Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis
Abstract
1. Introduction
2. Empirical Antimicrobial Therapy
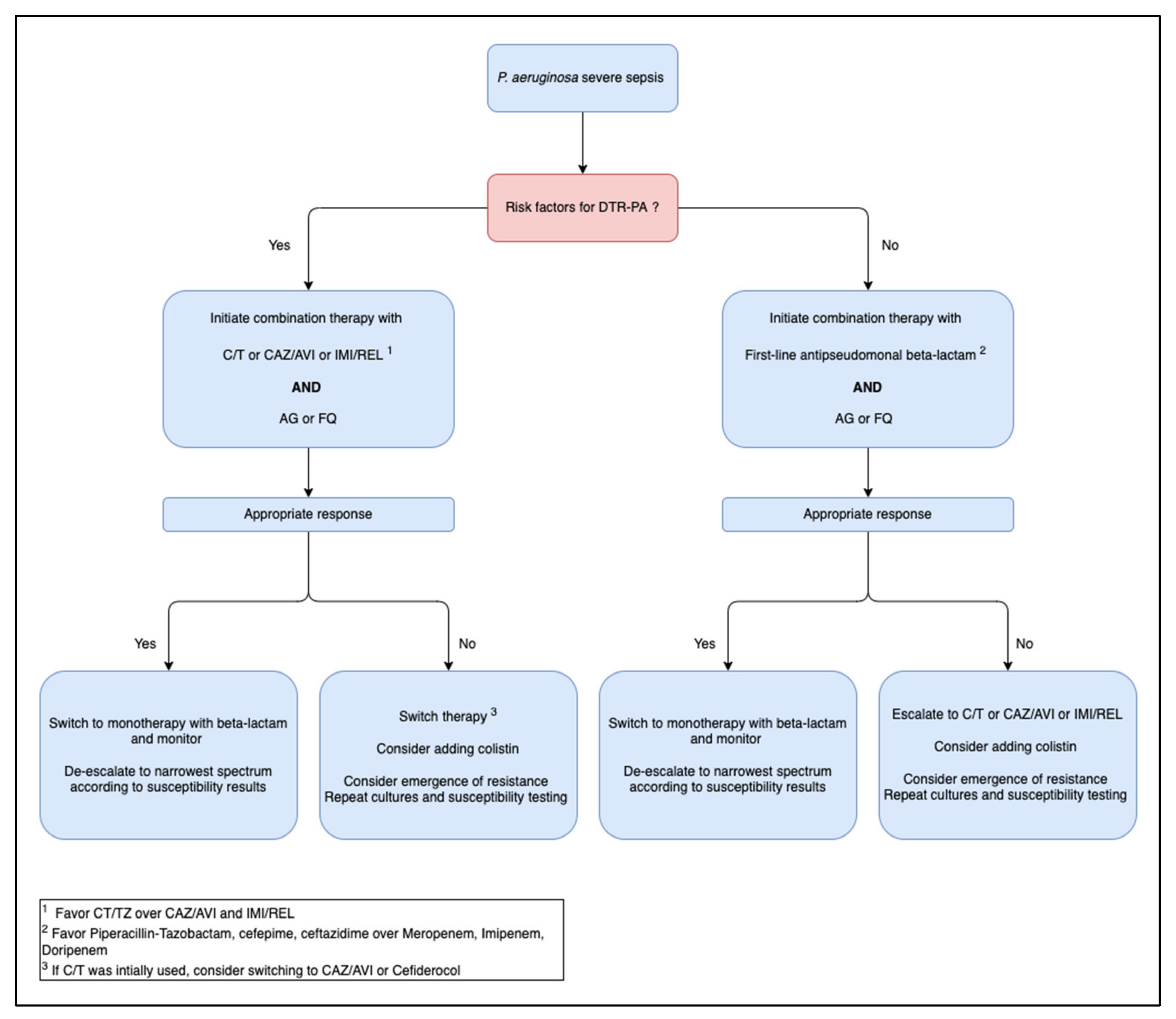
3. Targeted Therapy for P. aeruginosa Sepsis
3.1. P. aeruginosa Sensitive to First Line Antipseudomonal Agents
3.2. P. aeruginosa Resistant to First Line Therapy
4. Key Factors Related to Therapy
4.1. Antimicrobial Dosing
4.2. Infusion Rate
4.3. Duration of Therapy
5. Alternative Therapies
5.1. Phage Therapy
5.2. Antibodies/Vaccines
5.3. Quorum Sensing
5.4. Bacteriocins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tschudin-Sutter, S.; Fosse, N.; Frei, R.; Widmer, A.F. Combination therapy for treatment of Pseudomonas aeruginosa bloodstream infections. PLoS ONE 2018, 13, e0203295. [Google Scholar] [CrossRef] [PubMed]
- Horino, T.; Chiba, A.; Kawano, S.; Kato, T.; Sato, F.; Maruyama, Y.; Nakazawa, Y.; Yoshikawa, K.; Yoshida, M.; Hori, S. Clinical characteristics and risk factors for mortality in patients with bacteremia caused by Pseudomonas aeruginosa. Intern. Med. Tokyo Jpn. 2012, 51, 59–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, C.-I.; Kim, S.-H.; Kim, H.-B.; Park, S.-W.; Choe, Y.-J.; Oh, M.; Kim, E.-C.; Choe, K.-W. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G.; van Duin, D. Results from a 13-Year Prospective Cohort Study Show Increased Mortality Associated with Bloodstream Infections Caused by Pseudomonas aeruginosa Compared to Other Bacteria. Antimicrob. Agents Chemother. 2017, 61, e02671-16. [Google Scholar] [CrossRef]
- Cattaneo, C.; Antoniazzi, F.; Casari, S.; Ravizzola, G.; Gelmi, M.; Pagani, C.; D’Adda, M.; Morello, E.; Re, A.; Borlenghi, E.; et al. P. aeruginosa bloodstream infections among hematological patients: An old or new question? Ann. Hematol. 2012, 91, 1299–1304. [Google Scholar] [CrossRef]
- Tofas, P.; Samarkos, M.; Piperaki, E.-T.; Kosmidis, C.; Triantafyllopoulou, I.-D.; Kotsopoulou, M.; Pantazatou, A.; Perlorentzou, S.; Poulli, A.; Vagia, M.; et al. Pseudomonas aeruginosa bacteraemia in patients with hematologic malignancies: Risk factors, treatment and outcome. Diagn. Microbiol. Infect. Dis. 2017, 88, 335–341. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: A retrospective multicentre study. Int. J. Antimicrob. Agents 2020, 55, 105847. [Google Scholar] [CrossRef]
- Albasanz-Puig, A.; Durà-Miralles, X.; Laporte-Amargós, J.; Mussetti, A.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; Montejo, J.M.; et al. Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia. Microorganisms 2022, 10, 733. [Google Scholar] [CrossRef]
- Nakamura, A.; Miyake, K.; Misawa, S.; Kuno, Y.; Horii, T.; Kondo, S.; Tabe, Y.; Ohsaka, A. Meropenem as predictive risk factor for isolation of multidrug-resistant Pseudomonas aeruginosa. J. Hosp. Infect. 2013, 83, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.H.; Gurtman, A.; Szabo, S.; Neibart, E.; Meyers, B.R.; Policar, M.; Cheung, T.W.; Lillienfeld, D.; Hammer, G.; Reddy, S. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 18, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Stavola, J.J.; Noel, G.J. Bacteremia due to Pseudomonas aeruginosa in children with AIDS. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1993, 16, 706–708. [Google Scholar] [CrossRef]
- Zidaru, A.; Sofjan, A.K.; Devarajan, S.R.; Tam, V.H. Clinical outcomes of cystic fibrosis patients with Pseudomonas aeruginosa bloodstream infection. J. Glob. Antimicrob. Resist. 2022, 29, 551–552. [Google Scholar] [CrossRef]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: Impact of multidrug resistance. Crit. Care Lond. Engl. 2015, 19, 219. [Google Scholar] [CrossRef]
- Tumbarello, M.; De Pascale, G.; Trecarichi, E.M.; Spanu, T.; Antonicelli, F.; Maviglia, R.; Pennisi, M.A.; Bello, G.; Antonelli, M. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013, 39, 682–692. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Jabbour, J.-F.; Sharara, S.L.; Kanj, S.S. Treatment of multidrug-resistant Gram-negative skin and soft tissue infections. Curr. Opin. Infect. Dis. 2020, 33, 146–154. [Google Scholar] [CrossRef]
