Evaluation of Fosfomycin-Sulbactam Combination Therapy against Carbapenem-Resistant Acinetobacter baumannii Isolates in a Hollow-Fibre Infection Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antimicrobial Agents
2.3. In Vitro Susceptibility
2.4. Hollow-Fibre Infection Model
2.5. Quantification of Viable Bacterial Populations
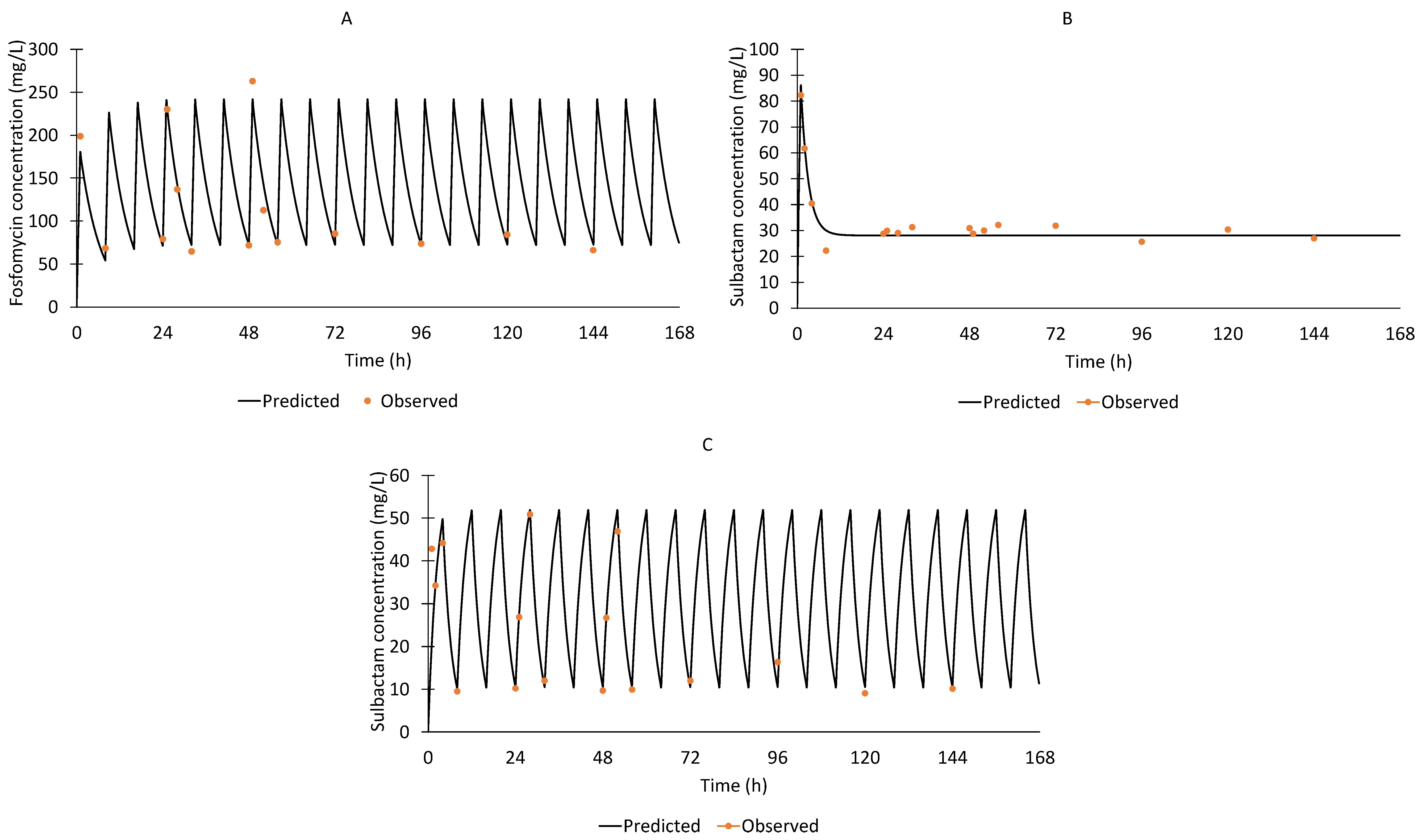
2.6. Fosfomycin and Sulbactam Assays for Pharmacokinetics
3. Results
3.1. In Vitro Susceptibility
3.2. Hollow-Fibre Infection Model
3.3. In Vitro Susceptibility Post-Drug Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef]
- Mohd Sazlly Lim, S.; Sime, F.B.; Roberts, J.A. Multidrug-resistant Acinetobacter baumannii infections: Current evidence on treatment options and the role of pharmacokinetics/pharmacodynamics in dose optimisation. Int. J. Antimicrob. Agents 2019, 53, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, F.; Feng, B.; Zhang, Z.; Liu, L.; Wang, G. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Glob. Antimicrob. Resist. 2021, 24, 136–147. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Douzinas, E.E. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J. Infect. 2008, 56, 432–436. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Georgiadis, G. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand. J. Infect. Dis. 2007, 39, 38–43. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kastoris, A.C.; Karageorgopoulos, D.E.; Rafailidis, P.I. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: A systematic review of microbiological, animal and clinical studies. Int. J. Antimicrob. Agents 2009, 34, 111–120. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Q.; Chen, Z.; Li, C. Efficacy of sulbactam for the treatment of Acinetobacter baumannii complex infection: A systematic review and meta-analysis. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2017, 23, 278–285. [Google Scholar] [CrossRef]
