“Growth-Promoting Effect” of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey
Abstract
1. Introduction
2. Objectives/Hypothesis
3. Materials and Methods
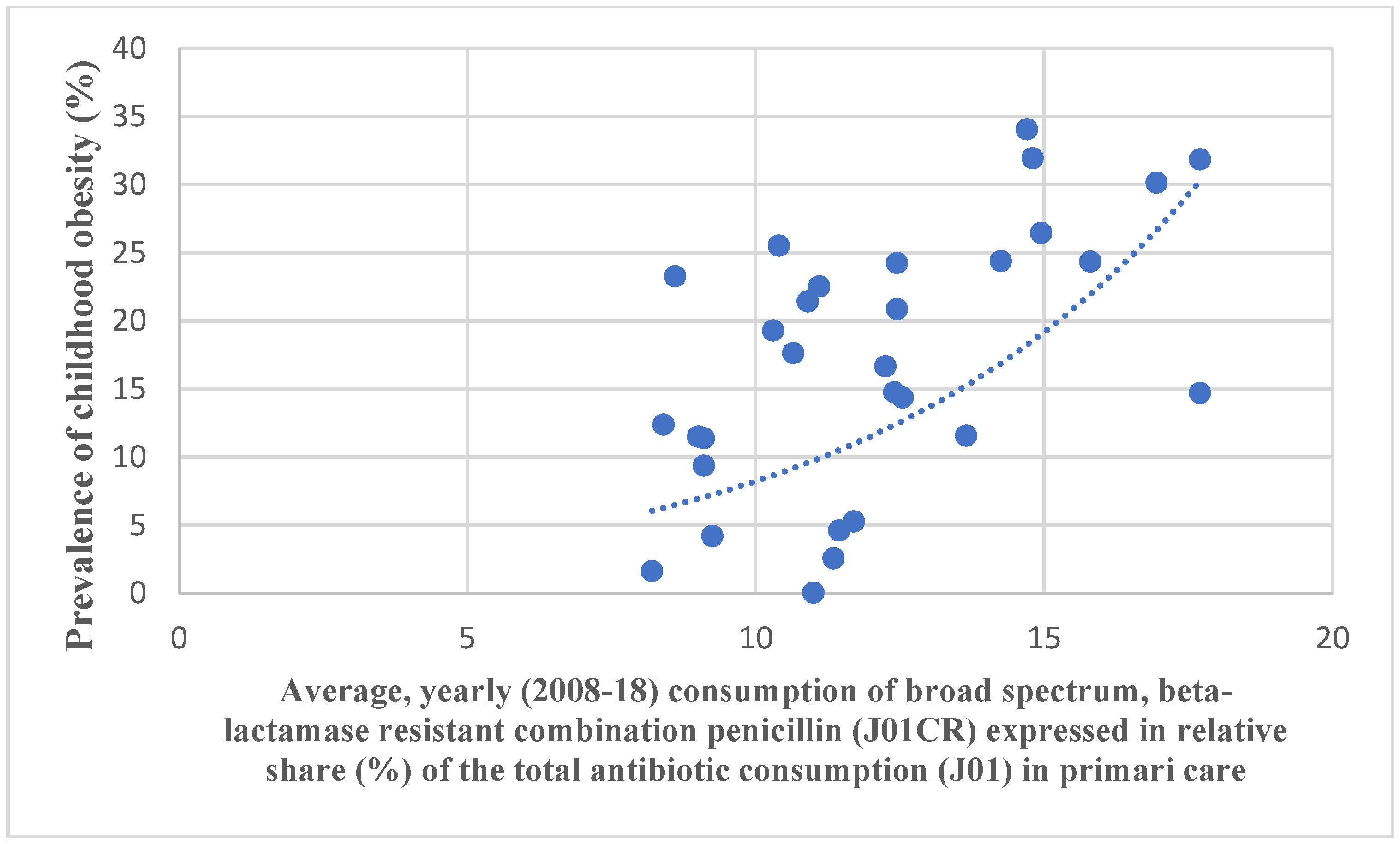
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, W.P.T. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Obesity in Perspective, Fogarty International Center Series on Preventive Medicine; DHEW Publication 75-708; Government Printing Office: Washington, DC, USA, 1973. [Google Scholar]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Frank, J. Origins of the obesity pandemic can be analyzed. Nature 2016, 532, 149. [Google Scholar] [CrossRef]
- Moore, P.R.; Evenson, A. Use of sulfapyridine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Riley, L.W.; Raphael, E.; Faerstein, E. Obesity in the United States—Dysbiosis from exposure to low-dose antibiotics? Front. Public Health 2013, 19, 69. [Google Scholar] [CrossRef]
- Ternak, G. Antibiotics may act as growth/obesity promoters in humans as an inadvertent result of antibiotic pollution? Med. Hypotheses 2005, 64, 14–16. [Google Scholar] [CrossRef]
- Lipstein, E.A.; Block, J.P.; Dodds, C.; Forrest, C.B.; Heerman, W.J.; Law, J.K.; Lunsford, D.; Winkler, P.; Finkelstein, J.A. Early Antibiotics and Childhood Obesity: Do Future Risks Matter to Parents and Physicians? Clin. Pediatr. 2019, 58, 191–198. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Derraik, J.G.B.; Hofman, P.L.; Cutfield, W.S. Antibiotics, gut microbiome, and obesity. Clin. Endocrinol. 2018, 88, 185–200. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Dalamaga, M.; Stratigou, T.; Karampela, I.; Tsigalou, C. Do Antibiotics Cause Obesity Through Long-term Alterations in the Gut Microbiome? A Review of Current Evidence. Curr. Obes. Rep. 2021, 10, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Dror, T.; Dickstein, Y.; Dubourg, G.; Paul, M. Microbiota manipulation for weight change. Microb. Pathog. 2017, 106, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut microbiota and obesity. Cell Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Bio. 2008, 6, e280. