ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone Harboring blaNDM Replaced a blaKPC Clone in a Tertiary Hospital in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Bacterial Isolates
2.2. Carbapenemase-Producing Phenotypic Tests
2.3. Antimicrobial Susceptibility Testing
2.4. Multi-Locus Sequence Typing (MLST)
2.5. WGS and Phylogenetic Analysis
2.6. Growth Assay and In Vitro Competition Experiment
2.7. Serum-Killing Assay
2.8. Biofilm Formation Assay
2.9. Extraction and Quantification of Capsules
2.10. Statistical Analysis
2.11. Ethics Statement
3. Results
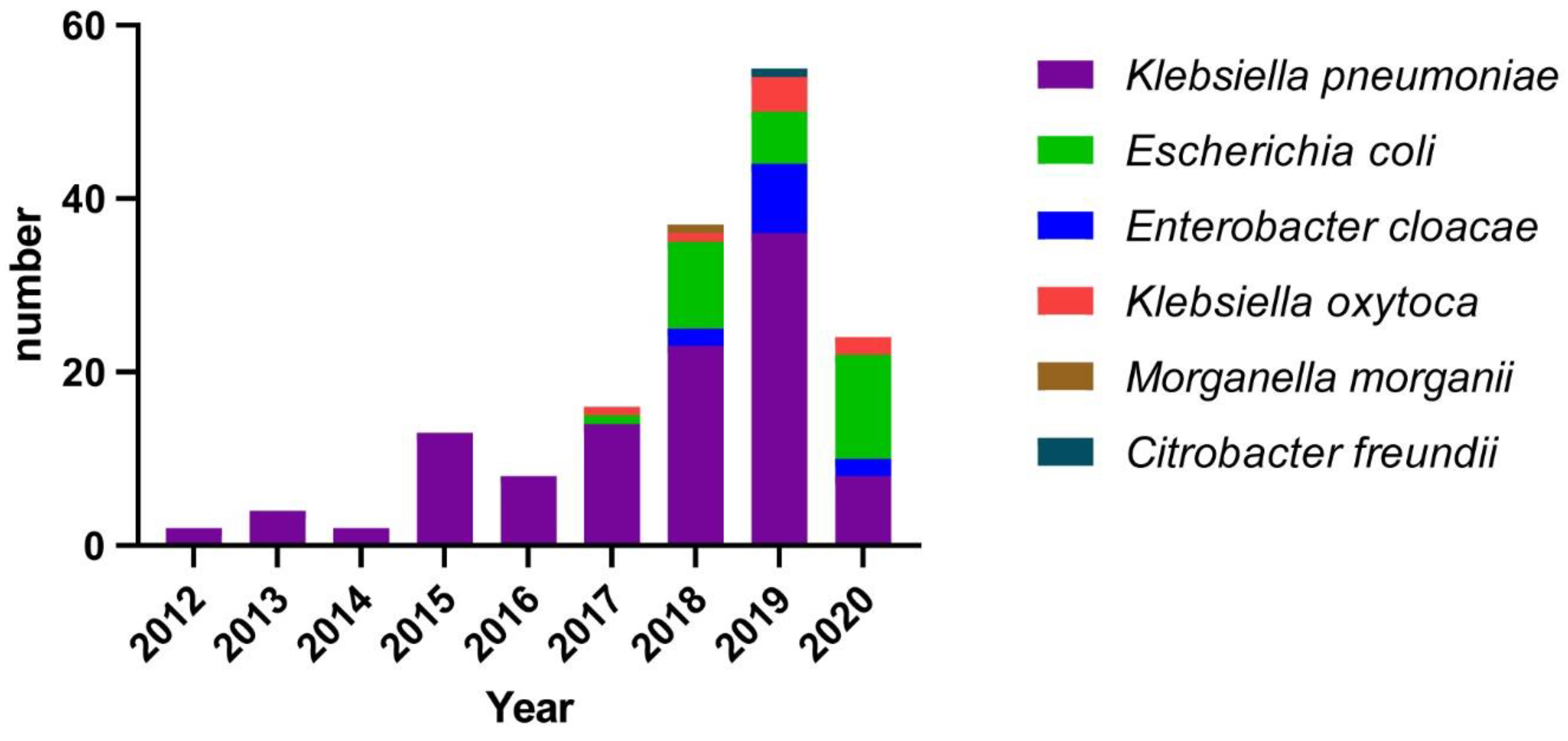
3.1. Distribution of Isolates from 2012 to 2020
3.2. Antimicrobial Susceptibility Testing
3.3. Distribution of STs and Carbapenemases
3.4. Phylogenetic Analysis of ST11 K. pneumoniae Isolates
3.5. Growth Assay and In Vitro Competition Experiment
3.6. Serum Killing Assay
3.7. Biofilm Formation Assay
3.8. Quantification of Capsule Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- De Oliveira, D.; Forde, B.; Kidd, T.; Harris, P.; Schembri, M.; Beatson, S.; Paterson, D.; Walker, M. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Luterbach, C.L.; Chen, L.; Komarow, L.; Ostrowsky, B.; Kaye, K.S.; Hanson, B.; Arias, C.A.; Desai, S.; Gallagher, J.C.; Novick, E.; et al. Transmission of Carbapenem-resistant Klebsiella pneumoniae in US hospitals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Wang, J.; Ouyang, P.; Jin, C.; Wang, R.; Zhang, Y.; Jin, L.; Chen, H.; Wang, Z.; et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae: Data From a Longitudinal Large-scale CRE Study in China (2012–2016). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, S196–S205. [Google Scholar] [CrossRef]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Philippon, A.; Slama, P.; Deny, P.; Labia, R. A Structure-Based Classification of Class A beta-Lactamases, a Broadly Diverse Family of Enzymes. Clin. Microbiol. Rev. 2016, 29, 29–57. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Li, X.Z.; Plesiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ferrer, S.; Penaloza, H.F.; Budnick, J.A.; Bain, W.G.; Nordstrom, H.R.; Lee, J.S.; Van Tyne, D. Finding Order in the Chaos: Outstanding Questions in Klebsiella pneumoniae Pathogenesis. Infect. Immun. 2021, 89, e00693-20. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.M.; Bergh, H.; Cuddihy, T.; Hajkowicz, K.; Hurst, T.; Playford, E.G.; Henderson, B.C.; Runnegar, N.; Clark, J.; Jennison, A.V.; et al. Clinical implementation of routine whole-genome sequencing for hospital infection control of multi-drug resistant pathogens. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022. [Google Scholar] [CrossRef]