- Ibrahim, D.; Jabbour, J.-F.; Kanj, S.S. Current choices of antibiotic treatment for Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2020, 33, 464–473. [Google Scholar] [CrossRef]
- Herrera, S.; Bodro, M.; Soriano, A. Predictors of multidrug resistant Pseudomonas aeruginosa involvement in bloodstream infections. Curr. Opin. Infect. Dis. 2021, 34, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Forkner, C.E.; Frei, E.; Edgcomb, J.H.; Utz, J.P. Pseudomonas septicemia; observations on twenty-three cases. Am. J. Med. 1958, 25, 877–889. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Carnelutti, A. Bloodstream infections in the Intensive Care Unit. Virulence 2016, 7, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Tansarli, G.S.; Andreatos, N.; Pliakos, E.E.; Mylonakis, E. A Systematic Review and Meta-analysis of Antibiotic Treatment Duration for Bacteremia Due to Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02495-18. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Kaye, K.S.; Neuner, E.A.; Bonomo, R.A. Carbapenem-resistant Enterobacteriaceae: A review of treatment and outcomes. Diagn. Microbiol. Infect. Dis. 2013, 75, 115–120. [Google Scholar] [CrossRef]
- Falcone, M.; Bassetti, M.; Tiseo, G.; Giordano, C.; Nencini, E.; Russo, A.; Graziano, E.; Tagliaferri, E.; Leonildi, A.; Barnini, S.; et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care Lond. Engl. 2020, 24, 29. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Russo, A.; Croxatto, A.; Calandra, T.; Guery, B. Rational approach in the management of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2018, 31, 578–586. [Google Scholar] [CrossRef]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. N. Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010-2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef]
- 2019 Antibiotic Resistance Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 31 July 2022).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. IDSA Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections: Version 1.0; IDSA: Arlington, VA, USA, 2022; Available online: https://www.idsociety.org/practice-guideline/amr-guidance/ (accessed on 31 July 2022).
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-To-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-Line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. Available online: https://pubmed.ncbi.nlm.nih.gov/30052813/ (accessed on 28 September 2022). [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Díaz-Cañestro, M.; Periañez, L.; Mulet, X.; Martin-Pena, M.L.; Fraile-Ribot, P.A.; Ayestarán, I.; Colomar, A.; Nuñez, B.; Maciá, M.; Novo, A.; et al. Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: Experience from the Balearic Islands. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2191–2200. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Tansarli, G.S.; Bliziotis, I.A.; Falagas, M.E. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: A meta-analysis. Int. J. Antimicrob. Agents 2013, 41, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Lador, A.; Grozinsky-Glasberg, S.; Leibovici, L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 2014, 2014, CD003344. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; Suarez, C.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; Calbo, E.; Rodríguez-Baño, J.; et al. Effect of Adequate Single-Drug vs Combination Antimicrobial Therapy on Mortality in Pseudomonas aeruginosa Bloodstream Infections: A Post Hoc Analysis of a Prospective Cohort. In Clinical Infectious Diseases; Oxford Academic: Oxford, UK, 2013; Available online: https://academic.oup.com/cid/article/57/2/208/313652 (accessed on 31 July 2022).