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4554–4561. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Bailey, L.C.; Forrest, C.B.; Zhang, P.; Richards, T.M.; Livshits, A.; DeRusso, P.A. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014, 168, 1063–1069. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; D’Alessio, D.A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008, 93, S37–S50. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.; Hanna, L.; Ellacott, K.L.J. CNS Targets of Adipokines. Compr. Physiol. 2017, 7, 1359–1406. [Google Scholar] [CrossRef]
- Benarroch, E.E. Neural control of feeding behavior: Overview and clinical correlations. Neurology 2010, 74, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Pocai, A.; Lam, T.K.T.; Gutierrez-Juarez, R.; Obici, S.; Schwartz, G.J.; Bryan, J.; Aguilar-Bryan, L.; Rossetti, L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005, 434, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, E.; Boem, F.; Russo, E.; Amedei, A. The Gut-Brain Axis in the Neuropsychological Disease Model of Obesity: A Classical Movie Revised by the Emerging Director “Microbiome”. Nutrients 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Gerber, J.S.; Bryan, M.; Ross, R.K.; Daymont, C.; Parks, E.P.; Localio, A.R.; Grundmeier, R.W.; Stallings, V.A.; Zaoutis, T.E. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA 2016, 315, 1258–1265. [Google Scholar] [CrossRef]
- Ternák, G.; Edel, Z.S.; Feiszt, Z.S.; Szekeres, J.; Visegrady, B.; Wittmann, I. Selective Association between Cephalosporin, Quinolone, Macrolide, and Penicillin Antibiotic Consumption and Childhood Obesity in Europe. Health 2015, 7, 1306–1314. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diabetes Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.T.; Reimer, R.A. Obesity, Early Life Gut Microbiota, and Antibiotics. Microorganisms 2021, 9, 413. [Google Scholar] [CrossRef] [PubMed]

| Countries | Childhood Obesity 5–9 Years in % (2019) | Average Yearly Antibiotic Consumption ECDC 2008–2018 | Defined Daily Dose/1000 Inhabitants/Day (100%) J01 | J01A % | J01C % | J01CA % | J01CR % | J01CE % | J01CF % | J01D % | J01F % | J01M % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 10.4 | Austria | 12.45 | 8.19 | 38.61 | 6.48 | 25.52 | 6.76 | 0.07 | 12.95 | 25.8 | 0.52 |