- Stanton, R.A.; Campbell, D.; McAllister, G.A.; Breaker, E.; Adamczyk, M.; Daniels, J.B.; Lutgring, J.D.; Karlsson, M.; Schutz, K.; Jacob, J.T.; et al. Whole-Genome Sequencing Reveals Diversity of Carbapenem-Resistant Pseudomonas aeruginosa Collected through CDC’s Emerging Infections Program, United States, 2016–2018. Antimicrob. Agents Chemother. 2022, 66, e0049622. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Heinz, E.; Holt, K.E.; Wyres, K.L. Kaptive Web: User-Friendly Capsule and Lipopolysaccharide Serotype Prediction for Klebsiella Genomes. J. Clin. Microbiol. 2018, 56, e00197-18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, K.; Gao, H.; Wang, Q.; Wang, X.; Li, H.; Wang, R.; Wang, H. Decreased Fitness and Virulence in ST10 Escherichia coli Harboring blaNDM-5 and mcr-1 against a ST4981 Strain with blaNDM-5. Front. Cell. Infect. Microbiol. 2017, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.S.; Hu, P.; Cai, L.; Fu, B.Q.; Li, Y.S.; Lu, S.Y.; Liu, N.N.; Ma, X.L.; Chi, D.; et al. Characterization of surface antigen protein 1 (SurA1) from Acinetobacter baumannii and its role in virulence and fitness. Vet. Microbiol. 2016, 186, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Koh, T.H.; Gardner, M.; Siu, L.K. Clinical and bacteriological characteristics of Klebsiella pneumoniae causing liver abscess with less frequently observed multi-locus sequences type, ST163, from Singapore and Missouri, US. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2012, 45, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, J.; Liu, W.; Zhao, F.; Hu, Z.; Zhao, C.; Wang, Q.; Wang, X.; Chen, H.; Li, H.; et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J. Infect. 2015, 71, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, M.S.; Hsu, J.; Rick, P.D.; Miller, V.L. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 2005, 58, 1054–1073. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fu, Y.; Fang, Y.; Xu, H.; Kong, H.; Liu, Y.; Chen, Y.; Li, L. High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect. Drug Resist. 2019, 12, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Fu, S.; Li, M.; Guo, Z.; Zhu, X.; Ren, J.; Hu, F. Microbiological Characteristics of Carbapenem-Resistant Enterobacteriaceae Clinical Isolates Collected from County Hospitals. Infect. Drug Resist. 2020, 13, 1163–1169. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Zhao, Y.; Qiu, Y.; Xiao, L.; He, H.; Zheng, D.; Li, X.; Huang, L.; Yu, X.; et al. Characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary hospital in Fuzhou, China. J. Appl. Microbiol. 2020, 129, 1220–1226. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, F.; Chen, W.; Zhao, Y.; Bai, G.; Zhang, Z. Molecular Epidemiology and Risk Factors of Ventilator-Associated Pneumonia Infection Caused by Carbapenem-Resistant Enterobacteriaceae. Front. Pharm. 2019, 10, 262. [Google Scholar] [CrossRef]
