Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains
4.3. Methods
4.3.1. Green Synthesis and Characterization of AuNFs
4.3.2. Antimicrobial Activity of AuNFs
4.3.3. Tolerance Level
4.3.4. Inhibitory Effect of AuNFs on Biofilm Formation
4.3.5. Effect of AuNFs on Disruption of Preformed Biofilm
4.3.6. Scanning Electron Microscopy of Biofilms
4.3.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef]
- Koca, F.D.; Demirezen Yilmaz, D.; Ertas Onmaz, N.; Yilmaz, E.; Ocsoy, I. Green Synthesis of Allicin Based Hybrid Nanoflowers with Evaluation of Their Catalytic and Antimicrobial Activities. Biotechnol. Lett. 2020, 42, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-González, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Apfalter, P.; Hartl, R.; et al. Emergence of Methicillin Resistance Predates the Clinical Use of Antibiotics. Nature 2022, 602, 135–141. [Google Scholar] [CrossRef]
- Nassima, B.; Nassima, B.; Riadh, K. Antimicrobial and Antibiofilm Activities of Phenolic Compounds Extracted from Populus Nigra and Populus Alba Buds (Algeria). Braz. J. Pharm. Sci. 2019, 55, 18114. [Google Scholar] [CrossRef]
- Sieniawska, E.; Los, R.; Baj, T.; Malm, A.; Glowniak, K. Antimicrobial Efficacy of Mutellina Purpurea Essential Oil and α-Pinene against Staphylococcus Epidermidis Grown in Planktonic and Biofilm Cultures. Ind. Crops Prod. 2013, 51, 152–157. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green Synthesis of Gold and Silver Nanoparticles from Cannabis Sativa (Industrial Hemp) and Their Capacity for Biofilm Inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Swolana, D.; Kępa, M.; Idzik, D.; Dziedzic, A.; Kabała-Dzik, A.; Wąsik, T.J.; Wojtyczka, R.D. The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability. Nanomaterials 2020, 10, 1010. [Google Scholar] [CrossRef]
- Ciotea, D.; Shamtsyan, M.; Popa, M.E. Antibacterial Activity of Peppermint, Basil and Rosemary Essential Oils Obtained by Steam Distillation. AgroLife Sci. J. 2021, 10, 75–82. [Google Scholar]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Nirmala Grace, A.; Pandian, K. Antibacterial Efficacy of Aminoglycosidic Antibiotics Protected Gold Nanoparticles—A Brief Study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of Bioinspired Metal and Metal Oxide Nanoparticles in Biomedical Sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef] [PubMed]
- Koca, F.D.; Halici, M.G.; Işik, Y.; Ünal, G. Green Synthesis of Ag-ZnO Nanocomposites by Using Usnea Florida and Pseudevernia Furfuracea Lichen Extracts and Evaluation of Their Neurotoxic Effects. Inorg. Nano Metal Chem. 2022, 1–8. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Umavathi, S.