| Belgium | 8.6 | Belgium | 22.52 | 9.17 | 46.1 | 21.28 | 23.27 | 0.21 | 1.13 | 6.64 | 14.5 | 10.69 |
| Bulgaria | 13.65 | Bulgaria | 17.54 | 9.98 | 31.83 | 19.28 | 11.57 | 1.36 | 0 | 17.9 | 20.2 | 14.22 |
| Croatia | 14.95 | Croatia | 17.86 | 6.96 | 42.57 | 11.74 | 26.46 | 4.32 | 0.05 | 17.24 | 16.7 | 8.13 |
| Cyprus | 15.8 | Cyprus | 26.77 | 12.12 | 34.94 | 10.39 | 24.35 | 0.32 | 0.09 | 21.11 | 11.5 | 16.77 |
| Czechia | 12.25 | Czechia | 16.51 | 13.38 | 35.88 | 7.16 | 16.67 | 11.61 | 0.23 | 10.17 | 22 | 6.35 |
| Denmark | 9.25 | Denmark | 15.24 | 10.75 | 63.09 | 20.54 | 4.21 | 29.46 | 8.73 | 0.21 | 13.2 | 3.26 |
| Estonia | 8.4 | Estonia | 10.23 | 16.58 | 31.73 | 17.36 | 12.38 | 2.05 | 0.01 | 10.26 | 22.8 | 8.2 |
| Finland | 11.7 | Finland | 16.16 | 24.72 | 29.75 | 16.24 | 5.27 | 7.87 | 0.22 | 13.54 | 7.55 | 4.93 |
| France | 10.3 | France | 23.62 | 13.69 | 50.54 | 29.3 | 19.3 | 0.67 | 1.17 | 9.27 | 14.7 | 7.32 |
| Germany | 11.35 | Germany | 13.23 | 17.73 | 25.15 | 16.72 | 2.56 | 5.87 | 0.08 | 20.56 | 18 | 10.03 |
| Greece | 17.7 | Greece | 32.22 | 7.42 | 29.26 | 13.66 | 14.7 | 0.87 | 0 | 24.53 | 26.1 | 8.12 |
| Hungary | 14.25 | Hungary | 13.65 | 8.95 | 33.95 | 7.03 | 24.39 | 2.61 | 0 | 14.27 | 21.7 | 15.83 |
| Iceland | 12.55 | Iceland | 18.76 | 20.3 | 47.87 | 16.45 | 14.36 | 11.66 | 5.56 | 2.88 | 8.42 | 4.18 |
| Ireland | 12.45 | Ireland | 19.51 | 14.2 | 47.34 | 14.22 | 20.87 | 6.24 | 6.73 | 6.28 | 21 | 4.52 |
| Italy | 17.7 | Italy | 22.23 | 2.41 | 45.4 | 12.9 | 31.85 | 0 | 0.04 | 10.68 | 20.8 | 14.67 |
| Latvia | 9 | Latvia | 10.75 | 21.22 | 38.21 | 26.26 | 11.51 | 0.64 | 0.01 | 5.02 | 14.5 | 9.27 |
| Lithuania | 9.1 | Lithuania | 14.97 | 10.75 | 45.78 | 33.26 | 9.37 | 3.27 | 0.04 | 9.67 | 13.3 | 7.05 |
| Luxembourg | 11.1 | Luxembourg | 23.35 | 7.89 | 37.14 | 12.95 | 22.53 | 0.18 | 0.89 | 14.9 | 17.5 | 11.41 |
| Malta | 16.95 | Malta | 19.18 | 6.56 | 33.08 | 2.94 | 30.15 | 0.38 | 0.24 | 23.48 | 20.5 | 23.14 |