- Salomao, M.C.; Freire, M.P.; Boszczowski, I.; Raymundo, S.F.; Guedes, A.R.; Levin, A.S. Increased Risk for Carbapenem-Resistant Enterobacteriaceae Colonization in Intensive Care Units after Hospitalization in Emergency Department. Emerg. Infect. Dis. 2020, 26, 1156–1163. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, K.; Chen, W.; Chen, J.; Zheng, J.; Liu, C.; Cheng, L.; Zhou, W.; Shen, H.; Cao, X. Epidemiological characteristics of carbapenem-resistant Enterobacteriaceae collected from 17 hospitals in Nanjing district of China. Antimicrob. Resist. Infect. Control 2020, 9, 15. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, Y.; Yu, J.; Li, S.; Zhang, Y.; Wang, H.; Lai, X.; Liu, D.; Mao, L.; Luo, Y.; et al. Bacterial characteristics of carbapenem-resistant Enterobacteriaceae (CRE) colonized strains and their correlation with subsequent infection. BMC Infect. Dis. 2021, 21, 638. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Wang, R.; Wang, Q.; Jin, L.; Wang, H. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 2020, 51, 102599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Xiao, T.; David, S.; Wang, Q.; Zhou, Y.; Guo, L.; Aanensen, D.; Holt, K.E.; Thomson, N.R.; Grundmann, H.; et al. Novel Subclone of Carbapenem-Resistant Klebsiella pneumoniae Sequence Type 11 with Enhanced Virulence and Transmissibility, China. Emerg. Infect. Dis. 2020, 26, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Marimuthu, K.; Teo, J.; Venkatachalam, I.; Cherng, B.P.Z.; De Wang, L.; Prakki, S.R.S.; Xu, W.; Tan, Y.H.; Nguyen, L.C.; et al. Acquisition of Plasmid with Carbapenem-Resistance Gene blaKPC2 in Hypervirulent Klebsiella pneumoniae, Singapore. Emerg. Infect. Dis. 2020, 26, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.; Moradi, M.; Kalantar-Neyestanaki, D.; Ali Golabi, D.; Hosseini-Nave, H. Virulence Factors, Capsular Serotypes and Antimicrobial Resistance of Hypervirulent Klebsiella pneumoniae and Classical Klebsiella pneumoniae in Southeast Iran. Infect. Chemother. 2019, 51, e39. [Google Scholar] [CrossRef]
- Cai, Z.; Jia, T.; Pu, M.; Zhang, S.; Zhang, J.; Geng, R.; Chen, S.; Li, Y.; Fan, H.; Tong, Y.; et al. Clinical and Molecular Analysis of ST11-K47 Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae: A Strain Causing Liver Abscess. Pathogens 2022, 11, 657. [Google Scholar] [CrossRef]
- Harada, S.; Doi, Y. Hypervirulent Klebsiella pneumoniae: A Call for Consensus Definition and International Collaboration. J. Clin. Microbiol. 2018, 56, e00959-18. [Google Scholar] [CrossRef]