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Virik, P.; Al-Mulhm, N.; Subash, M.; Gopinath, K.; Kavitha, C. Green Synthesis of ZnO Nanoparticles for Antimicrobial and Vegetative Growth Applications: A Novel Approach for Advancing Efficient High Quality Health Care to Human Wellbeing. Saudi J. Biol. Sci. 2021, 28, 1808–1815. [Google Scholar] [CrossRef]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The Green Synthesis of MgO Nano-Flowers Using Rosmarinus Officinalis L. (Rosemary) and the Antibacterial Activities against Xanthomonas Oryzae Pv. Oryzae. Biomed. Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef]
- Li, L.; Niu, R.; Zhang, Y. Ag–Au Bimetallic Nanocomposites Stabilized with Organic–Inorganic Hybrid Microgels: Synthesis and Their Regulated Optical and Catalytic Properties. RSC Adv. 2018, 8, 12428–12438. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sargent, E.H.; Kelley, S.O. One-Step DNA-Programmed Growth of Luminescent and Biofunctionalized Nanocrystals. Nat. Nanotechnol. 2009, 4, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 6588. [Google Scholar] [CrossRef]
- Borah, D.; Hazarika, M.; Tailor, P.; Silva, A.R.; Chetia, B.; Singaravelu, G.; Das, P. Starch-Templated Bio-Synthesis of Gold Nanoflowers for in Vitro Antimicrobial and Anticancer Activities. Appl. Nanosci. 2018, 8, 241–253. [Google Scholar] [CrossRef]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid Green Synthesis of Silver and Gold Nanoparticles Using Dendropanax Morbifera Leaf Extract and Their Anticancer Activities. Int. J. Nanomed. 2016, 11, 3691. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. Gold Nanoparticles Induce a Reactive Oxygen Species-Independent Apoptotic Pathway in Escherichia Coli. Colloids Surf. B Biointerfaces 2018, 167, 1–7. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant Extract Mediated Synthesis of Silver and Gold Nanoparticles and Its Antibacterial Activity against Clinically Isolated Pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef]
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-Inspired Synthesis of Metal Nanomaterials and Applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Helichrysum Italicum (Roth) G. Don Fil. (Asteraceae) from Montenegro. Nat. Prod. Res. 2020, 34, 445–448. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.-Y.; Chen, S.-M.; Ali, M.A.; Alhemaid, F.M.A. Green Synthesis and Electrochemical Characterizations of Gold Nanoparticles Using Leaf Extract of Magnolia Kobus. Int. J. Electrochem. Sci. 2012, 7, 12742–12751. [Google Scholar]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-Synthesized Gold Nanoparticles from Plumeria Alba Flower Extract to Augment Catalytic Degradation of Organic Dyes and Inhibit Bacterial Growth. Particuology 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Nagaraj, B.; Malakar, B.; Divya, T.K.; Krishnamurthy, N.B.; Liny, P.; Dinesh, R. Environmental Benign Synthesis of Gold Nanoparticles from the flower Extracts of Plumeria Alba Linn. (Frangipani) and Evaluation of Their Biological Activities. Int. J. Drug Dev. Res. 2011, 4, 144–150. [Google Scholar]