| Netherlands | 9.1 | Netherlands | 9.62 | 24.48 | 32.42 | 13.47 | 11.38 | 2.82 | 4.36 | 0.39 | 14.9 | 8.34 |
| Norway | 11 | Norway | 15.28 | 19.33 | 40.04 | 13.73 | 0.04 | 22.09 | 4 | 0.64 | 9.98 | 3.08 |
| Poland | 12.4 | Poland | 20.39 | 11.52 | 33.08 | 17.11 | 14.76 | 1.07 | 0.03 | 13.85 | 20.4 | 6.4 |
| Portugal | 14.7 | Portugal | 17.79 | 4.73 | 46.7 | 9.35 | 34.06 | 0.1 | 3.24 | 9.35 | 17.3 | 12.45 |
| Romania | 10.9 | Romania | 26.14 | 3.79 | 43.56 | 16.74 | 21.42 | 2.86 | 2.41 | 17.57 | 10.6 | 12.1 |
| Slovakia | 10.65 | Slovakia | 20.33 | 7.77 | 30.5 | 5.7 | 17.64 | 6.67 | 0 | 21.82 | 26.8 | 9.76 |
| Slovenia | 12.45 | Slovenia | 11.68 | 3 | 59.92 | 19.39 | 24.26 | 15.04 | 1.26 | 2.99 | 16.5 | 9.46 |
| Spain | 14.8 | Spain | 18.72 | 4.85 | 54.78 | 21.07 | 31.94 | 0.45 | 1.09 | 9.47 | 12.2 | 13.49 |
| Sweden | 8.2 | Sweden | 12.74 | 22.5 | 49.89 | 8.54 | 1.63 | 27.86 | 11.67 | 1.27 | 4.71 | 5.61 |
| UK | 11.45 | UK | 16.95 | 26.82 | 38.41 | 20.72 | 4.61 | 4.76 | 8.16 | 2.28 | 17 | 2.73 |
| Pearson’s, r | 0.517 | −0.497 | 0.141 | −0.369 | 0.573 | −0.375 | −0.339 | 0.536 | 0.3 | 0.554 | ||
| Pearson’s, p | 0.003 | 0.005 | 0.488 | 0.03 | 0.001 | 0.042 | 0.067 | 0.002 | 0.109 | 0.001 | ||
| ANOVA, p | 0.084 | 0.840 | 0.680 | 0.186 | 0.009 | 0.188 | 0.459 | 0.069 | 0.841 | 0.003 | ||
| OR | 1.128 | 0.846 | 1.013 | 0.898 | 1.175 | 0.932 | 0.853 | 1.084 | 1.106 | 1.252 | ||
| CI 95% | 0.965–1.319 | 0.733–0.977 | 0.937–1.096 | 0.792–1.019 | 1.044–1.323 | 0.833–1.043 | 0.652–1.118 | 0.973–1.208 | 0.959–1.275 | 1.017–1.540 | ||
| OR, p | 0.131 | 0.023 | 0.738 | 0.096 | 0.007 | 0.221 | 0.249 | 0.142 | 0.166 | 0.034 |
| Countries | Adult BMI ≥ 30, 2022 | Average Yearly Antibiotic Consumption ECDC 2008–2018 | Defined Daily Dose/1000 Inhabitants/Day (100%) J01 | J01A % | J01C % | J01CA % | J01CR % | J01CE % | J01CF % | J01D % | J01F % | J01M % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 20.1 | Austria | 12.45 | 8.19 | 38.61 | 6.48 | 25.52 | 6.76 | 0.07 | 12.95 | 25.8 | 0.52 |
| Belgium | 22.1 | Belgium | 22.52 | 9.17 | 46.1 | 21.28 | 23.27 | 0.21 | 1.13 | 6.64 | 14.5 | 10.69 |
| Bulgaria | 25 | Bulgaria | 17.54 | 9.98 | 31.83 | 19.28 | 11.57 | 1.36 | 0 | 17.9 | 20.2 | 14.22 |
