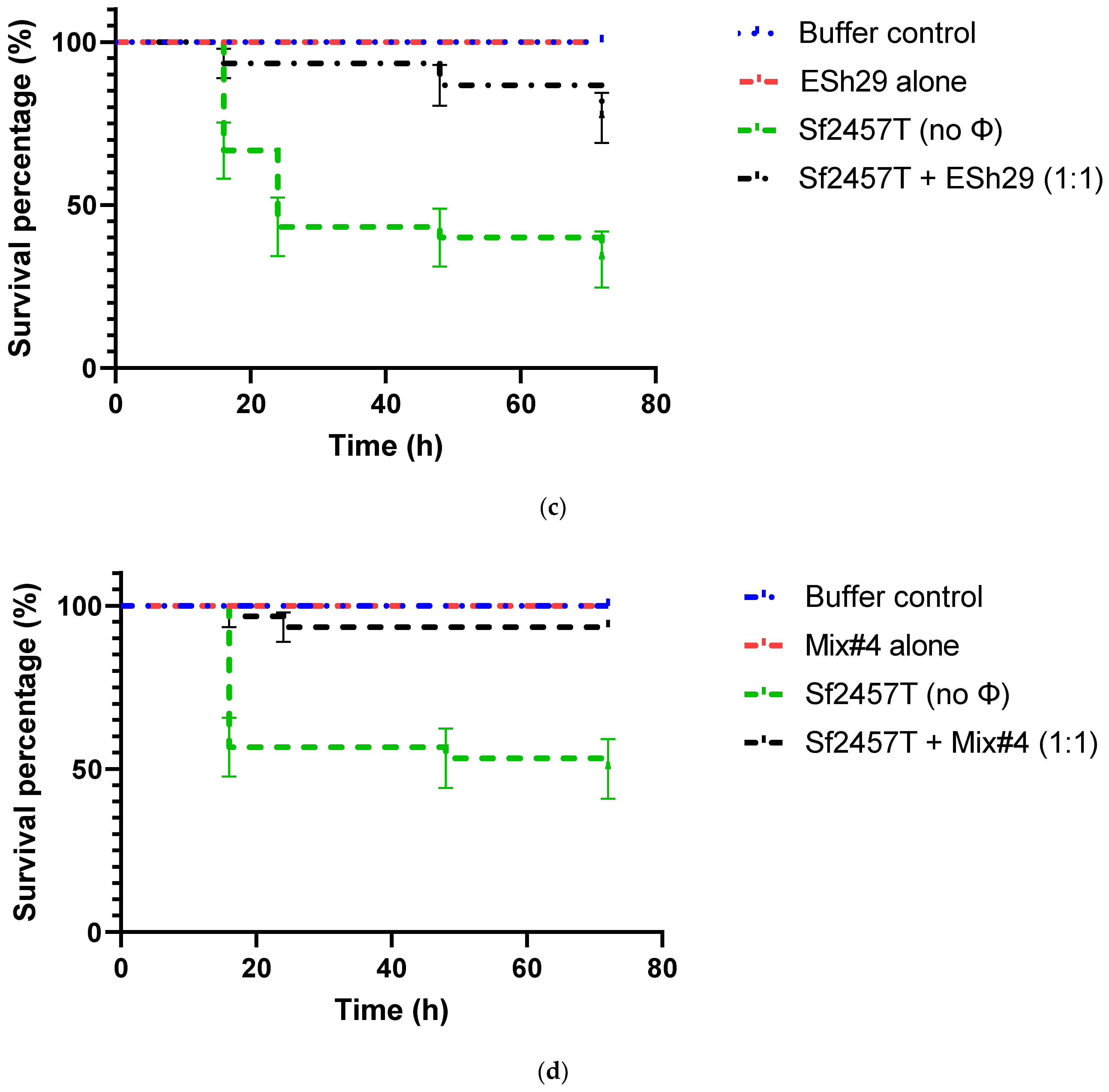
2.6. Phage Treatment of Shigella Infection of G. mellonella Larvae
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Media
4.2. Isolation of Phages
4.3. Phage Propagation
4.4. Phage DNA Isolation, Restriction Analysis and Genome Sequencing
4.5. Phage Phylogenetic Tree
4.6. Transmission Electron Microscopy
4.7. Phage Host Range Testing
4.8. Assessment of Phage Protection against Infection of G. mellonella Larvae with Shigella Strains
4.9. Statistical Analysis
4.10. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Baker, S.; The, H.C. Recent insights into Shigella. Curr. Opin. Infect. Dis. 2018, 31, 449–454. [Google Scholar] [CrossRef]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.; Hall, A.; Riddle, M.S.; Porter, C.K. Travelers’ diarrhea: Update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop. Dis. Travel Med. Vaccines 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Cassels, F.; Riddle, M.; Walker, R.; Wierzba, T. Vaccines against Shigella and enterotoxigenic Escherichia coli: A summary of the 2018 VASE Conference. Vaccine 2019, 37, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Chapartegui-González, I.; Bowser, S.; Torres, A.G.; Khakhum, N. Recent progress in Shigella and Burkholderia pseudomallei vaccines. Pathogens 2021, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public. Health 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Shahin, K.; Bouzari, M.; Komijani, M.; Wang, R. A new phage cocktail against multidrug, ESBL-producer isolates of Shigella sonnei and Shigella flexneri with highly efficient bacteriolytic activity. Microb. Drug Resist. 2020, 26, 831–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, B.A.; Riddle, M.S. Post-infectious sequelae of travelers’ diarrhea. J. Travel Med. 2013, 20, 303–312. [Google Scholar] [CrossRef]
- Sethuvel, D.P.M.; Ragupathi, N.K.D.; Anandan, S.; Veeraraghavan, B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett. Appl. Microbiol. 2017, 64, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, B.; Ochieng, J.B.; Ikumapayi, U.N.; Toure, A.; Ahmed, D.; Li, S.; Panchalingam, S.; Levine, M.M.; Kotloff, K.; Rasko, D.A.; et al. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J. Clin. Microbiol. 2013, 51, 1740–1746. [Google Scholar] [CrossRef] [Green Version]
- Pavlinac, P.B.; Platts-Mills, J.A.; Tickell, K.D.; Liu, J.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; et al. The clinical presentation of culture-positive and culture-negative, quantitative polymerase chain reaction (qPCR)-attributable shigellosis in the Global Enteric Multicenter Study and derivation of a Shigella severity score: Implications for pediatric Shigella vaccine trials. Clin. Infect. Dis. 2021, 73, e569–e579. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.W.; Putnam, S.D.; Gould, P.; Kolisnyk, J.; Merced, N.; Barthel, V.; Rozmajzl, P.J.; Shaheen, H.; Fouad, S.; Frenck, R.W. Diarrheal illness among deployed U.S. military personnel during Operation Bright Star 2001--Egypt. Diagn. Microbiol. Infect. Dis. 2005, 52, 85–90. [Google Scholar] [CrossRef]
- Porter, C.K.; Olson, S.; Hall, A.; Riddle, M.S. Travelers’ diarrhea: An update on the incidence, etiology, and risk in military deployments and similar travel populations. Mil. Med. 2017, 182, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodhidatta, L.; McDaniel, P.; Sornsakrin, S.; Srijan, A.; Serichantalergs, O.; Mason, C.J. Case-control study of diarrheal disease etiology in a remote rural area in Western Thailand. Am. J. Trop. Med. Hyg. 2010, 83, 1106–1109. [Google Scholar] [CrossRef] [Green Version]