- Flamm, R.K.; Rhomberg, P.R.; Lindley, J.M.; Sweeney, K.; Ellis-Grosse, E.J.; Shortridge, D. Evaluation of the Bactericidal Activity of Fosfomycin in Combination with Selected Antimicrobial Comparison Agents Tested against Gram-Negative Bacterial Strains by Using Time-Kill Curves. Antimicrob. Agents Chemother. 2019, 63, e02549-18. [Google Scholar] [CrossRef] [PubMed]
- Santimaleeworagun, W.; Wongpoowarak, P.; Chayakul, P.; Pattharachayakul, S.; Tansakul, P.; Garey, K.W. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J. Trop. Med. Public Health 2011, 42, 890. [Google Scholar] [PubMed]
- Zhu, W.; Wang, Y.; Cao, W.; Cao, S.; Zhang, J. In vitro evaluation of antimicrobial combinations against imipenem-resistant Acinetobacter baumannii of different MICs. J. Infect. Public Health 2018, 11, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, H.; Liu, Y.; Ye, Y.; Li, J. In vitro synergy of colistin combinations against extensively drug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase. J. Chemother. 2016, 28, 159–163. [Google Scholar] [CrossRef]
- Deveci, A.; Coban, A.Y.; Acicbe, O.; Tanyel, E.; Yaman, G.; Durupinar, B. In vitro effects of sulbactam combinations with different antibiotic groups against clinical Acinetobacter baumannii isolates. J. Chemother. 2012, 24, 247–252. [Google Scholar] [CrossRef]
- Mohd Sazlly Lim, S.; Naicker, S.; Ayfan, A.K.; Zowawi, H.; Roberts, J.A.; Sime, F.B. Non-polymyxin-based combinations as potential alternatives in treatment against carbapenem-resistant Acinetobacter baumannii infections. Int. J. Antimicrob. Agents 2020, 56, 106115. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Sartor, A.L.; Sidjabat, H.E.; Balkhy, H.H.; Walsh, T.R.; Al Johani, S.M.; AlJindan, R.Y.; Alfaresi, M.; Ibrahim, E.; Al-Jardani, A. Molecular Epidemiology of carbapenem resistant Acinetobacter baumannii in the Gulf Cooperation Council States. Dominance of OXA-23-type producers. J. Clin. Microbiol. 2015, 53, 896–903. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing 30th ed. Available online: http://em100.edaptivedocs.net/dashboard.aspx (accessed on 8 June 2020).