- Muthukumar, T.; Sudhakumari; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.-H. Green Synthesis of Gold Nanoparticles and Their Enhanced Synergistic Antitumor Activity Using HepG2 and MCF7 Cells and Its Antibacterial Effects. Process Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green Synthesized Silver Nanoparticles Destroy Multidrug Resistant Bacteria via Reactive Oxygen Species Mediated Membrane Damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Gold Nanoflowers Synthesized Using Acanthopanacis Cortex Extract Inhibit Inflammatory Mediators in LPS-Induced RAW264.7 Macrophages via NF-ΚB and AP-1 Pathways. Colloids Surf. B Biointerfaces 2017, 160, 423–428. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Semerdjieva, I.; Yankova-Tsvetkova, E.; Astatkie, T.; Stanev, S.; Dincheva, I.; Kačániová, M. Chemical Profile and Antimicrobial Activity of the Essential Oils of Helichrysum Arenarium (L.) Moench. and Helichrysum Italicum (Roth.) G. Don. Plants 2022, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus Officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Perrini, R.; Morone-Fortunato, I.; Lorusso, E.; Avato, P. Glands, Essential Oils and in Vitro Establishment of Helichrysum Italicum (Roth) G. Don Ssp. Microphyllum (Willd.) Nyman. Ind. Crops Prod. 2009, 29, 395–403. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Pióro-Jabrucka, E.; Przybył, J.L.; Gniewosz, M.; Kraśniewska, K.; Szyndel, M.S.; Costa, R.; Bączek, K.B. Antioxidant and Antibacterial Activity of Helichrysum Italicum (Roth) G. Don. from Central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus Officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Piacente, S. Metabolite Profiling of Helichrysum Italicum Derived Food Supplements by 1H-NMR-Based Metabolomics. Molecules 2021, 26, 6619. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Brewer, M.; Zhang, T.; Dong, W.; Rutherford, M.; Tian, Z.R. Future Approaches of Nanomedicine in Clinical Science. Med. Clin. N. Am. 2007, 91, 963–1016. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus Officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial Phenolics and Unusual Glycerides from Helichrysum Italicum Subsp. Microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The Potential of Use Basil and Rosemary Essential Oils as Effective Antibacterial Agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Sales, A.; Hossein-Nezhad, P. Antimicrobial Effects of Rosmarinus Officinalis Methanolic Extract on Staphylococcus Aureus, Bacillus Cereus, Escherichia Coli and Pseudomonas Aeruginosa in Laboratory Conditions. J. Med. Chem. Sci. 2020, 3, 103–108. [Google Scholar] [CrossRef]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Aklilu, A.; Seid, M.; Merdikios, B.; Tsegaye, B. In Vitro Antibacterial Activity of Medicinal Plants against Biofilm-Forming Methicillin-Resistant Staphylococcus Aureus: Efficacy of Moringa Stenopetala and Rosmarinus Officinalis Extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef]