- Onorato, L.; Macera, M.; Calò, F.; Cirillo, P.; Di Caprio, G.; Coppola, N. Beta-lactam monotherapy or combination therapy for bloodstream infections or pneumonia due to Pseudomonas aeruginosa: A meta-analysis. Int. J. Antimicrob. Agents 2022, 59, 106512. [Google Scholar] [CrossRef]
- Micek, S.T.; Lloyd, A.E.; Ritchie, D.J.; Reichley, R.M.; Fraser, V.J.; Kollef, M.H. Pseudomonas aeruginosa bloodstream infection: Importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2005, 49, 1306–1311. [Google Scholar] [CrossRef]
- Fiore, M.; Corrente, A.; Pace, M.C.; Alfieri, A.; Simeon, V.; Ippolito, M.; Giarratano, A.; Cortegiani, A. Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis; Antibiotics: Basel, Switzerland, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/33467508/ (accessed on 31 July 2022).
- Al Salman, J.; Al Dabal, L.; Bassetti, M.; Alfouzan, W.A.; Al Maslamani, M.; Alraddadi, B.; Elhoufi, A.; Enani, M.; Khamis, F.A.; Mokkadas, E.; et al. Management of infections caused by WHO critical priority Gram-negative pathogens in Arab countries of the Middle East: A consensus paper. Int. J. Antimicrob. Agents 2020, 56, 106104. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Paulsson, M.; Granrot, A.; Ahl, J.; Tham, J.; Resman, F.; Riesbeck, K.; Månsson, F. Antimicrobial combination treatment including ciprofloxacin decreased the mortality rate of Pseudomonas aeruginosa bacteraemia: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Puzniak, L.; DePestel, D.D.; Srinivasan, A.; Ye, G.; Murray, J.; Merchant, S.; DeRyke, C.A.; Gupta, V. A Combination Antibiogram Evaluation for Pseudomonas aeruginosa in Respiratory and Blood Sources from Intensive Care Unit (ICU) and Non-ICU Settings in U.S. Hospitals. Antimicrob. Agents Chemother. 2019, 63, e02564-18. [Google Scholar] [CrossRef] [PubMed]
- De Winter, S.; Wauters, J.; Meersseman, W.; Verhaegen, J.; Van Wijngaerden, E.; Peetermans, W.; Annaert, P.; Verelst, S.; Spriet, I. Higher versus standard amikacin single dose in emergency department patients with severe sepsis and septic shock: A randomised controlled trial. Int. J. Antimicrob. Agents 2018, 51, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Gonzalez, M.; Alekseeva, I.; Lopez, D.; Akrich, B.; DeRyke, C.A.; Chen, W.-T.; Pavia, J.; Palermo, B.; Hackel, M.; et al. Collective assessment of antimicrobial susceptibility among the most common Gram-negative respiratory pathogens driving therapy in the ICU. JAC-Antimicrob. Resist. 2021, 3, dlaa129. [Google Scholar] [CrossRef]
- Britt, N.S.; Ritchie, D.J.; Kollef, M.H.; Burnham, C.A.; Durkin, M.J.; Hampton, N.B.; Micek, S.T. Importance of Site of Infection and Antibiotic Selection in the Treatment of Carbapenem-Resistant Pseudomonas aeruginosa Sepsis. Antimicrob. Agents Chemother. 2018, 62, e02400-17. [Google Scholar] [CrossRef]
- Kaye, K.S.; Kanafani, Z.A.; Dodds, A.E.; Engemann, J.J.; Weber, S.G.; Carmeli, Y. Differential Effects of Levofloxacin and Ciprofloxacin on the Risk for Isolation of Quinolone-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2192–2196. [Google Scholar] [CrossRef]
- McKeage, K. Finafloxacin: First global approval. Drugs 2015, 75, 687–693. [Google Scholar] [CrossRef]
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef]
- Gimeno, C.; Cantón, R.; García, A.; Gobernado, M. Grupo Español de Estudio de Doripenem [Comparative activity of doripenem, meropenem, and imipenem in recent clinical isolates obtained during the COMPACT-Spain epidemiological surveillance study]. Rev. Espanola Quimioter. Publicacion Of. Soc. Espanola Quimioter. 2010, 23, 144–152. [Google Scholar]
- Pillar, C.M.; Torres, M.K.; Brown, N.P.; Shah, D.; Sahm, D.F. In vitro activity of doripenem, a carbapenem for the treatment of challenging infections caused by gram-negative bacteria, against recent clinical isolates from the United States. Antimicrob. Agents Chemother. 2008, 52, 4388–4399. [Google Scholar] [CrossRef][Green Version]
- Korten, V.; Söyletir, G.; Yalçın, A.N.; Oğünç, D.; Dokuzoğuz, B.; Esener, H.; Ulusoy, S.; Tünger, A.; Aygen, B.; Sümerkan, B.; et al. Comparative evaluation of in vitro activities of carbapenems against gram-negative pathogens: Turkish data of COMPACT study. Mikrobiyol. Bul. 2011, 45, 197–209. [Google Scholar] [PubMed]
- Kanj, S.S.; Kanafani, Z.A. Current Concepts in Antimicrobial Therapy Against Resistant Gram-Negative Organisms: Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae, Carbapenem-Resistant Enterobacteriaceae, and Multidrug-Resistant Pseudomonas aeruginosa. Mayo Clin. Proc. 2011, 86, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, Y.; Troillet, N.; Eliopoulos, G.M.; Samore, M.H. Emergence of antibiotic-resistant Pseudomonas aeruginosa: Comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 1999, 43, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Bowers, D.R.; Babic, J.T.; Tam, V.H. Outcomes of empiric aminoglycoside monotherapy for Pseudomonas aeruginosa bacteremia. Diagn. Microbiol. Infect. Dis. 2019, 93, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Antimicrobial Pharmacokinetic and Pharmacodynamic Issues in the Critically Ill with Severe Sepsis and Septic Shock-Critical Care Clinics; Elsevier: Amsterdam, The Netherlands, 2011; Available online: https://www.criticalcare.theclinics.com/article/S0749-0704(10)00068-0/fulltext (accessed on 16 September 2022).