- Cadwell, J. The Hollow Fiber Infection Model: Principles and Practice. Adv. Antibiot. Antibodies 2015, 1, 2. [Google Scholar]
- Blaser, J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 1985, 15, 125–130. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.-J.; Cuberos, L.; Pichardo, C.; Caballero, F.J.; Moreno, I.; Jiménez-Mejías, M.E.; García-Curiel, A.; Pachón, J. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J. Antimicrob. Chemother. 2001, 47, 479–482. [Google Scholar] [CrossRef]
- Wildfeuer, A.; Räder, K. Stability of β-lactamase inhibitors and β-lactam antibiotics in parenteral dosage forms and in body fluids and tissue homogenates: A comparative study of sulbactam, clavulanic acid, ampicillin and amoxycillin. Int. J. Antimicrob. Agents 1996, 6, S31–S34. [Google Scholar] [CrossRef]
- Pfausler, B.; Spiss, H.; Dittrich, P.; Zeitlinger, M.; Schmutzhard, E.; Joukhadar, C. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J. Antimicrob. Chemother. 2004, 53, 848–852. [Google Scholar] [CrossRef] [PubMed]
- VanScoy, B.D.; McCauley, J.; Ellis-Grosse, E.J.; Okusanya, O.O.; Bhavnani, S.M.; Forrest, A.; Ambrose, P.G. Exploration of the Pharmacokinetic-Pharmacodynamic Relationships for Fosfomycin Efficacy Using an In Vitro Infection Model. Antimicrob. Agents Chemother. 2015, 59, 7170–7177. [Google Scholar] [CrossRef] [PubMed]
- Zykov, I.N.; Samuelsen, Ø.; Jakobsen, L.; Småbrekke, L.; Andersson, D.I.; Sundsfjord, A.; Frimodt-Møller, N. Pharmacokinetics and Pharmacodynamics of Fosfomycin and Its Activity against Extended-Spectrum-β-Lactamase-, Plasmid-Mediated AmpC-, and Carbapenemase-Producing Escherichia coli in a Murine Urinary Tract Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02560-17. [Google Scholar] [CrossRef]
- Docobo-Pérez, F.; Drusano, G.L.; Johnson, A.; Goodwin, J.; Whalley, S.; Ramos-Martín, V.; Ballestero-Tellez, M.; Rodriguez-Martinez, J.M.; Conejo, M.C.; van Guilder, M.; et al. Pharmacodynamics of Fosfomycin: Insights into Clinical Use for Antimicrobial Resistance. Antimicrob. Agents Chemother. 2015, 59, 5602. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Klein, N.; Dittrich, P.; Zeitlinger, M.; Geppert, A.; Skhirtladze, K.; Frossard, M.; Heinz, G.; Müller, M. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 2003, 51, 1247–1252. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Nitchot, W.; Wongpoowarak, W.; Samaeng, M.; Nawakitrangsan, M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur. J. Pharm. Sci. 2019, 136, 104940. [Google Scholar] [CrossRef]
- Zeitlinger, M.; Sauermann, R.; Traunmüller, F.; Georgopoulos, A.; Müller, M.; Joukhadar, C. Impact of plasma protein binding on antimicrobial activity using time–killing curves. J. Antimicrob. Chemother. 2004, 54, 876–880. [Google Scholar] [CrossRef]
- Rolinson, G.; Sutherland, R. The binding of antibiotics to serum proteins. Br. J. Pharmacol. Chemother. 1965, 25, 638–650. [Google Scholar] [CrossRef]
- Abodakpi, H.; Chang, K.-T.; Gao, S.; Sánchez-Díaz, A.M.; Cantón, R.; Tam, V.H. Optimal piperacillin-tazobactam dosing strategies against extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e01906-18. [Google Scholar] [CrossRef]