- Almasoud, N.; Alhaik, H.; Almutairi, M.; Houjak, A.; Hazazi, K.; Alhayek, F.; Aljanoubi, S.; Alkhaibari, A.; Alghamdi, A.; Soliman, D.A.; et al. Green Nanotechnology Synthesized Silver Nanoparticles: Characterization and Testing Its Antibacterial Activity. Green Process. Synth. 2021, 10, 510–528. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Ierardi, V.; Longo, I.; Spepi, A.; Tiné, M.R.; Ferrari, C. Fast and Eco–Friendly Microwave-Assisted Synthesis of Silver Nanoparticles Using Rosemary Essential Oil as Renewable Reducing Agent. ChemistrySelect 2017, 2, 2131–2138. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the Characteristics of Gold Nanoparticles Synthesized Using Aqueous Plant Extracts and Natural Plant Essential Oils of Eucalyptus Globulus and Rosmarinus Officinalis. Arab. J. Chem. 2019, 12, 4795–4805. [Google Scholar] [CrossRef]
- ElMitwalli, O.S.; Barakat, O.A.; Daoud, R.M.; Akhtar, S.; Henari, F.Z. Green Synthesis of Gold Nanoparticles Using Cinnamon Bark Extract, Characterization, and Fluorescence Activity in Au/Eosin Y Assemblies. J. Nanoparticle Res. 2020, 22, 309. [Google Scholar] [CrossRef]
- Dash, S.S.; Bag, B.G. Synthesis of Gold Nanoparticles Using Renewable Punica Granatum Juice and Study of Its Catalytic Activity. Appl. Nanosci. 2014, 4, 55–59. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Anticancer Activity of Eco-Friendly Gold Nanoparticles against Lung and Liver Cancer Cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef]
- Naz, F.; Koul, V.; Srivastava, A.; Gupta, Y.K.; Dinda, A.K. Biokinetics of Ultrafine Gold Nanoparticles (AuNPs) Relating to Redistribution and Urinary Excretion: A Long-Term in Vivo Study. J. Drug Target. 2016, 24, 720–729. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. Microbial Synthesis of Flower-Shaped Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1469–1474. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; Özdemir, N.; Ocsoy, I. A New Generation Approach in Enzyme Immobilization: Organic-Inorganic Hybrid Nanoflowers with Enhanced Catalytic Activity and Stability. Enzym. Microb. Technol. 2016, 93–94, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Otari, S.V.; Chan Kang, Y.; Lee, J.K. Protein–Inorganic Hybrid System for Efficient His-Tagged Enzymes Immobilization and Its Application in L-Xylulose Production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef]
- Tran, T.D.; Kim, M. Organic-Inorganic Hybrid Nanoflowers as Potent Materials for Biosensing and Biocatalytic Applications. Biochip J. 2018, 12, 268–279. [Google Scholar] [CrossRef]
