Early Years of Carbapenem-Resistant Enterobacterales Epidemic in Abu Dhabi
Abstract
1. Introduction
2. Results
2.1. Characteristics of Collection and Coverage of CRE Studied
2.2. Characterization of Major Clones Present
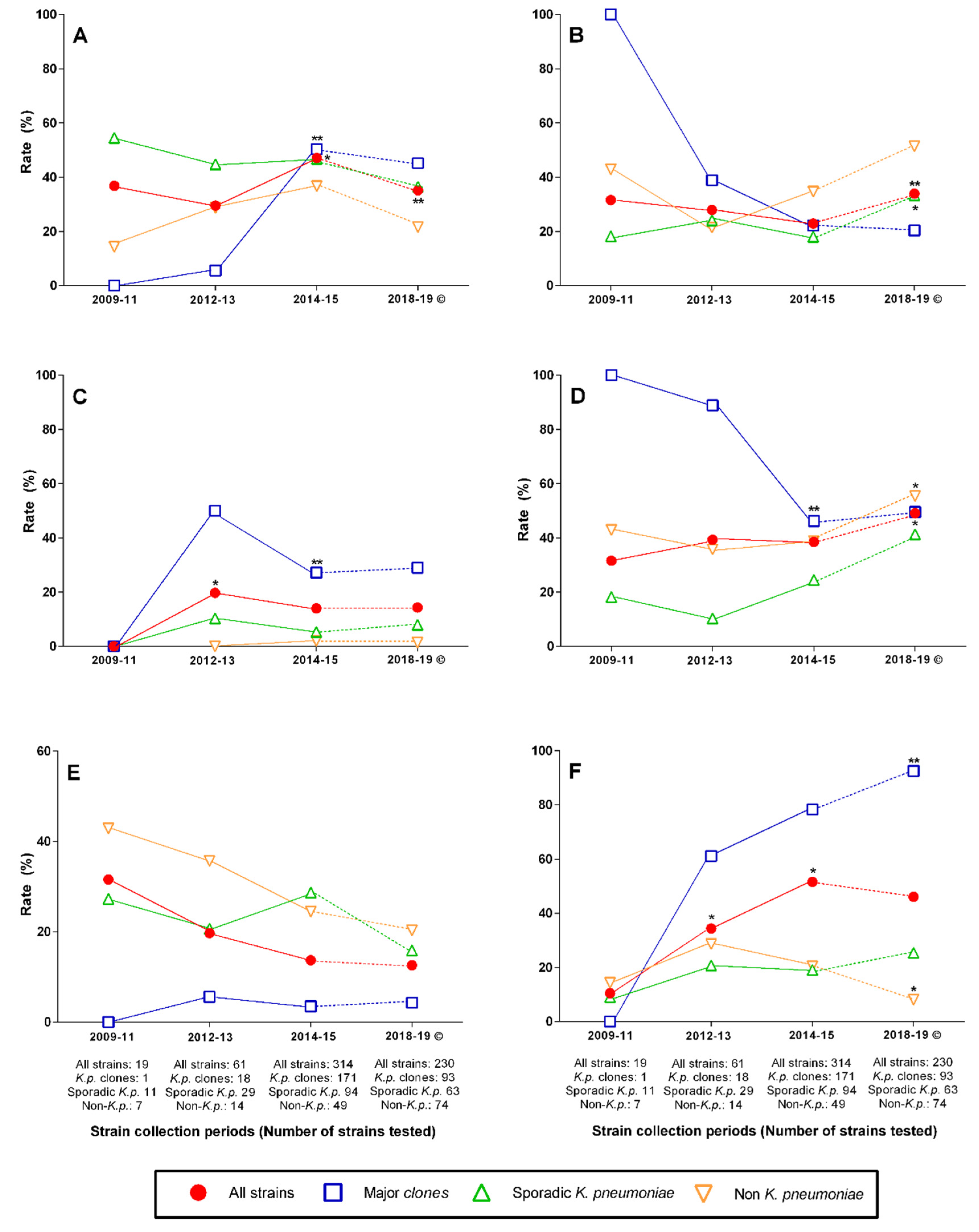
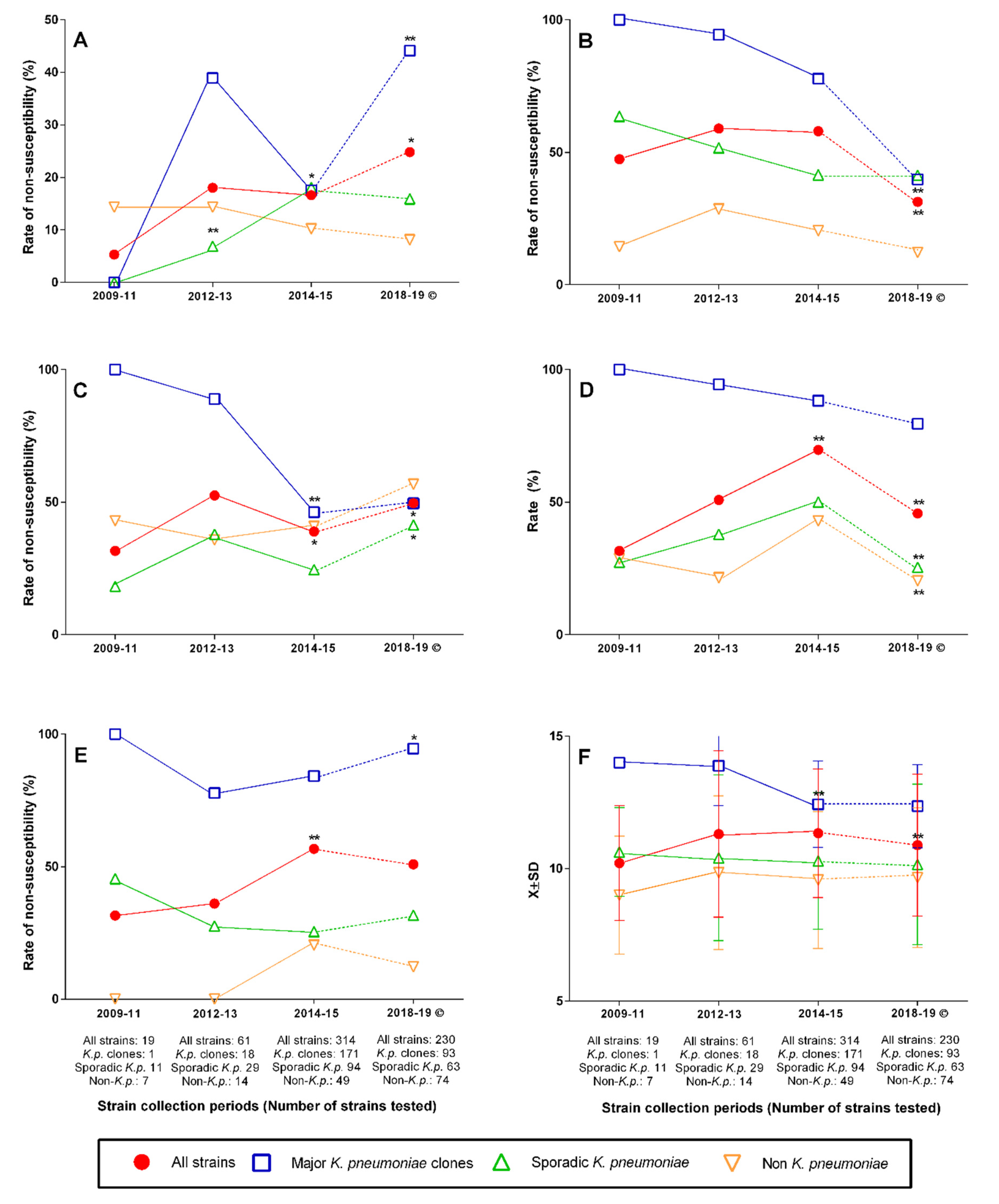
2.3. Changing Rates of Major Clones, Resistance Genes, and Antibiotic Nonsusceptibility over Time
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Al Mana, H.; Sundararaju, S.; Tsui, C.K.M.; Perez-Lopez, A.; Yassine, H.; Al Thani, A.; Al-Ansari, K.; Eltai, N.O. Whole-Genome Sequencing for Molecular Characterization of Carbapenem-Resistant Enterobacteriaceae Causing Lower Urinary Tract Infection among Pediatric Patients. Antibiotics 2021, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Abid, F.B.; Tsui, C.K.M.; Doi, Y.; Deshmukh, A.; McElheny, C.L.; Bachman, W.C.; Fowler, E.L.; Albishawi, A.; Mushtaq, K.; Ibrahim, E.B.; et al. Molecular characterization of clinical carbapenem-resistant Enterobacterales from Qatar. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1779–1785. [Google Scholar] [CrossRef]
- Abd El Ghany, M.; Sharaf, H.; Al-Agamy, M.H.; Shibl, A.; Hill-Cawthorne, G.A.; Hong, P.Y. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS ONE 2018, 13, e0201613. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdely, H.; AlHababi, R.; Dada, H.M.; Roushdy, H.; Alanazi, M.M.; Alessa, A.A.; Gad, N.M.; Alasmari, A.M.; Radwan, E.E.; Al-Dughmani, H.; et al. Molecular characterization of carbapenem-resistant Enterobacterales in thirteen tertiary care hospitals in Saudi Arabia. Ann. Saudi Med. 2021, 41, 63–70. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Aljallal, A.; Radwan, H.H.; Shibl, A.M. Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J. Infect. Public Health 2018, 11, 64–68. [Google Scholar] [CrossRef]
- Al-Baloushi, A.E.; Pal, T.; Ghazawi, A.; Sonnevend, A. Genetic support of carbapenemases in double carbapenemase producer Klebsiella pneumoniae isolated in the Arabian Peninsula. Acta Microbiol. Immunol. Hung. 2018, 65, 135–150. [Google Scholar] [CrossRef]