- Gil-Marqués, M.L.; Moreno-Martínez, P.; Costas, C.; Pachón, J.; Blázquez, J.; McConnell, M.J. Peptidoglycan recycling contributes to intrinsic resistance to fosfomycin in Acinetobacter baumannii. J. Antimicrob. Chemother. 2018, 73, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, J.R.; Smith, N.M.; Bulman, Z.P.; Tao, X.; Thamlikitkul, V.; Shin, B.S.; Nation, R.L.; Li, J.; Bulitta, J.B.; Tsuji, B.T. High-dose ampicillin-sulbactam combinations combat polymyxin-resistant Acinetobacter baumannii in a hollow-fiber infection model. Antimicrob. Agents Chemother. 2017, 61, e01268-16. [Google Scholar] [CrossRef]
- Sirijatuphat, R.; Thamlikitkul, V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014, 58, 5598–5601. [Google Scholar] [CrossRef]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Cattaneo, F.M.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2020, 10, 187–200. [Google Scholar] [CrossRef]
- Grabein, B.; Graninger, W.; Baño, J.R.; Dinh, A.; Liesenfeld, D. Intravenous fosfomycin—Back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2017, 23, 363–372. [Google Scholar] [CrossRef]
- Iarikov, D.; Wassel, R.; Farley, J.; Nambiar, S. Adverse events associated with fosfomycin use: Review of the literature and analyses of the FDA adverse event reporting system database. Infect. Dis. Ther. 2015, 4, 433–458. [Google Scholar] [CrossRef]
- Kahan, F.M.; Kahan, J.S.; Cassidy, P.J.; Kropp, H. The mechanism of action of fosfomycin (phosphonomycin). Ann. N. Y. Acad. Sci. 1974, 235, 364–386. [Google Scholar] [CrossRef]
- Noguchi, J.; Gill, M. Sulbactam: A beta-lactamase inhibitor. Clin. Pharm. 1988, 7, 37–51. [Google Scholar]
- Papp-Wallace, K.M.; Senkfor, B.; Gatta, J.; Chai, W.; Taracila, M.A.; Shanmugasundaram, V.; Han, S.; Zaniewski, R.P.; Lacey, B.M.; Tomaras, A.P. Early insights into the interactions of different β-lactam antibiotics and β-lactamase inhibitors against soluble forms of Acinetobacter baumannii PBP1a and Acinetobacter sp. PBP3. Antimicrob. Agents Chemother. 2012, 56, 5687–5692. [Google Scholar] [CrossRef]
- Cantón, R.; Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef]
- Oliveira, M.S.d.; Costa, S.F.; Pedri, E.d.; van der Heijden, I.; Levin, A.S.S. The minimal inhibitory concentration for sulbactam was not associated with the outcome of infections caused by carbapenem-resistant Acinetobacter sp. treated with ampicillin/sulbactam. Clinics 2013, 68, 569–573. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Sheng, W.-H.; Lauderdale, T.-L.; Li, S.-Y.; Wang, J.-T.; Chen, Y.-C.; Chang, S.-C. Molecular epidemiology, antimicrobial susceptibility and carbapenemase resistance determinants among Acinetobacter baumannii clinical isolates in Taiwan. J. Microbiol. Immunol. Infect. 2014, 47, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Oliveira, M.S.; Perdigão-Neto, L.V.; Rocha, C.K.D.; Guimarães, T.; Rizek, C.; Levin, A.S.; Costa, S.F. Antimicrobial Combinations against Pan-Resistant Acinetobacter baumannii Isolates with Different Resistance Mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef]