- Celik, C.; Ildiz, N.; Ocsoy, I. Building Block and Rapid Synthesis of Catecholamines-Inorganic Nanoflowers with Their Peroxidase-Mimicking and Antimicrobial Activities. Sci. Rep. 2020, 10, 2903. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, S.K.; Yun, S. il Fungus Mediated Synthesis of Gold Nanoparticles and Their Conjugation with Genomic DNA Isolated from Escherichia Coli and Staphylococcus Aureus. Process Biochem. 2012, 47, 701–711. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Rajeshkumar, S.; Venkat Kumar, S. Phyto-Assisted Synthesis, Characterization and Applications of Gold Nanoparticles—A Review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lomeli-Marroquin, D.; Medina Cruz, D.; Nieto-Arguello, A.; Vernet Crua, A.; Chen, J.; Torres-Castro, A.; Webster, T.J.; CholulaDiaz, J.L. Starch-mediated synthesis of mono- and bimetallic silver/gold nanoparticles as antimicrobial and anticancer agents. Int. J. Nanomed. 2019, 14, 2171–2190. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q.; Lee, J.Y.; Wang, D.I.C. The Synthesis of SERS-Active Gold Nanoflower Tags for in Vivo Applications. ACS Nano 2008, 2, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Peter, Y.A.; Nadeau, J.L. Facile Biosynthesis, Separation and Conjugation of Gold Nanoparticles to Doxorubicin. Nanotechnology 2008, 19, 495101. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Hoag, G.; Collins, J.; Varma, R.S. Green Synthesis of Au Nanostructures at Room Temperature Using Biodegradable Plant Surfactants. Cryst. Growth Des. 2009, 9, 4979–4983. [Google Scholar] [CrossRef]
- He, Z.; Zang, S.; Liu, Y.; He, Y.; Lei, H. A Multi-Walled Carbon Nanotubes-Poly(l-Lysine) Modified Enantioselective Immunosensor for Ofloxacin by Using Multi-Enzyme-Labeled Gold Nanoflower as Signal Enhancer. Biosens. Bioelectron. 2015, 73, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Piktel, E.; Oscilowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Głuszek, K.; Klimek, J.; Zieliński, P.M.; et al. Peanut-Shaped Gold Nanoparticles with Shells of Ceragenin CSA-131 Display the Ability to Inhibit Ovarian Cancer Growth In Vitro and in a Tumor Xenograft Model. Cancers 2021, 13, 5424. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green Synthesis of Gold Nanoparticles Using an Aqueous Root Extract of Morinda Citrifolia L. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef]
- Wani, K.; Chikate, R.; Kaul-Ghanekar, R. Synthesis and Characterization of Gold Nanoparticles Using Ficus Religiosa Extract. Carbon Sci. Technol. 2013, 5, 203–210. [Google Scholar]
- Abbasi, T.; Anuradha, J.; Ganaie, S.U.; Abbasi, S.A. Gainful Utilization of the Highly Intransigent Weed Ipomoea in the Synthesis of Gold Nanoparticles. J. King Saud. Univ. Sci. 2015, 27, 15–22. [Google Scholar] [CrossRef]