- Swierczewski, B.E.; Odundo, E.A.; Koech, M.C.; Ndonye, J.N.; Kirera, R.K.; Odhiambo, C.P.; Cheruiyot, E.K.; Wu, M.T.; Lee, J.E.; Zhang, C.; et al. Surveillance for enteric pathogens in a case-control study of acute diarrhea in Western Kenya. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage therapy: Developments and directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, E208–E220. [Google Scholar] [CrossRef]
- Llanos-Chea, A.; Citorik, R.J.; Nickerson, K.P.; Ingano, L.; Serena, G.; Senger, S.; Lu, T.K.; Fasano, A.; Faherty, C.S. Bacteriophage therapy testing against Shigella flexneri in a novel human intestinal organoid-derived infection model. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Ukhanova, M.; Reinhard, M.K.; Li, M.; Sulakvelidze, A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015, 5, e1088124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, V.; Ukhanova, M.; Visone, L.; Abuladze, T.; Sulakvelidze, A. Bacteriophage administration reduces the concentration of Listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int. J. Microbiol. 2010, 2010, 624234. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages reduce pathogenic Escherichia coli counts in mice without distorting gut microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.; Cairns, L.S.; Camilli, A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat. Commun. 2017, 8, 14187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodridge, L.D. Bacteriophages for managing Shigella in various clinical and non-clinical settings. Bacteriophage 2013, 3, e25098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.S.; Biswas, S.K.; Tan, W.S.; Saha, A.K.; Leo, B.F. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. PeerJ 2019, 7, e6225. [Google Scholar] [CrossRef] [Green Version]
- Solodovnikov, I.P.; Pavlova, L.I.; Emel’ianov, P.I.; Garnova, N.A.; Nogteva, I.B.; Sotemskiĭ, I.S.; Bogdashich, O.M.; Arshinova, V.V. The prophylactic use of dry polyvalent dysentery bacteriophage with pectin in preschool children’s institutions. I. Results of a strictly controlled epidemiologic trial (Yaroslavl, 1968). Zh. Mikrobiol. Epidemiol. Immunobiol. 1970, 47, 131–137. [Google Scholar] [PubMed]
- Solodovnikov, I.P.; Pavlova, L.I.; Garnova, N.A.; Nogteva, I.B.; Sotemskiĭ, I.S. Preventive use of dry polyvalent dysentery bacteriophage in preschool institutions. II. Principles of present-day tactics and application schedule of bacteriophage. Zh. Mikrobiol. Epidemiol. Immunobiol. 1971, 48, 123–127. [Google Scholar] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L. Novel “superspreader” bacteriophages promote horizontal gene transfer by transformation. mBio 2017, 8, e02115-16. [Google Scholar] [CrossRef] [Green Version]
- Maurelli, A.T.; Blackmon, B.; Curtiss, R., III. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect. Immun. 1984, 43, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formal, S.B.; Oaks, E.V.; Olsen, R.E.; Wingfield-Eggleston, M.; Snoy, P.J.; Cogan, J.P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 1991, 164, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, T.H.; Brinkac, L.M.; Sutton, G.; Fouts, D.E. GGRaSP: A R-package for selecting representative genomes using Gaussian mixture models. Bioinformatics 2018, 34, 3032–3034. [Google Scholar] [CrossRef]