- Kluge, R.M.; Standiford, H.C.; Tatem, B.; Young, V.M.; Greene, W.H.; Schimpff, S.C.; Calia, F.M.; Hornick, R.B. Comparative activity of tobramycin, amikacin, and gentamicin alone and with carbenicillin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1974, 6, 442–446. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawson, C.D.; Zelenitsky, S.; Findlay, B.; Schweizer, F.; Adam, H.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev. Anti Infect. Ther. 2012, 10, 459–473. [Google Scholar] [CrossRef]
- Anderson, D.J.; Miller, B.; Marfatia, R.; Drew, R. Ability of an antibiogram to predict Pseudomonas aeruginosa susceptibility to targeted antimicrobials based on hospital day of isolation. Infect. Control Hosp. Epidemiol. 2012, 33, 589–593. [Google Scholar] [CrossRef]
- Riou, M.; Carbonnelle, S.; Avrain, L.; Mesaros, N.; Pirnay, J.-P.; Bilocq, F.; De Vos, D.; Simon, A.; Piérard, D.; Jacobs, F.; et al. In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. Int. J. Antimicrob. Agents 2010, 36, 513–522. [Google Scholar] [CrossRef]
- De Matos, E.C.O.; De Matos, H.J.; da Conceição, M.L.; Rodrigues, Y.C.; Carneiro, I.C.D.R.S.; Lima, K.V.B. Clinical and microbiological features of infections caused by Pseudomonas aeruginosa in patients hospitalized in intensive care units. Rev. Soc. Bras. Med. Trop. 2016, 49, 305–311. [Google Scholar] [CrossRef]
- Peng, Y.; Bi, J.; Shi, J.; Li, Y.; Ye, X.; Chen, X.; Yao, Z. Multidrug-resistant Pseudomonas aeruginosa infections pose growing threat to health care–associated infection control in the hospitals of Southern China: A case-control surveillance study. Am. J. Infect. Control 2014, 42, 1308–1311. [Google Scholar] [CrossRef]
- Jeong, S.J.; Yoon, S.S.; Bae, I.K.; Jeong, S.H.; Kim, J.M.; Lee, K. Risk factors for mortality in patients with bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa: Clinical impact of bacterial virulence and strains on outcome. Diagn. Microbiol. Infect. Dis. 2014, 80, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fetroja® (Cefiderocol). Antimicrobial Activity. Available online: https://www.fetroja.com/antimicrobial-activity (accessed on 22 September 2022).
- Torrens, G.; Cabot, G.; Ocampo-Sosa, A.A.; Conejo, M.C.; Zamorano, L.; Navarro, F.; Pascual, Á.; Martínez-Martínez, L.; Oliver, A. Activity of Ceftazidime-Avibactam against Clinical and Isogenic Laboratory Pseudomonas aeruginosa Isolates Expressing Combinations of Most Relevant β-Lactam Resistance Mechanisms. Antimicrob. Agents Chemother. 2016, 60, 6407–6410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iregui, A.; Khan, Z.; Landman, D.; Quale, J. Activity of Cefiderocol Against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii Endemic to Medical Centers in New York City. Microb. Drug Resist. 2020, 26, 722–726. [Google Scholar] [CrossRef]
- Fraile-Ribot, P.A.; Zamorano, L.; Orellana, R.; del Barrio-Tofiño, E.; Sánchez-Diener, I.; Cortes-Lara, S.; López-Causapé, C.; Cabot, G.; Bou, G.; Martínez-Martínez, L.; et al. Activity of Imipenem-Relebactam against a Large Collection of Pseudomonas aeruginosa Clinical Isolates and Isogenic β-Lactam-Resistant Mutants. Antimicrob. Agents Chemother. 2020, 64, e02165-19. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. In Clinical Infectious Diseases; Oxford Academic: Oxford, UK, 2019; Available online: https://academic.oup.com/cid/article/69/Supplement_7/S565/5623998 (accessed on 22 September 2022).
- EMA. Zerbaxa. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zerbaxa (accessed on 28 September 2022).
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.-F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): A randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Solomkin, J.; Hershberger, E.; Miller, B.; Popejoy, M.; Friedland, I.; Steenbergen, J.; Yoon, M.; Collins, S.; Yuan, G.; Barie, P.S.; et al. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, L.M.; Umeh, O.; Steenbergen, J.; Yuan, G.; Darouiche, R.O. Ceftolozane-TAZOBACTAM Compared with Levofloxacin in the Treatment of Complicated Urinary-Tract Infections, Including Pyelonephritis: A Randomised, Double-Blind, Phase 3 Trial (ASPECT-cUTI). Lancet 2015, 385, 1949–1956. Available online: https://pubmed.ncbi.nlm.nih.gov/25931244/ (accessed on 28 September 2022).