- de Canha, M.N.; Thipe, V.C.; Katti, K.V.; Mandiwana, V.; Kalombo, M.L.; Ray, S.S.; Rikhotso, R.; Janse van Vuuren, A.; Lall, N. The Activity of Gold Nanoparticles Synthesized Using Helichrysum Odoratissimum Against Cutibacterium Acnes Biofilms. Front. Cell Dev. Biol. 2021, 9, 2288. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus Aureus and Pseudomonas Aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, X.-J.; Li, Q.; Li, J.; Xu, D. Facile Synthesis of Gold Nanoflowers with High Surface-Enhanced Raman Scattering. Nanotechnology 2011, 22, 385601. [Google Scholar] [CrossRef] [PubMed]
- Coman, C.; Leopold, L.F.; Ruginǎ, O.D.; Barbu-Tudoran, L.; Leopold, N.; Tofanǎ, M.; Socaciu, C. Green Synthesis of Gold Nanoparticles by Allium Sativum Extract and Their Assessment as SERS Substrate. J. Nanoparticle Res. 2014, 16, 2158. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Ayat, M.S.; Shabrawy, M.A.; Millet, P. Green Synthesis of Gold Nanoparticles Using Parsley Leaves Extract and Their Applications as an Alternative Catalytic, Antioxidant, Anticancer, and Antibacterial Agents. Adv. Powder Technol. 2020, 31, 4390–4400. [Google Scholar] [CrossRef]
- Su, C.; Huang, K.; Li, H.-H.; Lu, Y.-G.; Zheng, D.-L. Antibacterial Properties of Functionalized Gold Nanoparticles and Their Application in Oral Biology. J. Nanomater. 2020, 2020, 5616379. [Google Scholar] [CrossRef]
- Eskikaya, O.; Ozdemir, S.; Tollu, G.; Dizge, N.; Ramaraj, R.; Manivannan, A.; Balakrishnan, D. Synthesis of Two Different Zinc Oxide Nanoflowers and Comparison of Antioxidant and Photocatalytic Activity. Chemosphere 2022, 306, 135389. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Jeon, K.; Choi, Y.; Kim, H.Y. Carbon Nanofibers Decorated with Binary Semiconductor (TiO2/ZnO) Nanocomposites for the Effective Removal of Organic Pollutants and the Enhancement of Antibacterial Activities. Ceram Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, K.; He, J.; Zhang, Y.; Chen, S.; Ma, G.; Cui, Y.; Wang, L.; Gao, D. Synthesis of Gold Nanoflowers Stabilized with Amphiphilic Daptomycin for Enhanced Photothermal Antitumor and Antibacterial Effects. Int. J. Pharm. 2020, 580, 119231. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial Properties and Toxicity from Metallic Nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of Aflatoxin B1 with Immunochromatographic Test Strips: Enhanced Signal Sensitivity Using Gold Nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Malaikozhundan, B.; Lakshmi, V.N.; Krishnamoorthi, R. Copper Oxide Nanoparticles Using Mentha Spicata Leaves as Antibacterial, Antibiofilm, Free Radical Scavenging Agent and Efficient Photocatalyst to Degrade Methylene Blue Dyes. Mater. Today Commun. 2022, 33, 104348. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Krishnamoorthi, R.; Vinodhini, J.; Nambi, K.S.N.; Palanisamy, S. Multifunctional Iron Oxide Nanoparticles Using Carica Papaya Fruit Extract as Antibacterial, Antioxidant and Photocatalytic Agent to Remove Industrial Dyes. Inorg. Chem. Commun. 2022, 144, 109843. [Google Scholar] [CrossRef]