- Alotaibi, F.E.; Bukhari, E.E.; Al-Mohizea, M.M.; Hafiz, T.; Essa, E.B.; AlTokhais, Y.I. Emergence of carbapenem-resistant Enterobacteriaceae isolated from patients in a university hospital in Saudi Arabia. Epidemiology, clinical profiles and outcomes. J. Infect. Public Health 2017, 10, 667–673. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Al Yaqoubi, F.; Nordmann, P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin. Microbiol. Infect. 2012, 18, E144–E148. [Google Scholar] [CrossRef]
- Pal, T.; Ghazawi, A.; Darwish, D.; Villa, L.; Carattoli, A.; Hashmey, R.; Aldeesi, Z.; Jamal, W.; Rotimi, V.; Al-Jardani, A.; et al. Characterization of NDM-7 Carbapenemase-Producing Escherichia coli Isolates in the Arabian Peninsula. Microb. Drug Resist. 2017, 23, 871–878. [Google Scholar] [CrossRef]
- Poirel, L.; Al Maskari, Z.; Al Rashdi, F.; Bernabeu, S.; Nordmann, P. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J. Antimicrob. Chemother. 2011, 66, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Sonnevend, A.; Ghazawi, A.; Darwish, D.; AlDeesi, Z.; Kadhum, A.F.; Pal, T. Characterization of KPC-type carbapenemase-producing Klebsiella pneumoniae strains isolated in the Arabian Peninsula. J. Antimicrob. Chemother. 2015, 70, 1592–1593. [Google Scholar] [CrossRef] [PubMed]
- Sonnevend, A.; Ghazawi, A.; Yahfoufi, N.; Al-Baloushi, A.; Hashmey, R.; Mathew, M.; Tariq, W.Z.; Pal, T. VIM-4 carbapenemase-producing Enterobacter cloacae in the United Arab Emirates. Clin. Microbiol. Infect. 2012, 18, E494–E496. [Google Scholar] [CrossRef] [PubMed]
- Sonnevend, A.; Ghazawi, A.A.; Hashmey, R.; Jamal, W.; Rotimi, V.O.; Shibl, A.M.; Al-Jardani, A.; Al-Abri, S.S.; Tariq, W.U.; Weber, S.; et al. Characterization of Carbapenem-Resistant Enterobacteriaceae with High Rate of Autochthonous Transmission in the Arabian Peninsula. PLoS ONE 2015, 10, e0131372. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Sartor, A.L.; Balkhy, H.H.; Walsh, T.R.; Al Johani, S.M.; AlJindan, R.Y.; Alfaresi, M.; Ibrahim, E.; Al-Jardani, A.; Al-Abri, S.; et al. Molecular characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the countries of the Gulf cooperation council: Dominance of OXA-48 and NDM producers. Antimicrob. Agents Chemother. 2014, 58, 3085–3090. [Google Scholar] [CrossRef]