- AVYCAZ. Safely and Effectively. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf (accessed on 28 September 2022).
- EMA. Zavicefta. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zavicefta (accessed on 29 September 2022).
- Lasarte-Monterrubio, C.; Fraile-Ribot, P.A.; Vázquez-Ucha, J.C.; Cabot, G.; Guijarro-Sánchez, P.; Alonso-García, I.; Rumbo-Feal, S.; Galán-Sánchez, F.; Beceiro, A.; Arca-Suárez, J.; et al. Activity of cefiderocol, imipenem/relebactam, cefepime/taniborbactam and cefepime/zidebactam against ceftolozane/tazobactam- and ceftazidime/avibactam-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2022, 77, 2809–2815. [Google Scholar] [CrossRef]
- Skoglund, E.; Abodakpi, H.; Rios, R.; Diaz, L.; De La Cadena, E.; Dinh, A.Q.; Ardila, J.; Miller, W.R.; Munita, J.M.; Arias, C.A.; et al. In Vivo Resistance to Ceftolozane/Tazobactam in Pseudomonas aeruginosa Arising by AmpC- and Non-AmpC-Mediated Pathways. Case Rep. Infect. Dis. 2018, 2018, 9095203. [Google Scholar] [CrossRef]
- Tamma, P.D.; Beisken, S.; Bergman, Y.; Posch, A.E.; Avdic, E.; Sharara, S.L.; Cosgrove, S.E.; Simner, P.J. Modifiable Risk Factors for the Emergence of Ceftolozane-tazobactam Resistance. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e4599–e4606. [Google Scholar] [CrossRef]
- Sid Ahmed, M.A.; Hamid, J.M.; Husain, A.A.; Hadi, H.A.; Skariah, S.; Sultan, A.A.; Ibrahim, E.B.; Al Khal, A.L.; Soderquist, B.; Jass, J.; et al. Clinical outcomes, molecular epidemiology and resistance mechanisms of multidrug-resistant Pseudomonas aeruginosa isolated from bloodstream infections from Qatar. Ann. Med. 2021, 53, 2345–2353. [Google Scholar] [CrossRef]
- Garazzino, S.; Altieri, E.; Silvestro, E.; Pruccoli, G.; Scolfaro, C.; Bignamini, E. Ceftolozane/Tazobactam for Treating Children With Exacerbations of Cystic Fibrosis Due to Pseudomonas aeruginosa: A Review of Available Data. Front. Pediatr. 2020, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Shortridge, D.; Harris, K.A.; Garrison, M.W.; DeRyke, C.A.; DePestel, D.D.; Moise, P.A.; Sader, H.S. Ceftolozane-tazobactam activity against clinical isolates of Pseudomonas aeruginosa from ICU patients with pneumonia: United States, 2015–2018. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 112, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Stone, G.G.; Newell, P.; Broadhurst, H.; Wardman, A.; MacPherson, M.; Yates, K.; Riccobene, T.; Critchley, I.A.; Das, S. Ceftazidime-Avibactam Susceptibility Breakpoints against Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e02590-17. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Q.-M.; Lim, J.C.; Tang, C.Y.; Lee, S.J.-Y.; Tan, S.H.; Sim, J.H.-C.; Ong, R.T.-H.; Kwa, A.L.-H. Ceftolozane/Tazobactam Resistance and Mechanisms in Carbapenem-Nonsusceptible Pseudomonas aeruginosa. mSphere 2021, 6, e01026-20. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs. Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 70, 1799–1808. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; Puzniak, L.A.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 304–310. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, e00472-17. Available online: https://pubmed.ncbi.nlm.nih.gov/28630192/ (accessed on 22 September 2022).