- Denou, E.; Bruttin, A.; Barretto, C.; Ngom-Bru, C.; Brüssow, H.; Zuber, S. T4 phages against Escherichia coli diarrhea: Potential and problems. Virology 2009, 388, 21–30. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Kumlien, A.C.M.; Pérez-Vega, C.; González-Villalobos, E.; Borrego, C.M.; Balcázar, J.L. Genome analysis of a new Escherichia phage vB_EcoM_C2-3 with lytic activity against multidrug-resistant Escherichia coli. Virus Res. 2022, 307, 198623. [Google Scholar] [CrossRef] [PubMed]
- Doore, S.M.; Schrad, J.R.; Dean, W.F.; Dover, J.A.; Parent, K.N. Shigella phages isolated during a dysentery outbreak reveal uncommon structures and broad species diversity. J. Virol. 2018, 92, e02117-17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, W.; Liu, W.; Zou, L.; Yan, C.; Lu, K.; Ren, H. T4-like phage Bp7, a potential antimicrobial agent for controlling drug-resistant Escherichia coli in chickens. Appl. Environ. Microbiol. 2013, 79, 5559–5565. [Google Scholar] [CrossRef]
- Kaczorowska, J.; Casey, E.; Neve, H.; Franz, C.M.A.P.; Noben, J.-P.; Lugli, G.A.; Ventura, M.; van Sinderen, D.; Mahony, J. A quest of great importance-developing a broad spectrum Escherichia coli phage collection. Viruses 2019, 11, 899. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.D.; Liu, H.; Du, H.; Meng, R.; Mahmoud, E.S.; Wang, G.; McAllister, T.A.; Stanford, K. Efficacy of individual bacteriophages does not predict efficacy of bacteriophage cocktails for control of Escherichia coli O157. Front. Microbiol. 2021, 12, 616712. [Google Scholar] [CrossRef]
- Pham-Khan, N.H.; Sunahara, H.; Yamadeya, H.; Sakai, M.; Nakayama, T.; Yamamoto, H.; Bich, V.T.T.; Miyanaga, K.; Kamei, K. Isolation, characterisation and complete genome sequence of a Tequatrovirus phage, Escherichia phage KIT03, which simultaneously infects Escherichia coli O157:H7 and Salmonella enterica. Curr. Microbiol. 2019, 76, 1130–1137. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Han, K.; Wang, L.; Cao, Y.; Ma, D.; Wang, X. A polyvalent broad-spectrum Escherichia phage Tequatrovirus EP01 capable of controlling Salmonella and Escherichia coli contamination in foods. Viruses 2022, 14, 286. [Google Scholar] [CrossRef]
- Hamdi, S.; Rousseau, G.M.; Labrie, S.J.; Tremblay, D.M.; Kourda, R.S.; Slama, K.B.; Moineau, S. Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci. Rep. 2017, 7, 40349. [Google Scholar] [CrossRef] [Green Version]
- Skurnik, M.; Jaakkola, S.; Mattinen, L.; von Ossowski, L.; Nawaz, A.; Pajunen, M.I.; Happonen, L.J. Bacteriophages fEV-1 and fD1 infect Yersinia pestis. Viruses 2021, 13, 1384. [Google Scholar] [CrossRef]
- Jansen, M.; Wahida, A.; Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 14140. [Google Scholar] [CrossRef] [Green Version]
- Peters, D.L.; Stothard, P.; Dennis, J.J. The isolation and characterization of Stenotrophomonas maltophilia T4-like bacteriophage DLP6. PLoS ONE 2017, 12, e0173341. [Google Scholar] [CrossRef] [Green Version]
- Schofield, D.A.; Wray, D.J.; Molineux, I.J. Isolation and development of bioluminescent reporter phages for bacterial dysentery. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 395–403. [Google Scholar] [CrossRef]