- Fuursted, K.; Scholer, L.; Hansen, F.; Dam, K.; Bojer, M.S.; Hammerum, A.M.; Dagnaes-Hansen, F.; Olsen, A.; Jasemian, Y.; Struve, C. Virulence of a Klebsiella pneumoniae strain carrying the New Delhi metallo-beta-lactamase-1 (NDM-1). Microbes Infect. 2012, 14, 155–158. [Google Scholar] [CrossRef]
- Montanari, S.; Oliver, A.; Salerno, P.; Mena, A.; Bertoni, G.; Tummler, B.; Cariani, L.; Conese, M.; Doring, G.; Bragonzi, A. Biological cost of hypermutation in Pseudomonas aeruginosa strains from patients with cystic fibrosis. Microbiology 2007, 153, 1445–1454. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, Y.; Li, X.; Liu, Y.; Ye, Y.; Guan, S.; Li, J. Identification and Characterization of NDM-1-producing Hypervirulent (Hypermucoviscous) Klebsiella pneumoniae in China. Ann. Lab Med. 2019, 39, 167–175. [Google Scholar] [CrossRef]
- Wei, D.D.; Wan, L.G.; Liu, Y. Draft Genome Sequence of an NDM-1- and KPC-2-Coproducing Hypervirulent Carbapenem-Resistant Klebsiella pneumoniae Strain Isolated from Burn Wound Infections. Genome Announc. 2018, 6, e00192-18. [Google Scholar] [CrossRef]





| Antibiotic Name | All Isolates (n = 160) | Klebsiella pneumoniae (n = 109) | Escherichia coli (n = 29) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %R | %S | MIC50 | MIC90 | %R | %S | MIC50 | MIC90 | %R | %S | MIC50 | MIC90 | |
| Amikacin | 35 | 65 | 4 | >256 | 42.2 | 57.8 | 4 | >256 | 24.1 | 75.9 | 4 | 256 |
| Aztreonam | 82.5 | 14.4 | 128 | >256 | 89.9 | 6.4 | 256 | >256 | 55.2 | 41.4 | 16 | >256 |
| Cefepime | 94.4 | 1.2 | 128 | >256 | 95.4 | 1.8 | 128 | 256 | 96.6 | 0 | 256 | >256 |
| Cefoperazone-sulbactam | 98.1 | 0.6 | >256 | >256 | 97.2 | 0.9 | >256 | >256 | 100 | 0 | >256 | >256 |
| Ceftazidime | 97.5 | 1.9 | >256 | >256 | 96.3 | 2.8 | 256 | >256 | 100 | 0 | >256 | >256 |
| Ciprofloxacin | 86.9 | 10.6 | 64 | 128 | 93.6 | 5.5 | 64 | 128 | 89.7 | 6.9 | 64 | 128 |
| Colistin | 1.2 | 98.8 | 0.25 | 0.5 | 0 | 100 | 0.25 | 0.5 | 3.4 | 96.6 | 0.25 | 0.25 |
| Ertapenem | 96.9 | 1.9 | 32 | 256 | 97.2 | 1.8 | 64 | 256 | 93.1 | 3.4 | 32 | 64 |
| Fosfomycin | 41.5 | 52.2 | 64 | >256 | 55.6 | 36.1 | 256 | >256 | 13.8 | 86.2 | 4 | >256 |
| Imipenem | 87.5 | 8.1 | 16 | 64 | 89.9 | 6.4 | 16 | 64 | 79.3 | 10.3 | 4 | 16 |
| Levofloxacin | 85 | 14.4 | 32 | 128 | 90.8 | 8.3 | 32 | 128 | 89.7 | 10.3 | 32 | 64 |
| Meropenem | 85 | 9.4 | 16 | 128 | 90.8 | 5.5 | 32 | 128 | 72.4 | 20.7 | 4 | 16 |
| Minocycline | 41.9 | 49.4 | 8 | 32 | 38.5 | 53.2 | 4 | 32 | 48.3 | 41.4 | 8 | 128 |
| Piperacillin-tazobactam | 95 | 2.5 | >256 | >256 | 96.3 | 2.8 | >256 | >256 | 93.1 | 3.4 | 256 | >256 |
| Tigecycline | 1.9 | 90 | 0.5 | 2 | 0.9 | 88.1 | 0.5 | 4 | 0 | 100 | 0.25 | 0.5 |
| Organism | ST | No. | IMP-4 n (%) | KPC-2 n (%) | NDM-1 n (%) | NDM-4 n (%) | NDM-5 n (%) |