- Lee, M.; Abbey, T.; Biagi, M.; Wenzler, E. Activity of aztreonam in combination with ceftazidime–avibactam against serine- and metallo-β-lactamase–producing Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2021, 99, 115227. Available online: https://www.sciencedirect.com/science/article/pii/S0732889320306040 (accessed on 22 September 2022). [CrossRef]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019, 79, 271–289. Available online: https://link.springer.com/article/10.1007/s40265-019-1055-2 (accessed on 7 September 2022). [CrossRef]
- Kallel, H.; Bahloul, M.; Hergafi, L.; Akrout, M.; Ketata, W.; Chelly, H.; Hamida, C.B.; Rekik, N.; Hammami, A.; Bouaziz, M. Colistin as a salvage therapy for nosocomial infections caused by multidrug-resistant bacteria in the ICU. Int. J. Antimicrob. Agents 2006, 28, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Giske, C.G.; Ciamarellou, H. When and How to Cover for Resistant Gram-Negative Bacilli in Severe Sepsis and Septic Shock. Curr. Infect. Dis. Rep. 2011, 13, 416. Available online: https://link.springer.com/article/10.1007/s11908-011-0200-1 (accessed on 29 September 2022). [CrossRef] [PubMed]
- Pontikis, K.; Karaiskos, I.; Bastani, S.; Dimopoulos, G.; Kalogirou, M.; Katsiari, M.; Oikonomou, A.; Poulakou, G.; Roilides, E.; Giamarellou, H. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int. J. Antimicrob. Agents 2014, 43, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Sa-Borges, M.; Sole-Violan, J.; Barcenilla, F.; Escoresca-Ortega, A.; Ochoa, M.; Cayuela, A.; Rello, J. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: An observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit. Care Med. 2007, 35, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Crit. Care Lond. Engl. 2011, 15, R206. [Google Scholar] [CrossRef]
- Carlier, M.; Carrette, S.; Roberts, J.A.; Stove, V.; Verstraete, A.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; Lipman, J.; Wallis, S.C.; et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: Does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit. Care Lond. Engl. 2013, 17, R84. [Google Scholar] [CrossRef]
- De Waele, J.; Carlier, M.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Extended versus bolus infusion of meropenem and piperacillin: A pharmacokinetic analysis. Minerva Anestesiol. 2014, 80, 1302–1309. [Google Scholar]
- Taccone, F.S.; Cotton, F.; Roisin, S.; Vincent, J.; Jacobs, F. Optimal Meropenem Concentrations To Treat Multidrug-Resistant Pseudomonas aeruginosa Septic Shock. Antimicrob. Agents Chemother. 2012, 56, 2129–2131. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3318384/ (accessed on 16 September 2022). [CrossRef]
- Kuti, J.L.; Dandekar, P.K.; Nightingale, C.H.; Nicolau, D.P. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharmacol. 2003, 43, 1116–1123. [Google Scholar] [CrossRef]
- Kothekar, A.T.; Divatia, J.V.; Myatra, S.N.; Patil, A.; Nookala Krishnamurthy, M.; Maheshwarappa, H.M.; Siddiqui, S.S.; Gurjar, M.; Biswas, S.; Gota, V. Clinical pharmacokinetics of 3-h extended infusion of meropenem in adult patients with severe sepsis and septic shock: Implications for empirical therapy against Gram-negative bacteria. Ann. Intensive Care 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Nasomsong, W.; Nulsopapon, P.; Changpradub, D.; Pungcharoenkijkul, S.; Hanyanunt, P.; Chatreewattanakul, T.; Santimaleeworagun, W. Optimizing Doses of Ceftolozane/Tazobactam as Monotherapy or in Combination with Amikacin to Treat Carbapenem-Resistant Pseudomonas aeruginosa. Antibiotics 2022, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Giannella, M.; Raschi, E.; Viale, P.; De Ponti, F. Ceftolozane/tazobactam exposure in critically ill patients undergoing continuous renal replacement therapy: A PK/PD approach to tailor dosing. J. Antimicrob. Chemother. 2021, 76, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Ferrada, A.; Salavert, M.; Gordon, M.; Villarreal, E.; Castellanos-Ortega, Á.; Ramirez, P. Ceftolozane/Tazobactam Dosing Requirements Against Pseudomonas aeruginosa Bacteremia. Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325819885790. [Google Scholar] [CrossRef]
- Xiao, A.J.; Miller, B.W.; Huntington, J.A.; Nicolau, D.P. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J. Clin. Pharmacol. 2016, 56, 56–66. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Drusano, G.L. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 357–363. [Google Scholar] [CrossRef]
- Bauer, K.A.; West, J.E.; O’Brien, J.M.; Goff, D.A. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 2013, 57, 2907–2912. [Google Scholar] [CrossRef]
- Abrar, K.; Thabit, P.D.; Hobbs, A.L.V.; Guzman, O.E.; Shea, K.M. The pharmacodynamics of prolonged infusion blactams for the treatment of Pseudomonas aeruginosa infections: A systematic review. Clin. Ther. 2019, 41, 2397–2415.e8. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. Available online: https://pubmed.ncbi.nlm.nih.gov/26200166/ (accessed on 16 September 2022). [CrossRef]
- Shiu, J.; Wang, E.; Tejani, A.M.; Wasdell, M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst. Rev. 2013, 2013, CD008481. [Google Scholar] [CrossRef]
- Roberts, J.A.; Webb, S.; Paterson, D.; Ho, K.M.; Lipman, J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit. Care Med. 2009, 37, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Kasiakou, S.K.; Sermaides, G.J.; Michalopoulos, A.; Soteriades, E.S.; Falagas, M.E. Continuous versus intermittent intravenous administration of antibiotics: A meta-analysis of randomised controlled trials. Lancet Infect. Dis. 2005, 5, 581–589. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. Available online: https://europepmc.org/article/med/26754759 (accessed on 16 September 2022). [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. Available online: https://pubmed.ncbi.nlm.nih.gov/23074313/ (accessed on 16 September 2022). [CrossRef]