- Mohd Sazlly Lim, S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Pharmacodynamic Analysis of Meropenem and Fosfomycin Combination Against Carbapenem-Resistant Acinetobacter baumannii in Patients with Normal Renal Clearance: Can It Be a Treatment Option? Microb. Drug Resist. 2020, 27, 546–552. [Google Scholar] [CrossRef]
- Lim, S.M.S.; Heffernan, A.; Roberts, J.; Sime, F. Semi-mechanistic PK/PD modelling of fosfomycin and sulbactam combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, 5. [Google Scholar] [CrossRef]
- Louie, A.; Brown, D.; Baluya, D.; Rodriquez, J.; Robbins, N.; Kurhanewicz, S.; Fikes, S.; Liu, W.; Drusano, G.L. Cirz R: Interaction of Drug- and Granulocyte-Mediated Killing of Pseudomonas aeruginosa in a Murine Pneumonia Model. J. Infect. Dis. 2014, 210, 1319–1324. [Google Scholar]

| Isolates | Pre-Exposure MIC (mg/L) | Antibiotic Regimen | Post-Exposure MIC (mg/L) | ||
|---|---|---|---|---|---|
| Fosfomycin | Sulbactam | Fosfomycin | Sulbactam | ||
| #75 | 128 | 64 | Fosfomycin monotherapy | 2048 | 32 |
| Sulbactam CI monotherapy | 128 | 64 | |||
| Sulbactam EI monotherapy | 128 | 256 | |||
| Fosfomycin + Sulbactam CI | 512 | 32 | |||
| Fosfomycin + Sulbactam EI | - | - | |||
| #98 | 256 | 64 | Fosfomycin monotherapy | 2048 | 64 |
| Sulbactam CI monotherapy | 256 | 128 | |||
| Sulbactam EI monotherapy | 128 | 128 | |||
| Fosfomycin + Sulbactam CI | - | - | |||
| Fosfomycin + Sulbactam EI | - | - | |||
| #102 | 256 | 64 | Fosfomycin monotherapy | 1024 | 32 |
| Sulbactam CI monotherapy | 128 | 256 | |||
| Sulbactam EI monotherapy | 128 | 256 | |||
| Fosfomycin + Sulbactam CI | 512 | 32 | |||
| Fosfomycin + Sulbactam EI | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Sazlly Lim, S.; Heffernan, A.; Naicker, S.; Wallis, S.; Roberts, J.A.; Sime, F.B. Evaluation of Fosfomycin-Sulbactam Combination Therapy against Carbapenem-Resistant Acinetobacter baumannii Isolates in a Hollow-Fibre Infection Model. Antibiotics 2022, 11, 1578. https://doi.org/10.3390/antibiotics11111578
Mohd Sazlly Lim S, Heffernan A, Naicker S, Wallis S, Roberts JA, Sime FB. Evaluation of Fosfomycin-Sulbactam Combination Therapy against Carbapenem-Resistant Acinetobacter baumannii Isolates in a Hollow-Fibre Infection Model. Antibiotics. 2022; 11(11):1578. https://doi.org/10.3390/antibiotics11111578
Chicago/Turabian StyleMohd Sazlly Lim, Sazlyna, Aaron Heffernan, Saiyuri Naicker, Steven Wallis, Jason A. Roberts, and Fekade Bruck Sime. 2022. "Evaluation of Fosfomycin-Sulbactam Combination Therapy against Carbapenem-Resistant Acinetobacter baumannii Isolates in a Hollow-Fibre Infection Model" Antibiotics 11, no. 11: 1578. https://doi.org/10.3390/antibiotics11111578
APA StyleMohd Sazlly Lim, S., Heffernan, A., Naicker, S., Wallis, S., Roberts, J. A., & Sime, F. B. (2022). Evaluation of Fosfomycin-Sulbactam Combination Therapy against Carbapenem-Resistant Acinetobacter baumannii Isolates in a Hollow-Fibre Infection Model. Antibiotics, 11(11), 1578. https://doi.org/10.3390/antibiotics11111578