- Jamal, W.Y.; Albert, M.J.; Rotimi, V.O. High Prevalence of New Delhi Metallo-βLactamase-1 (NDM-1) Producers among Carbapenem-Resistant Enterobacteriaceae in Kuwait. PLoS ONE 2016, 11, e0152638. [Google Scholar] [CrossRef]
- Jamal, W.; Rotimi, V.O.; Albert, M.J.; Khodakhast, F.; Nordmann, P.; Poirel, L. High prevalence of VIM-4 and NDM-1 metallo-blactamase among carbapenem-resistant Enterobacteriaceae. J. Med. Microbiol. 2013, 62, 1239–1244. [Google Scholar] [CrossRef]
- Saeed, N.K.; Alkhawaja, S.; Azam, N.F.A.E.M.; Alaradi, K.; Al-Biltagi, M. Epidemiology of carbapenem-resistant Enterobacteriaceae in a Tertiary Care Center in the Kingdom of Bahrain. J. Lab. Physicians 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Mouftah, S.F.; Pal, T.; Ghazawi, A.; Halat, D.H.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Peters, C.C.; Celiloglu, H.; et al. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int. J. Antimicrob. Agents 2018, 52, 90–95. [Google Scholar] [CrossRef]
- Sonnevend, A.; Abdulrazzaq, N.; Ghazawi, A.; Thomsen, J.; Bharathan, G.; Makszin, L.; Rizvi, T.A.; Pal, T.; Group, U.C.S. The first nationwide surveillance of carbapenem-resistant Enterobacterales in the United Arab Emirates—Increased association of Klebsiella pneumoniae CC14 clone with Emirati patients. Int. J. Infect. Dis. 2022, 120, 103–112. [Google Scholar] [CrossRef]
- Sonnevend, A.; Ghazawi, A.; Darwish, D.; Barathan, G.; Hashmey, R.; Ashraf, T.; Rizvi, T.A.; Pal, T. In vitro efficacy of ceftazidime-avibactam, aztreonam-avibactam and other rescue antibiotics against carbapenem-resistant Enterobacterales from the Arabian Peninsula. Int. J. Infect. Dis. 2020, 99, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Bartzavali, C.; Lambropoulou, A.; Solomou, A.; Tsiata, E.; Anastassiou, E.D.; Fligou, F.; Marangos, M.; Spiliopoulou, I.; Christofidou, M. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J. Antimicrob. Chemother. 2019, 74, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, A.; Papadimitriou-Olivgeris, M.; Bartzavali, C.; Vamvakopoulou, S.; Marangos, M.; Spiliopoulou, I.; Anastassiou, E.D.; Christofidou, M. A ten-year surveillance study of carbapenemase-producing Klebsiella pneumoniae in a tertiary care Greek university hospital: Predominance of KPC- over VIM- or NDM-producing isolates. J. Med. Microbiol. 2016, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Yu, W.L. Klebsiella pneumoniae Harboring Carbapenemase Genes in Taiwan: Its Evolution over 20 Years, 1998–2019. Int. J. Antimicrob. Agents 2021, 58, 106354. [Google Scholar] [CrossRef]
- Sonnevend, A.; Alali, W.Q.; Mahmoud, S.A.; Ghazawi, A.; Bharathan, G.; Melegh, S.; Rizvi, T.A.; Pal, T. Molecular Characterization of MCR-1 Producing Enterobacterales Isolated in Poultry Farms in the United Arab Emirates. Antibiotics 2022, 11, 305. [Google Scholar] [CrossRef]
- Mouftah, S.F.; Pal, T.; Higgins, P.G.; Ghazawi, A.; Idaghdour, Y.; Alqahtani, M.; Omrani, A.S.; Rizvi, T.A.; Sonnevend, A. Diversity of carbapenem-resistant Klebsiella pneumoniae ST14 and emergence of a subgroup with KL64 capsular locus in the Arabian Peninsula. Eur J. Clin. Microbiol Infect. Dis. 2021. online, ahead of print. [Google Scholar] [CrossRef]
- Chief Executive Officer Office Health Authority Abu Dhabi, U.A.E. Emergence of Carbapenem-Resistant Enterobacteriaceae (CRE). 2013; Circular No. DG 46/13. 2013. [Google Scholar]
- Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI Supplement M100; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Davis, M.A.; Baker, K.N.; Orfe, L.H.; Shah, D.H.; Besser, T.E.; Call, D.R. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 2666–2669. [Google Scholar] [CrossRef]
- Hidalgo, L.; Hopkins, K.L.; Gutierrez, B.; Ovejero, C.M.; Shukla, S.; Douthwaite, S.; Prasad, K.N.; Woodford, N.; Gonzalez-Zorn, B. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J. Antimicrob. Chemother. 2013, 68, 1543–1550. [Google Scholar] [CrossRef]