- Chytra, I.; Stepan, M.; Benes, J.; Pelnar, P.; Zidkova, A.; Bergerova, T.; Pradl, R.; Kasal, E. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit. Care 2012, 16, R113. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Li, Q.; Qi, S.; Zhou, F. Short-course versus long-course antibiotic treatment in patients with uncomplicated gram-negative bacteremia: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 173–180. [Google Scholar] [CrossRef]
- Haddad, S.F.; Allaw, F.; Kanj, S.S. Duration of antibiotic therapy in Gram-negative infections with a particular focus on multidrug-resistant pathogens. Curr. Opin. Infect. Dis. 2022, 10, 1097. [Google Scholar] [CrossRef]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Duration of Treatment for Pseudomonas aeruginosa Bacteremia: A Retrospective Study. Infect. Dis. Ther. 2022, 11, 1505–1519. [Google Scholar] [CrossRef]
- Fabre, V.; Amoah, J.; Cosgrove, S.E.; Tamma, D.P. Antibiotic Therapy for Pseudomonas aeruginosa Bloodstream Infections: How Long Is Long Enough? Clin. Infect. Dis. 2019, 69, 2011–2014. Available online: https://click.endnote.com/viewer?doi=10.1093%2Fcid%2Fciz223&token=WzM4MDQ0MjQsIjEwLjEwOTMvY2lkL2NpejIyMyJd.1inrEbGS-99J69wEqiNKGyN3dNc (accessed on 16 September 2022). [CrossRef]
- Olearo, F.; Kronig, I.; Masouridi-Levrat, S.; Chalandon, Y.; Khanna, N.; Passweg, J.; Medinger, M.; Mueller, N.J.; Schanz, U.; van Delden, C.; et al. Optimal Treatment Duration of Pseudomonas aeruginosa Infections in Allogeneic Hematopoietic Cell Transplant Recipients. Open Forum Infect. Dis. 2020, 7, ofaa246. Available online: https://click.endnote.com/viewer?doi=10.1093%2Fofid%2Fofaa246&token=WzM4MDQ0MjQsIjEwLjEwOTMvb2ZpZC9vZmFhMjQ2Il0.ajHGcE3LARNjbass_CrEl20bAtY (accessed on 16 September 2022). [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.; Grant, C.; Cooke, R.P.D.; Dempsey, G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst. Rev. 2015, 2015, CD007577. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.; Nepal, R.; Dhungana, G.; Parajuli, A.; Regmi, M.; Upadhyaya, E.; Mandal, D.; Shrestha, M.; Pradhan, P.; Manandhar, K.D.; et al. Isolation and Characterization of Lytic Bacteriophage Against Multi-drug Resistant Pseudomonas aeruginosa. J. Nepal Health Res. Counc. 2022, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Waters, E.M.; Neill, D.R.; Kaman, B.; Sahota, J.S.; Clokie, M.R.J.; Winstanley, C.; Kadioglu, A. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 2017, 72, 666–667. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.-J. Activity of Bacteriophages in Removing Biofilms of Pseudomonas aeruginosa Isolates from Chronic Rhinosinusitis Patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Cook, R.; Brown, N.; Redgwell, T.; Rihtman, B.; Barnes, M.; Clokie, M.; Stekel, D.J.; Hobman, J.; Jones, M.A.; Millard, A. INfrastructure for a PHAge REference Database: Identification of Large-Scale Biases in the Current Collection of Cultured Phage Genomes. PHAGE 2021, 2, 214–223. [Google Scholar] [CrossRef]
- Nordstrom, H.R.; Evans, D.R.; Finney, A.G.; Westbrook, K.J.; Zamora, P.F.; Hofstaedter, C.E.; Yassin, M.H.; Pradhan, A.; Iovleva, A.; Ernst, R.K.; et al. Genomic characterization of lytic bacteriophages targeting genetically diverse Pseudomonas aeruginosa clinical isolates. iScience 2022, 25, 104372. [Google Scholar] [CrossRef]
- Cafora, M.; Deflorian, G.; Forti, F.; Ferrari, L.; Binelli, G.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci. Rep. 2019, 9, 1527. [Google Scholar] [CrossRef]
- Adaptive Phage Therapeutics, Inc. Expanded Access Study of Phage Treatment in COVID-19 Patients on Anti-Microbials for Pneumonia or Bacteremia/Septicemia Due to A. Baumannii, P. Aeruginosa or S. Aureus; Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2021. [Google Scholar]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.-M.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—A case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.S.; de Vries, C.R.; Khosravi, A.; Sweere, J.M.; Popescu, M.C.; Chen, Q.; Demirdjian, S.; Hargil, A.; Van Belleghem, J.D.; Kaber, G.; et al. Filamentous bacteriophage delays healing of Pseudomonas-infected wounds. Cell Rep. Med. 2022, 3, 100656. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hawkins, C.H.; Anggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef]
- Holger, D.J.; Lev, K.L.; Kebriaei, R.; Morrisette, T.; Shah, R.; Alexander, J.; Lehman, S.M.; Rybak, M.J. Bacteriophage-antibiotic combination therapy for multidrug-resistant Pseudomonas aeruginosa: In vitro synergy testing. J. Appl. Microbiol. 2022, 133, 1636–1649. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Duplessis, C.; Biswas, B.; Hanisch, B.; Perkins, M.; Henry, M.; Quinones, J.; Wolfe, D.; Estrella, L.; Hamilton, T. Refractory Pseudomonas Bacteremia in a 2-Year-Old Sterilized by Bacteriophage Therapy. J. Pediatr. Infect. Dis. Soc. 2018, 7, 253–256. [Google Scholar] [CrossRef]
- Simner, P.J.; Cherian, J.; Suh, G.A.; Bergman, Y.; Beisken, S.; Fackler, J.; Lee, M.; Hopkins, R.J.; Tamma, P.D. Combination of phage therapy and cefiderocol to successfully treat Pseudomonas aeruginosa cranial osteomyelitis. JAC-Antimicrob. Resist. 2022, 4, dlac046. [Google Scholar] [CrossRef]