- Veena, S.; Devasena, T.; Sathak, S.S.M.; Yasasve, M.; Vishal, L.A. Green Synthesis of Gold Nanoparticles from Vitex Negundo Leaf Extract: Characterization and In Vitro Evaluation of Antioxidant–Antibacterial Activity. J. Clust. Sci. 2019, 30, 1591–1597. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green Synthesis of Gold Nanoparticles Using Jatropha Integerrima Jacq. Flower Extract and Their Antibacterial Activity. J. King Saud. Univ. Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial Activities of Gold and Silver Nanoparticles against Escherichia Coli and Bacillus Calmette-Guérin. J. Nanobiotechnology 2012, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green Synthesis of Gold Nanoparticles Using Curcuma Pseudomontana Isolated Curcumin: Its Characterization, Antimicrobial, Antioxidant and Anti- Inflammatory Activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and Gold Nanoparticles for Antimicrobial Purposes against Multi-Drug Resistance Bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Perveen, K.; Husain, F.M.; Qais, F.A.; Khan, A.; Razak, S.; Afsar, T.; Alam, P.; Almajwal, A.M.; Abulmeaty, M.M.A. Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum Ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules 2021, 11, 197. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of Gold Nanoparticles (AuNPs) on Pathogenic Biofilm Formation and Invasion to Host Cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.H.; Lee, J. Direct One-Pot Synthesis of Cinnamaldehyde Immobilized on Gold Nanoparticles and Their Antibiofilm Properties. Colloids Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Alyahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic Gold Nanoparticles as Potent Antibacterial and Antibiofilm Nano-Antibiotics against Pseudomonas Aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef]
- Cherian, T.; Maity, D.; Kumar, R.T.R.; Balasubramani, G.; Ragavendran, C.; Yalla, S.; Mohanraju, R.; Peijnenburg, W.J.G.M. Green Chemistry Based Gold Nanoparticles Synthesis Using the Marine Bacterium Lysinibacillus Odysseyi PBCW2 and Their Multitudinous Activities. Nanomaterials 2022, 12, 2940. [Google Scholar] [CrossRef]
- Khosravi, M.; Mirzaie, A.; Kashtali, A.B.; Noorbazargan, H. Antibacterial, Anti-Efflux, Anti-Biofilm, Anti-Slime (Exopolysaccharide) Production and Urease Inhibitory Efficacies of Novel Synthesized Gold Nanoparticles Coated Anthemis Atropatana Extract against Multidrug- Resistant Klebsiella Pneumoniae Strains. Arch. Microbiol. 2020, 202, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Shannon, K.; King, A.; French, G. Glycopeptide Tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Enhancement of Antibiofilm Activity of Ciprofloxacin against Staphylococcus aureus by Administration of Antimicrobial Peptides. Antibiotics 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Nayeri Fasaei, B. Selected Plant Essential Oils Inhibit Biofilm Formation and LuxS- and Pfs-Mediated Quorum Sensing by Escherichia coli O157:H7. Lett. Appl. Microbiol. 2022, 74, 916–923. [Google Scholar] [CrossRef]