- Whichard, J.M.; Sriranganathan, N.; Pierson, F.W. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food Prot. 2003, 66, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Parent, K.N.; Doore, S.M. Ecology, Structure, and Evolution of Shigella Phages. Annu. Rev. Virol. 2020, 7, 121–141. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Ma, H.; Bao, R.; Liu, H.; Yang, L.; Liang, B.; Jia, L.; Xie, J.; Xiang, Y.; et al. Characterization and genomic analysis of SFPH2, a novel T7virus infecting Shigella. Front. Microbiol. 2018, 9, 3027. [Google Scholar] [CrossRef]
- Goebel, W.F.; Jesaitis, M.A. The somatic antigen of a phage-resistant variant of phase II Shigella sonnei. J. Exp. Med. 1952, 96, 425–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, R. The genetics of T-odd phages. Annu. Rev. Microbiol. 1973, 27, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.H.; Schroeder, C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol. Rev. 1981, 45, 9–51. [Google Scholar] [CrossRef]
- Dunn, J.J.; Studier, F.W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 1983, 166, 477–535. [Google Scholar] [CrossRef]
- Pajunen, M.I.; Elizondo, M.R.; Skurnik, M.; Kieleczawa, J.; Molineux, I.J. Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J. Mol. Biol. 2002, 319, 1115–1132. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y.; Xu, J.; Yu, X.; Huang, X.; Liu, G.; Liu, X. Identification of novel bacteriophage vB_EcoP-EG1 with lytic activity against planktonic and biofilm forms of uropathogenic Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 315–326. [Google Scholar] [CrossRef]
- Sofy, A.R.; El-Dougdoug, N.K.; Refaey, E.E.; Dawoud, R.A.; Hmed, A.A. Characterization and full genome sequence of novel KPP-5 lytic phage against Klebsiella pneumoniae responsible for recalcitrant Infection. Biomedicines 2021, 9, 342. [Google Scholar] [CrossRef]
- Garcia, E.; Elliott, J.M.; Ramanculov, E.; Chain, P.S.; Chu, M.C.; Molineux, I.J. The genome sequence of Yersinia pestis bacteriophage φA1122 reveals an intimate history with the coliphage T3 and T7 genomes. J. Bacteriol. 2003, 185, 5248–5262. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Characterization and complete genome sequence of the Shigella bacteriophage pSf-1. Res. Microbiol. 2013, 164, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.; Resch, G.; Pirnay, J.-P.; Blasdel, B.G.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E.; et al. Expert opinion on three phage therapy related topics: Bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernasconi, O.J.; Donà, V.; Tinguely, R.; Endimiani, A. In vitro activity of 3 commercial bacteriophage cocktails against Salmonella and Shigella spp. isolates of human origin. Pathog. Immun. 2018, 3, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a suitable model of bacterial infection: Past, present and future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Barnoy, S.; Gancz, H.; Zhu, Y.; Honnold, C.L.; Zurawski, D.V.; Venkatesan, M.M. The Galleria mellonella larvae as an in vivo model for evaluation of Shigella virulence. Gut Microbes 2017, 8, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Seed, K.D.; Dennis, J.J. Experimental bacteriophage therapy increases survival of Galleria mellonella larvae infected with clinically relevant strains of the Burkholderia cepacia complex. Antimicrob. Agents Chemother. 2009, 53, 2205–2208. [Google Scholar] [CrossRef] [Green Version]