- Khawaldeh, A.; Morales, S.; Dillon, B.; Alavidze, Z.; Ginn, A.N.; Thomas, L.; Chapman, S.J.; Dublanchet, A.; Smithyman, A.; Iredell, J.R. Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 2011, 60, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Racenis, K.; Rezevska, D.; Madelane, M.; Lavrinovics, E.; Djebara, S.; Petersons, A.; Kroica, J. Use of Phage Cocktail BFC 1.10 in Combination With Ceftazidime-Avibactam in the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Femur Osteomyelitis-A Case Report. Front. Med. 2022, 9, 851310. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.-L.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am. J. Transplant. 2019, 19, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Z.; Tan, X.; Wang, H.; Liang, Y.; Kong, Y.; Sun, W.; Sun, L.; Ma, Y.; Lu, H. Bacteriophage therapy for empyema caused by carbapenem-resistant Pseudomonas aeruginosa. Biosci. Trends 2022, 16, 158–162. [Google Scholar] [CrossRef]
- Hoggarth, A.; Weaver, A.; Pu, Q.; Huang, T.; Schettler, J.; Chen, F.; Yuan, X.; Wu, M. Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa. Drug Des. Devel. Ther. 2019, 13, 909–924. [Google Scholar] [CrossRef]
- Hart, R.J.; Morici, L.A. Vaccination to Prevent Pseudomonas aeruginosa Bloodstream Infections. Front. Microbiol. 2022, 13, 870104. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Wurm, R.; Depuydt, P.; Spapen, H.; Lorente, J.A.; Staudinger, T.; Creteur, J.; Zauner, C.; Meier-Hellmann, A.; Eller, P.; et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients—A randomized clinical trial. Crit. Care 2020, 24, 74. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Sawa, T.; Shimizu, M.; Moriyama, K.; Wiener-Kronish, J.P. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: A review. Crit. Care Lond. Engl. 2014, 18, 668. [Google Scholar] [CrossRef]
- François, B.; Luyt, C.-E.; Dugard, A.; Wolff, M.; Diehl, J.-L.; Jaber, S.; Forel, J.-M.; Garot, D.; Kipnis, E.; Mebazaa, A.; et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: A randomized, double-blind, placebo-controlled trial. Crit. Care Med. 2012, 40, 2320–2326. [Google Scholar] [CrossRef]
- Song, Y.; Baer, M.; Srinivasan, R.; Lima, J.; Yarranton, G.; Bebbington, C.; Lynch, S.V. PcrV antibody-antibiotic combination improves survival in Pseudomonas aeruginosa-infected mice. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Lindorfer, M.A.; Nardin, A.; Foley, P.L.; Solga, M.D.; Bankovich, A.J.; Martin, E.N.; Henderson, A.L.; Price, C.W.; Gyimesi, E.; Wozencraft, C.P.; et al. Targeting of Pseudomonas aeruginosa in the Bloodstream with Bispecific Monoclonal Antibodies. J. Immunol. 2001, 167, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, R.; Nguyen, S.; Nowak, E.; Kipnis, E.; Pierre, M.; Ader, F.; Courcol, R.; Guery, B.P.; Faure, K. Pyopneumagen Group Quorum-sensing activity and related virulence factor expression in clinically pathogenic isolates of Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2008, 14, 337–343. [Google Scholar] [CrossRef]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Molin, S.; Givskov, M.; Høiby, N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 2004, 53, 1054–1061. [Google Scholar] [CrossRef]
- Hoffmann, N.; Lee, B.; Hentzer, M.; Rasmussen, T.B.; Song, Z.; Johansen, H.K.; Givskov, M.; Høiby, N. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) mice. Antimicrob. Agents Chemother. 2007, 51, 3677–3687. [Google Scholar] [CrossRef]
- Adonizio, A.; Kong, K.-F.; Mathee, K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef]
- Smyth, A.R.; Cifelli, P.M.; Ortori, C.A.; Righetti, K.; Lewis, S.; Erskine, P.; Holland, E.D.; Givskov, M.; Williams, P.; Cámara, M.; et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—A pilot randomized controlled trial. Pediatr. Pulmonol. 2010, 45, 356–362. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Al-Mathkhury, H.J.F.; Ali, A.S.; Ghafil, J.A. Antagonistic effect of bacteriocin against urinary catheter associated Pseudomonas aeruginosa biofilm. N. Am. J. Med. Sci. 2011, 3, 367–370. [Google Scholar] [CrossRef]
- Snopkova, K.; Dufkova, K.; Klimesova, P.; Vanerkova, M.; Ruzicka, F.; Hola, V. Prevalence of bacteriocins and their co-association with virulence factors within Pseudomonas aeruginosa catheter isolates. Int. J. Med. Microbiol. 2020, 310, 151454. [Google Scholar] [CrossRef] [PubMed]
| C/T | CAZ/AVI | IMI/REL | Cefiderocol | Pazomicin | Fosfomycin | Colistin | |
|---|---|---|---|---|---|---|---|
| Carbapenemase | |||||||
| Class A | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Class B | No | No | No | Yes | Variable | Yes | Yes |
| Class D | No | Yes | No | Yes | Yes | Yes | Yes |
| OprD | Yes | Yes | Yes | Yes | |||
| MexAB | Yes | No | Yes | Yes | |||
| MexXY | Yes | No | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakhour, J.; Sharara, S.L.; Hindy, J.-R.; Haddad, S.F.; Kanj, S.S. Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics 2022, 11, 1432. https://doi.org/10.3390/antibiotics11101432
Zakhour J, Sharara SL, Hindy J-R, Haddad SF, Kanj SS. Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics. 2022; 11(10):1432. https://doi.org/10.3390/antibiotics11101432
Chicago/Turabian StyleZakhour, Johnny, Sima L. Sharara, Joya-Rita Hindy, Sara F. Haddad, and Souha S. Kanj. 2022. "Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis" Antibiotics 11, no. 10: 1432. https://doi.org/10.3390/antibiotics11101432
APA StyleZakhour, J., Sharara, S. L., Hindy, J.-R., Haddad, S. F., & Kanj, S. S. (2022). Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics, 11(10), 1432. https://doi.org/10.3390/antibiotics11101432