- Gautom, R.K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 1997, 35, 2977–2980. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent clones of Klebsiella pneumoniae: Identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Genes, Clones or Resistance Features | % within the Collection | Alleles | % within the Enzyme Group | Year Encountered | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |||||
| Resistance genes | blaNDM | 24.9 | blaNDM-1 | 89.8 | + | + | + | + | + | + | + |
| blaNDM-4 | 1.0 | + | |||||||||
| blaNDM-5 | 8.2 | + | + | ||||||||
| blaNDM-7 | 1.0 | + | |||||||||
| blaOXA-48-like | 43.9 | blaOXA-48 | 37.0 | + | + | + | + | + | + | + | |
| blaOXA-162 | 1.7 | + | + | ||||||||
| blaOXA-181 | 16.8 | + | + | + | |||||||
| blaOXA-232 | 42.8 | + | + | + | |||||||
| blaOXA-244 | 1.7 | + | + | + | |||||||
| blaVIM | 0.8 | blaVIM-4 | 66.6 | + | + | ||||||
| blaVIM-55 | 33.3 | + | |||||||||
| blaNDM + blaOXA-48-like | 14.5 | blaNDM-1 + blaOXA-48 | 8.9 | + | + | ||||||
| blaNDM-1 + blaOXA-162 | 1.8 | + | |||||||||
| blaNDM-1 + blaOXA-181 | 3.6 | + | + | ||||||||
| blaNDM-1 + blaOXA-232 | 73.2 | + | + | + | |||||||
| blaNDM-5 + blaOXA-181 | 12.5 | + | + | + | |||||||
| blaNDM-1 + blaVIM-55 | 0.3 | blaNDM-1 + blaVIM-55 | 100.0 | + | |||||||
| Any MBL 1 | 40.4 | - | - | + | + | + | + | + | + | + | |
| Any 16S methylase | 49.6 | armA | 30.5 | + | + | + | + | + | |||
| rmtB | 1.3 | + | + | + | |||||||
| rmtC | 1.3 | + | + | ||||||||
| rmtF | 15.7 | + | + | + | + | ||||||
| Resistance-related characteristics | XDR | 9.1 | - | - | + | + | + | + | + | + | |
| PDR | 2.5 | - | - | + | + | + | + | ||||
| Colistin R | 16.2 | - | - | + | + | + | + | + | |||
| Tigecycline R | 57.6 | - | - | + | + | + | + | + | + | + | |
| Ceftazidime–avibactam R | 40.6 | - | - | + | + | + | + | + | + | + | |
| K. pneumoniae major clones | ST14 | 28.2 | - | - | + | + | + | + | |||
| CC147 2 | 10.9 | - | - | + | + | + | + | ||||
| ST231 | 11.1 | - | - | + | + | ||||||
| Group | N | Rate (%) among | Presence in Hospitals 3 | |
|---|---|---|---|---|
| K. pneumoniae | All Strains | |||