- Rafaque, Z.; Abid, N.; Liaqat, N.; Afridi, P.; Siddique, S.; Masood, S.; Kanwal, S.; Dasti, J.I. In-Vitro Investigation of Antibiotics Efficacy Against Uropathogenic Escherichia coli Biofilms and Antibiotic Induced Biofilm Formation at Sub-Minimum Inhibitory Concentration of Ciprofloxacin. Infect. Drug Resist. 2020, 13, 2801. [Google Scholar] [CrossRef]

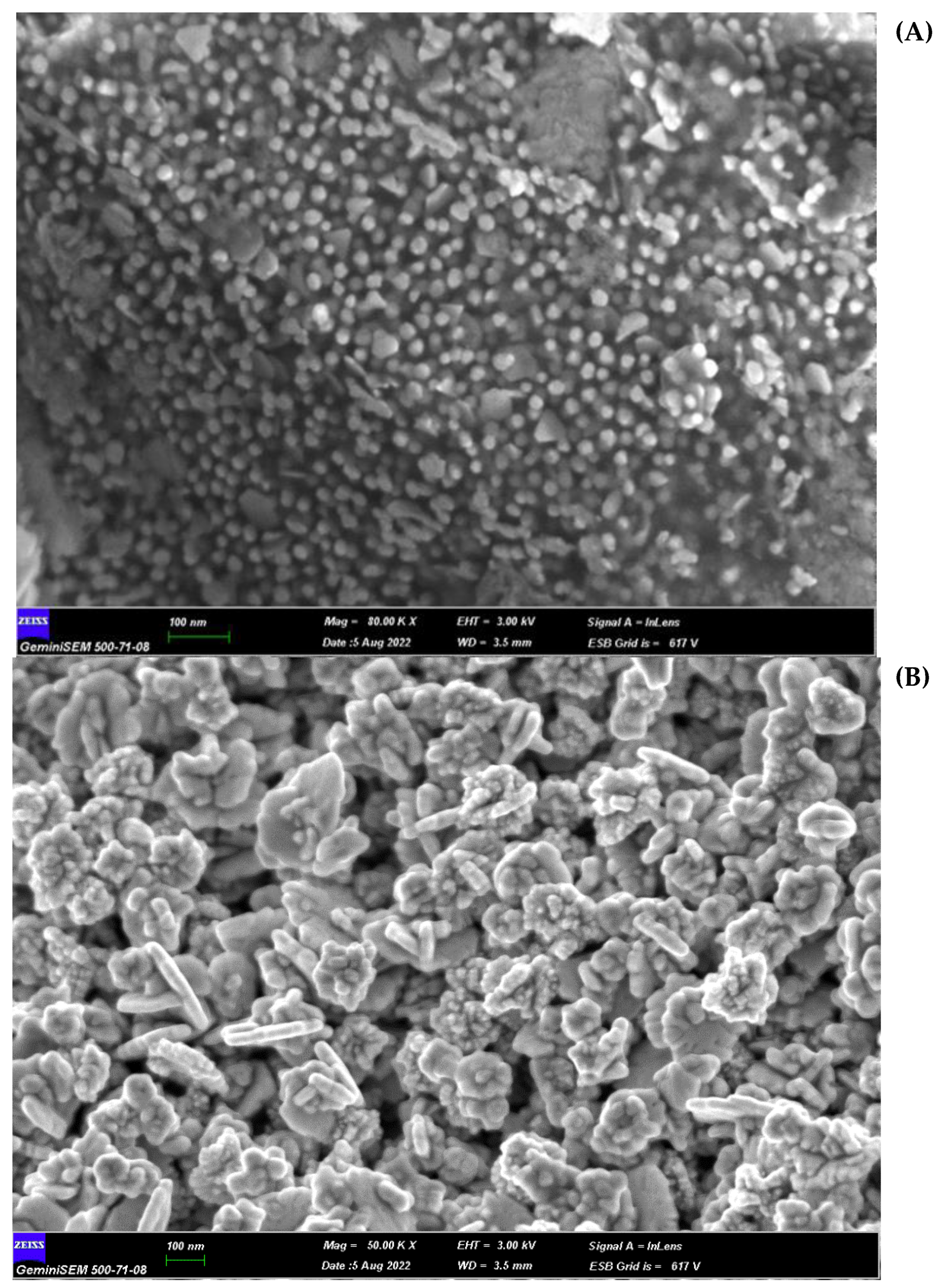



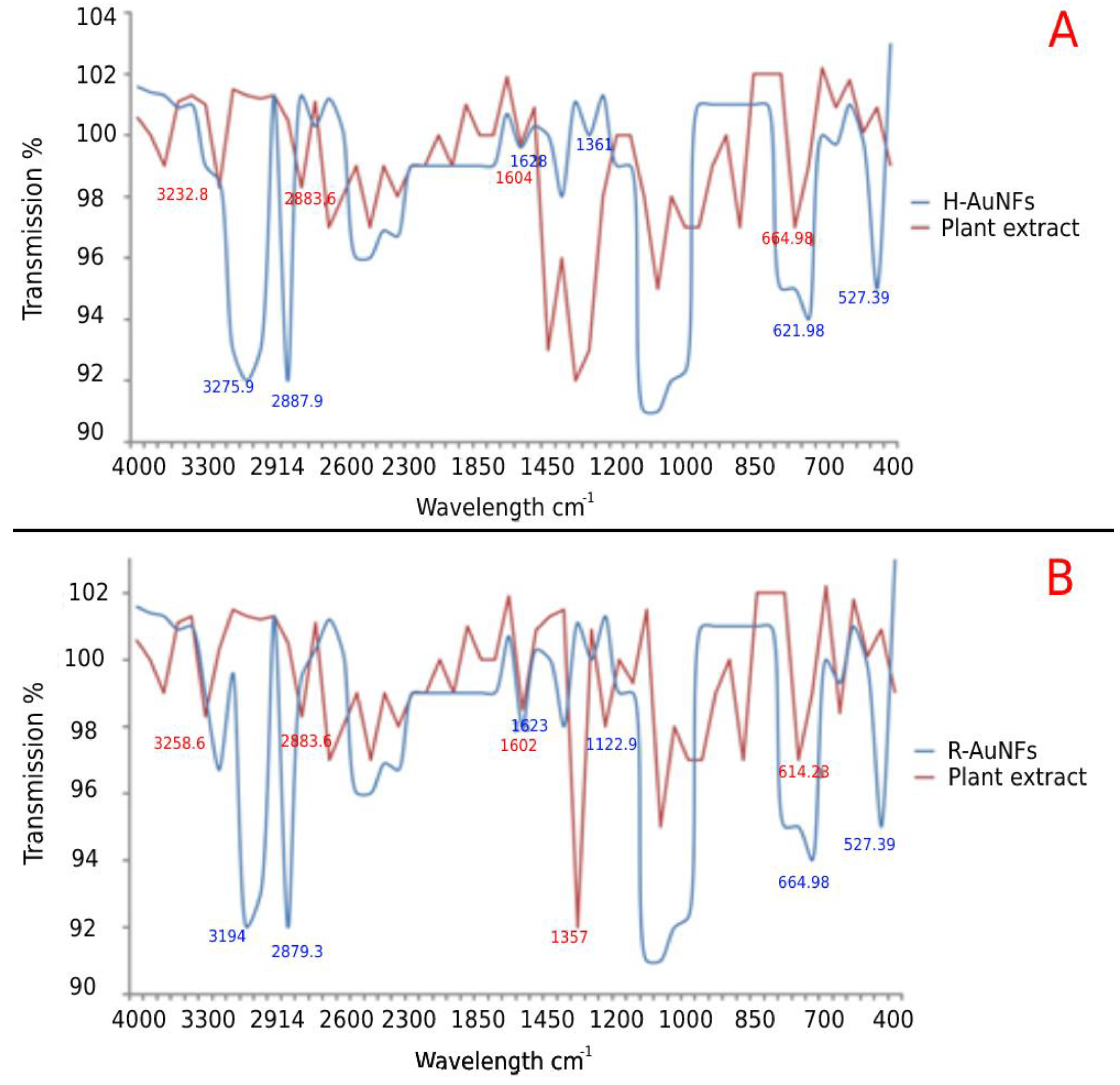
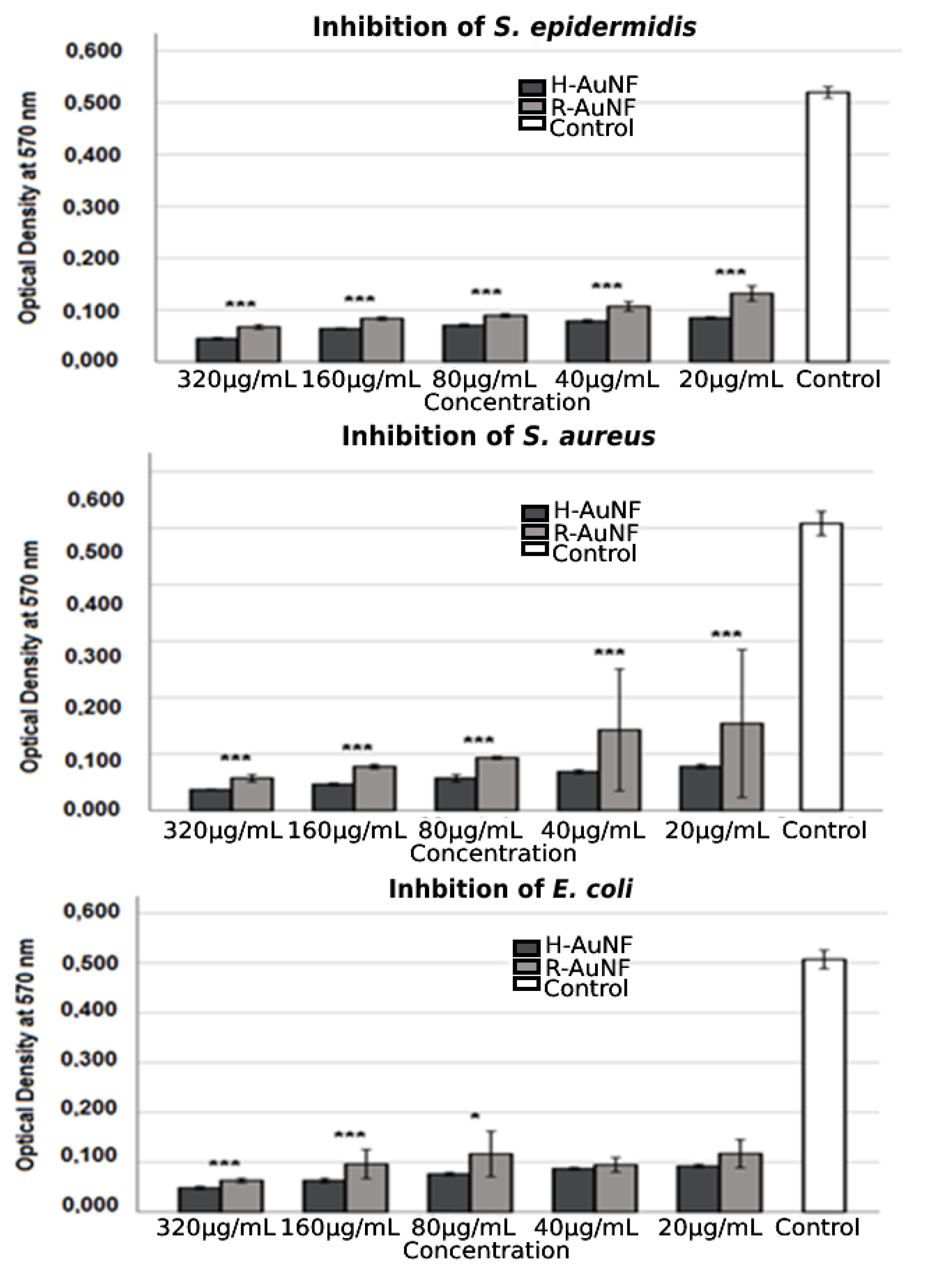
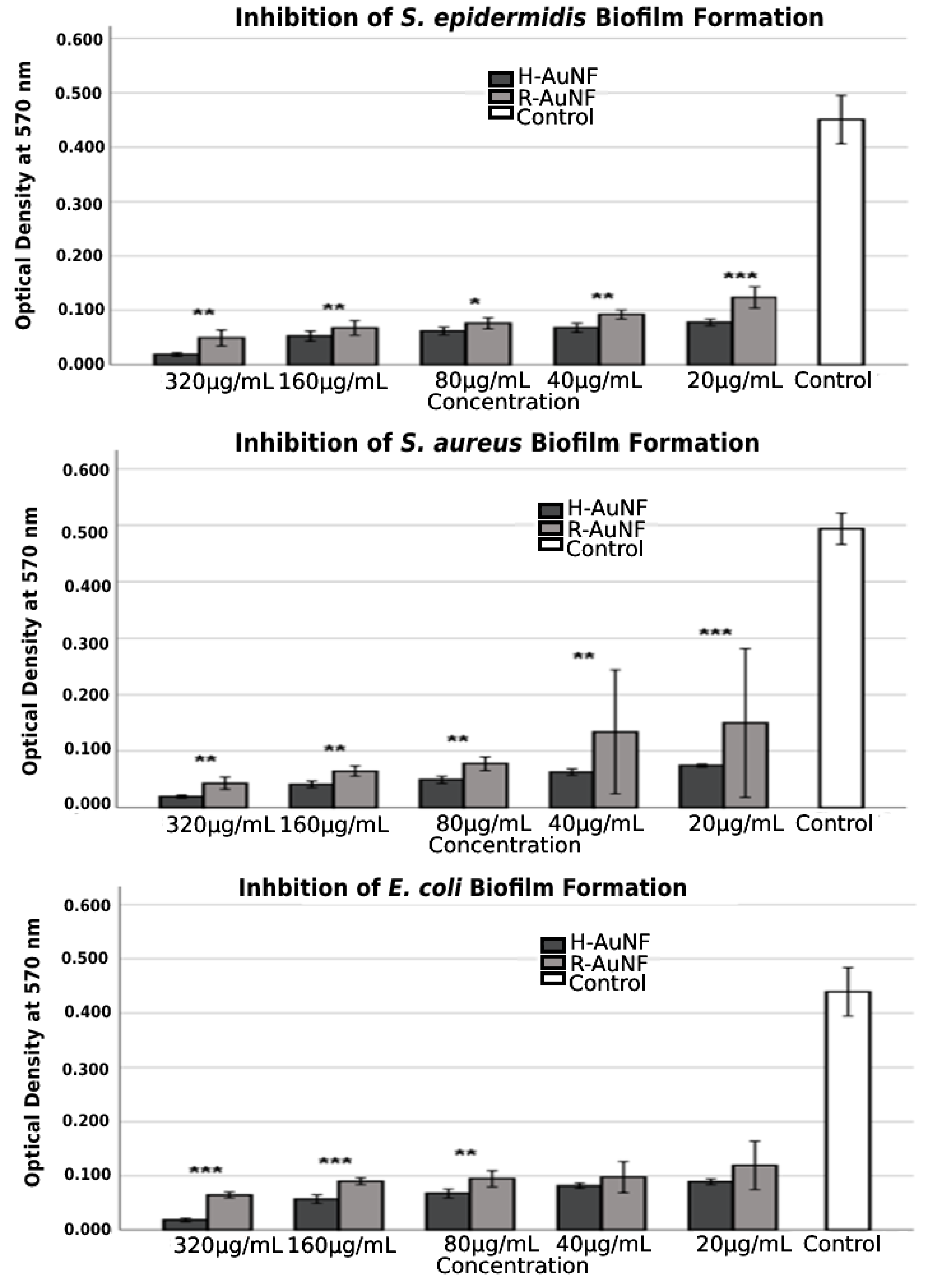
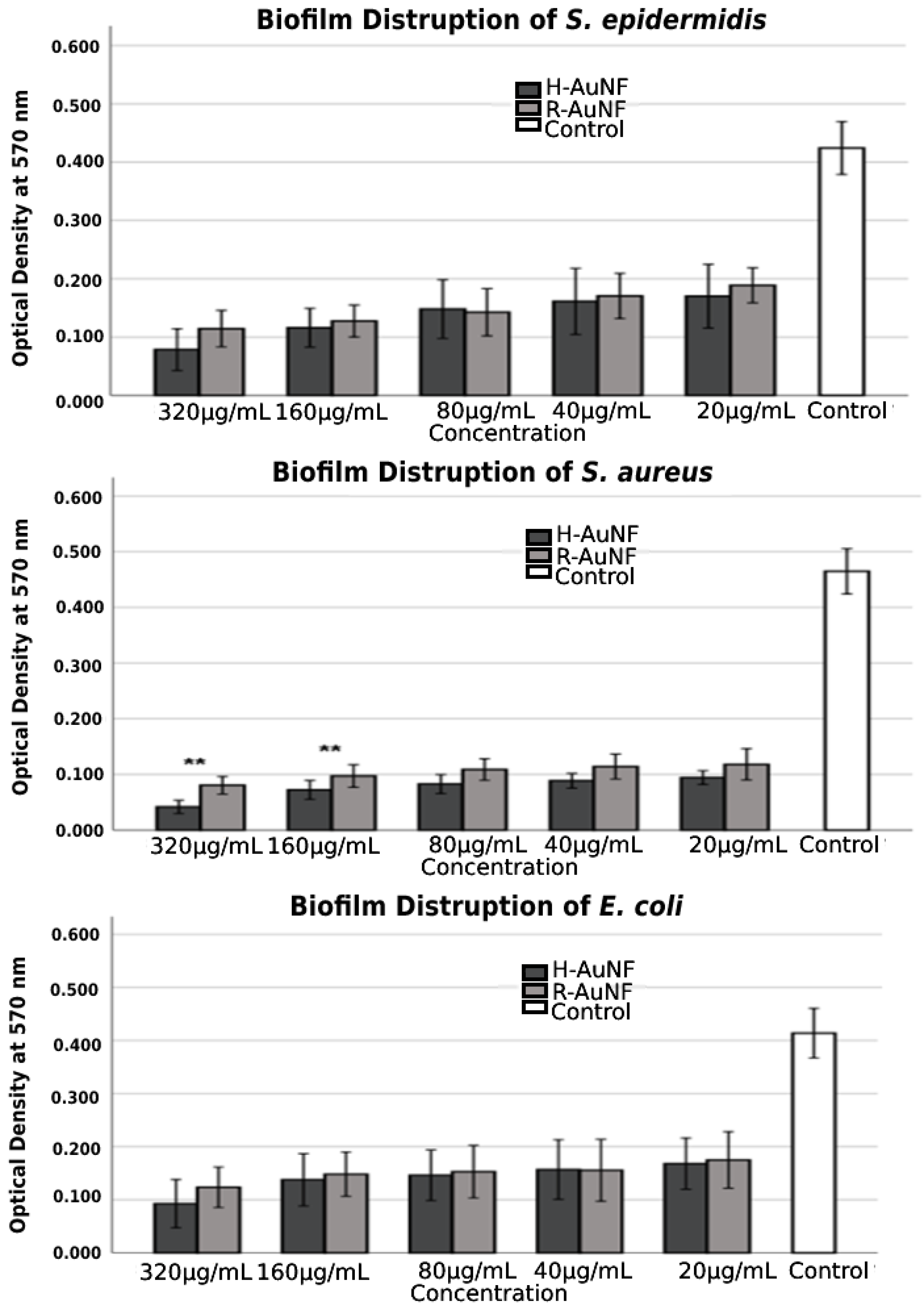
| Bacteria | MIC (µg/mL) | MBC (µg/mL) | Tolerance (MBC/MIC Ratio) | |||
|---|---|---|---|---|---|---|
| H-AuNFs | R-AuNFs | H-AuNFs | R-AuNFs | H-AuNFs | R-AuNFs | |
| S. aureus | 20 | 160 | 20 | 320 | 1 | 2 |
| S. epidermidis | 20 | 40 | 20 | 80 | 1 | 2 |
| E. coli | 20 | 40 | 20 | 80 | 1 | 2 |
| AuNFs | Concentration (µg/mL) | Biofilm Inhibition Rate (%) | Biofilm Disruption Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. coli | S. aureus | S. epidermidis | E. coli | ||
| H-AuNFs | 320 | 96.0 | 95.8 | 95.8 | 91.0 | 81.5 | 77.5 |
| 160 | 91.6 | 88.3 | 87.0 | 84.4 | 72.6 | 66.7 | |
| 80 | 90.0 | 86.2 | 84.6 | 82.2 | 65.0 | 64.6 | |
| 40 | 87.2 | 84.9 | 81.4 | 80.9 | 61.9 | 62.0 | |
| 20 | 84.9 | 82.7 | 79.7 | 79.7 | 59.8 | 59.3 | |
| R-AuNFs | 320 | 91.2 | 89.1 | 85.2 | 82.7 | 73.0 | 70.1 |
| 160 | 86.9 | 85.0 | 79.5 | 79.0 | 69.9 | 64.1 | |
| 80 | 84.2 | 83.1 | 78.4 | 76.6 | 66.4 | 63.0 | |
| 40 | 72.8 | 79.5 | 77.7 | 75.4 | 59.7 | 62.3 | |
| 20 | 69.6 | 72.5 | 72.8 | 74.6 | 55.5 | 57.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertas Onmaz, N.; Demirezen Yilmaz, D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1466. https://doi.org/10.3390/antibiotics11111466
Ertas Onmaz N, Demirezen Yilmaz D, Imre K, Morar A, Gungor C, Yilmaz S, Gundog DA, Dishan A, Herman V, Gungor G. Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics. 2022; 11(11):1466. https://doi.org/10.3390/antibiotics11111466
Chicago/Turabian StyleErtas Onmaz, Nurhan, Dilek Demirezen Yilmaz, Kálmán Imre, Adriana Morar, Candan Gungor, Seyda Yilmaz, Dursun Alp Gundog, Adalet Dishan, Viorel Herman, and Guven Gungor. 2022. "Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities" Antibiotics 11, no. 11: 1466. https://doi.org/10.3390/antibiotics11111466
APA StyleErtas Onmaz, N., Demirezen Yilmaz, D., Imre, K., Morar, A., Gungor, C., Yilmaz, S., Gundog, D. A., Dishan, A., Herman, V., & Gungor, G. (2022). Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics, 11(11), 1466. https://doi.org/10.3390/antibiotics11111466