|---|---|---|---|---|---|---|---|
| Klebsiella pneumoniae (n = 102) | ST11 | 71 | 55 (77.5) | 16 (22.5) | |||
| ST15 | 14 | 7 (50) | 5 (35.7) | 2 (14.3) | |||
| ST25 | 1 | 1 (100) | |||||
| ST48 | 6 | 6 (100) | |||||
| ST86 | 1 | 1 (100) | |||||
| ST143 | 1 | 1 (100) | |||||
| ST357 | 1 | 1 (100) | |||||
| ST412 | 1 | 1 (100) | |||||
| ST685 | 1 | 1 (100) | |||||
| ST784 | 1 | 1 (100) | |||||
| ST896 | 1 | 1 (100) | |||||
| Other STs | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| Escherichia coli (n = 25) | ST410 | 7 | 7 (100) | ||||
| ST167 | 4 | 4 (100) | |||||
| ST617 | 3 | 3 (100) | |||||
| ST10 | 2 | 2 (100) | |||||
| ST354 | 2 | 2 (100) | |||||
| ST744 | 2 | 2 (100) | |||||
| ST46 | 1 | 1 (100) | |||||
| ST48 | 1 | 1 (100) | |||||
| ST69 | 1 | 1 (100) | |||||
| ST648 | 1 | 1 (100) | |||||
| ST10565 | 1 | 1 (100) | |||||
| Enterobacter cloacae (n = 12) | ST175 | 5 | 5 (100) | ||||
| ST97 | 2 | 2 (100) | |||||
| ST171 | 2 | 2 (100) | |||||
| ST116 | 1 | 1 (100) | |||||
| ST177 | 1 | 1 (100) | |||||
| ST231 | 1 | 1 (100) |
| Isolate | Hour (h) | Grade | Interpretive | ||
|---|---|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | |||
| C7676-blaKPC | 119.40 | 229.70 | 175.67 | 6 | R |
| C5343-blaKPC | 100.00 | 59.03 | 27.26 | 3 | I |
| C7673-blaKPC | 103.94 | 118.29 | 106.10 | 5 | R |
| C7674-blaNDM | 54.21 | 0.86 | 0.86 | 2 | S |
| C7664-blaNDM | 99.08 | 0.33 | 0.00 | 2 | S |
| C7662-blaNDM | 99.56 | 0.00 | 0.00 | 2 | S |
| K2044 | 108.80 | 107.23 | 101.16 | 5 | R |
| ATCC®13883 | 1.95 | 0.00 | 0.00 | 1 | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Q.; Wang, Q.; Sun, S.; Cui, Q.; Ding, Q.; Wang, R.; Wang, H. ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone Harboring blaNDM Replaced a blaKPC Clone in a Tertiary Hospital in China. Antibiotics 2022, 11, 1373. https://doi.org/10.3390/antibiotics11101373
Duan Q, Wang Q, Sun S, Cui Q, Ding Q, Wang R, Wang H. ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone Harboring blaNDM Replaced a blaKPC Clone in a Tertiary Hospital in China. Antibiotics. 2022; 11(10):1373. https://doi.org/10.3390/antibiotics11101373
Chicago/Turabian StyleDuan, Qiaoyan, Qi Wang, Shijun Sun, Qiaozhen Cui, Qi Ding, Ruobing Wang, and Hui Wang. 2022. "ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone Harboring blaNDM Replaced a blaKPC Clone in a Tertiary Hospital in China" Antibiotics 11, no. 10: 1373. https://doi.org/10.3390/antibiotics11101373
APA StyleDuan, Q., Wang, Q., Sun, S., Cui, Q., Ding, Q., Wang, R., & Wang, H. (2022). ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone Harboring blaNDM Replaced a blaKPC Clone in a Tertiary Hospital in China. Antibiotics, 11(10), 1373. https://doi.org/10.3390/antibiotics11101373