| ST14 | 111 | 34.3 | 28.2 | A, B, C, D, F |
| CC147 1 | 43 | 13.3 | 10.9 | A, B, C, D, F |
| ST231 | 36 | 11.1 | 9.1 | A, B, C, D, E |
| All major clones | 190 | 58.6 | 48.2 | A, B, C, D, E, F |
| Sporadic 2 K. pneumoniae | 134 | 41.4 | 34.0 | A, B, C, D, E, F |
| Groups | N | Rate (%) of | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance Genes | Nonsusceptibility to | Resistance-Related Parameters | ||||||||||||
| blaNDM | blaOXA-48-like | blaNDM + blaOXA-48-like | MBL 3 | Any 16S Methylase | armA | rmtF | Ceftazidime-Avibactam | Colistin | Tigecycline | Meropenem MIC > 8 mg/L | XDR or PDR | R Index | ||
| ST14 | 111 | 30.3 | 36.9 | 32.4 | 63.1 | 78.4 | 78.4 | 0 | 67.6 | 26.1 | 75.7 | 89.2 | 33.3 | 13.02 ± 1.29 |
| ST231 | 36 | 0.0 | 97.2 | 0.0 | 0.0 | 100.0 | 5.6 | 97.2 | 0.0 | 5.6 | 100.0 | 91.7 | 0.0 | 12.27 ± 0.77 |
| CC147 1 | 43 | 34.9 | 37.2 | 14.0 | 48.8 | 51.2 | 11.6 | 39.5 | 48.8 | 14.0 | 72.1 | 86.1 | 9.3 | 11.67 ± 2.51 |
| Sporadic 2 strains | 134 | 12.8 | 30.1 | 3.9 | 17.2 | 12.2 | 7.8 | 2.5 | 17.7 | 9.3 | 30.0 | 30.0 | 2.0 | 10.35 ± 2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pál, T.; Butt, A.B.; Ghazawi, A.; Thomsen, J.; Rizvi, T.A.; Sonnevend, Á. Early Years of Carbapenem-Resistant Enterobacterales Epidemic in Abu Dhabi. Antibiotics 2022, 11, 1435. https://doi.org/10.3390/antibiotics11101435
Pál T, Butt AB, Ghazawi A, Thomsen J, Rizvi TA, Sonnevend Á. Early Years of Carbapenem-Resistant Enterobacterales Epidemic in Abu Dhabi. Antibiotics. 2022; 11(10):1435. https://doi.org/10.3390/antibiotics11101435
Chicago/Turabian StylePál, Tibor, Aqdas B. Butt, Akela Ghazawi, Jens Thomsen, Tahir A. Rizvi, and Ágnes Sonnevend. 2022. "Early Years of Carbapenem-Resistant Enterobacterales Epidemic in Abu Dhabi" Antibiotics 11, no. 10: 1435. https://doi.org/10.3390/antibiotics11101435
APA StylePál, T., Butt, A. B., Ghazawi, A., Thomsen, J., Rizvi, T. A., & Sonnevend, Á. (2022). Early Years of Carbapenem-Resistant Enterobacterales Epidemic in Abu Dhabi. Antibiotics, 11(10), 1435. https://doi.org/10.3390/antibiotics11101435