- Nale, J.Y.; Chutia, M.; Carr, P.; Hickenbotham, P.T.; Clokie, M.R.J. ‘Get in early’; biofilm and wax moth (Galleria mellonella) models reveal new insights into the therapeutic potential of Clostridium difficile bacteriophages. Front. Microbiol. 2016, 7, 1383. [Google Scholar] [CrossRef] [Green Version]
- Thiry, D.; Passet, V.; Danis-Wlodarczyk, K.; Lood, C.; Wagemans, J.; De Sordi, L.; van Noort, V.; Dufour, N.; Debarbieux, L.; Mainil, J.G.; et al. New bacteriophages against emerging lineages ST23 and ST258 of Klebsiella pneumoniae and efficacy assessment in Galleria mellonella larvae. Viruses 2019, 11, 411. [Google Scholar] [CrossRef] [Green Version]
- El Haddad, L.; Harb, C.P.; Stibich, M.; Chemaly, R.F.; Chemaly, R.F. 735. Bacteriophage therapy improves survival of Galleria mellonella larvae injected with vancomycin-resistant Enterococcus faecium. Open Forum Infect. Dis. 2019, 6, S329. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Wang, L.; Tavanti, A.; Trampuz, A.; Di Luca, M. Antibacterial efficacy of two commercially available bacteriophage formulations, Staphylococcal bacteriophage and PYO bacteriophage, against methicillin-resistant Staphylococcus aureus: Prevention and eradication of biofilm formation and control of a systemic infection of Galleria mellonella larvae. Front. Microbiol. 2020, 11, 110. [Google Scholar] [CrossRef]
- Jeon, J.; Yong, D. Two novel bacteriophages improve survival in Galleria mellonella infection and mouse acute pneumonia models infected with extensively drug-resistant Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2019, 85, e02900-18. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Park, J.H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Formal, S.B.; Kent, T.H.; May, H.C.; Palmer, A.; Falkow, S.; LaBrec, E.H. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J. Bacteriol. 1966, 92, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Hromockyj, A.E.; Coker, C.; Maurelli, A.T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect. Immun. 1991, 59, 1997–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigyi, Z.; Kishore, A.R.; Maeland, J.A.; Forsgren, A.; Naidu, A.S. Lactoferrin-binding proteins in Shigella flexneri. Infect. Immun. 1992, 60, 2619–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, A.B.; Venkatesan, M.M. Construction of a stable attenuated Shigella sonnei ΔvirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect. Immun. 1998, 66, 4572–4576. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.L.; Guerry, P.; Seid, R.C., Jr.; Kapfer, C.; Wingfield, M.E.; Reaves, C.B.; Baron, L.S.; Formal, S.B. Expression of lipopolysaccharide O antigen in Escherichia coli K-12 hybrids containing plasmid and chromosomal genes from Shigella dysenteriae 1. Infect. Immun. 1984, 46, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Mencke, J.L.; He, Y.; Filippov, A.A.; Nikolich, M.P.; Belew, A.T.; Fouts, D.E.; McGann, P.T.; Swierczewski, B.E.; Getnet, D.; Ellison, D.W.; et al. Identification and characterization of vB_PreP_EPr2, a lytic bacteriophage of pan-drug resistant Providencia rettgeri. Viruses 2022, 14, 708. [Google Scholar] [CrossRef]
- Sergueev, K.V.; Filippov, A.A.; Farlow, J.; Su, W.; Kvachadze, L.; Balarjishvili, N.; Kutateladze, M.; Nikolich, M.P. Correlation of host range expansion of therapeutic bacteriophage Sb-1 with allele state at a hypervariable repeat locus. Appl. Environ. Microbiol. 2019, 85, e0109-19. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. Basic phage electron microscopy. Methods Mol. Biol. 2009, 501, 113–126. [Google Scholar] [CrossRef]



| Phage | Bacterial Host | Plaque Phenotype | |||||
|---|---|---|---|---|---|---|---|
| S. flexneri 2a | S. flexneri 3a | S. flexneri 5 | S. flexneri 6 | S. sonnei | S. dysenteriae 1 | ||
| ESh1 | + | + | + | − | − | + | Large |
| ESh9 | + | + | + | − | − | + | Large |
| ESh12 | + | + | + | − | − | + | Very large |
| ESh16 | + | + | + | − | + | + | Large turbid |
| ESh17 | + | + | + | + | + | − | Small turbid |
| ESh18 | + | + | + | + | + | − | Small turbid |
| ESh22 | + | + | + | − | − | + | Large, halo |
| ESh27 | + | + | + | − | + | + | Small turbid |
| ESh29 | + | + | + | − | + | + | Small turbid |
| ESh31 | + | + | + | − | + | + | Small clear |
| ESh33 | + | + | + | + | + | − | Small clear |
| ESh35 | + | + | + | − | − | + | Small clear |
| Mixture | Phage Components | Sterility Test Result after 24 h Incubation | ||
|---|---|---|---|---|
| #1 | ESh1 | ESh18 | ESh27 | Sterile |
| #2 | ESh12 | ESh18 | ESh27 | Sterile |
| #4 | ESh12 | ESh18 | ESh29 | Sterile |
| #15 | ESh1 | ESh31 | ESh33 | Low secondary growth |
| Bacterial Isolates | Lytic Activity of Phage Mixtures (%) | ||||
|---|---|---|---|---|---|
| n | #1 | #2 | #4 | #15 | |
| S. sonnei | 7 | 100 | 100 | 100 | 100 |
| S. flexneri | 75 | 85.9 | 81.7 | 83.1 | 97.2 |
| S. dysenteriae 1, 2 | 4 | 100 | 100 | 100 | 100 |
| S. dysenteriae 9, 12 | 3 | 0 | 0 | 0 | 0 |
| S. boydii | 6 | 16.7 | 16.7 | 16.7 | 16.7 |
| Overall Shigella collection | 95 | 76.4 | 76.4 | 77.5 | 88.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippov, A.A.; Su, W.; Sergueev, K.V.; Kevorkian, R.T.; Snesrud, E.C.; Srijan, A.; He, Y.; Fouts, D.E.; Lurchachaiwong, W.; McGann, P.T.; et al. Design of a Bacteriophage Cocktail Active against Shigella Species and Testing of Its Therapeutic Potential in Galleria mellonella. Antibiotics 2022, 11, 1659. https://doi.org/10.3390/antibiotics11111659
Filippov AA, Su W, Sergueev KV, Kevorkian RT, Snesrud EC, Srijan A, He Y, Fouts DE, Lurchachaiwong W, McGann PT, et al. Design of a Bacteriophage Cocktail Active against Shigella Species and Testing of Its Therapeutic Potential in Galleria mellonella. Antibiotics. 2022; 11(11):1659. https://doi.org/10.3390/antibiotics11111659
Chicago/Turabian StyleFilippov, Andrey A., Wanwen Su, Kirill V. Sergueev, Richard T. Kevorkian, Erik C. Snesrud, Apichai Srijan, Yunxiu He, Derrick E. Fouts, Woradee Lurchachaiwong, Patrick T. McGann, and et al. 2022. "Design of a Bacteriophage Cocktail Active against Shigella Species and Testing of Its Therapeutic Potential in Galleria mellonella" Antibiotics 11, no. 11: 1659. https://doi.org/10.3390/antibiotics11111659
APA StyleFilippov, A. A., Su, W., Sergueev, K. V., Kevorkian, R. T., Snesrud, E. C., Srijan, A., He, Y., Fouts, D. E., Lurchachaiwong, W., McGann, P. T., Ellison, D. W., Swierczewski, B. E., & Nikolich, M. P. (2022). Design of a Bacteriophage Cocktail Active against Shigella Species and Testing of Its Therapeutic Potential in Galleria mellonella. Antibiotics, 11(11), 1659. https://doi.org/10.3390/antibiotics11111659