| Croatia | 24.4 | Croatia | 17.86 | 6.96 | 42.57 | 11.74 | 26.46 | 4.32 | 0.05 | 17.24 | 16.7 | 8.13 |
| Cyprus | 21.8 | Cyprus | 26.77 | 12.12 | 34.94 | 10.39 | 24.35 | 0.32 | 0.09 | 21.11 | 11.5 | 16.77 |
| Czechia | 26 | Czech Rep. | 16.51 | 13.38 | 35.88 | 7.16 | 16.67 | 11.61 | 0.23 | 10.17 | 22 | 6.35 |
| Denmark | 19.7 | Denmark | 15.24 | 10.75 | 63.09 | 20.54 | 4.21 | 29.46 | 8.73 | 0.21 | 13.2 | 3.26 |
| Estonia | 21.2 | Estonia | 10.23 | 16.58 | 31.73 | 17.36 | 12.38 | 2.05 | 0.01 | 10.26 | 22.8 | 8.2 |
| Finland | 22.2 | Finland | 16.16 | 24.72 | 29.75 | 16.24 | 5.27 | 7.87 | 0.22 | 13.54 | 7.55 | 4.93 |
| France | 21.6 | France | 23.62 | 13.69 | 50.54 | 29.3 | 19.3 | 0.67 | 1.17 | 9.27 | 14.7 | 7.32 |
| Germany | 22.3 | Germany | 13.23 | 17.73 | 25.15 | 16.72 | 2.56 | 5.87 | 0.08 | 20.56 | 18 | 10.03 |
| Greece | 24.9 | Greece | 32.22 | 7.42 | 29.26 | 13.66 | 14.7 | 0.87 | 0 | 24.53 | 26.1 | 8.12 |
| Hungary | 26.4 | Hungary | 13.65 | 8.95 | 33.95 | 7.03 | 24.39 | 2.61 | 0 | 14.27 | 21.7 | 15.83 |
| Iceland | 21.9 | Iceland | 18.76 | 20.3 | 47.87 | 16.45 | 14.36 | 11.66 | 5.56 | 2.88 | 8.42 | 4.18 |
| Ireland | 25.3 | Ireland | 19.51 | 14.2 | 47.34 | 14.22 | 20.87 | 6.24 | 6.73 | 6.28 | 21 | 4.52 |
| Italy | 19.9 | Italy | 22.23 | 2.41 | 45.4 | 12.9 | 31.85 | 0 | 0.04 | 10.68 | 20.8 | 14.67 |
| Latvia | 23.6 | Latvia | 10.75 | 21.22 | 38.21 | 26.26 | 11.51 | 0.64 | 0.01 | 5.02 | 14.5 | 9.27 |
| Lithuania | 26.3 | Lithuania | 14.97 | 10.75 | 45.78 | 33.26 | 9.37 | 3.27 | 0.04 | 9.67 | 13.3 | 7.05 |
| Luxembourg | 22.6 | Luxembourg | 23.35 | 7.89 | 37.14 | 12.95 | 22.53 | 0.18 | 0.89 | 14.9 | 17.5 | 11.41 |
| Malta | 28.9 | Malta | 19.18 | 6.56 | 33.08 | 2.94 | 30.15 | 0.38 | 0.24 | 23.48 | 20.5 | 23.14 |
| Netherlands | 20.4 | Netherlands | 9.62 | 24.48 | 32.42 | 13.47 | 11.38 | 2.82 | 4.36 | 0.39 | 14.9 | 8.34 |
| Norway | 23.1 | Norway | 15.28 | 19.33 | 40.04 | 13.73 | 0.04 | 22.09 | 4 | 0.64 | 9.98 | 3.08 |
| Poland | 23.1 | Poland | 20.39 | 11.52 | 33.08 | 17.11 | 14.76 | 1.07 | 0.03 | 13.85 | 20.4 | 6.4 |
| Portugal | 20.8 | Portugal | 17.79 | 4.73 | 46.7 | 9.35 | 34.06 | 0.1 | 3.24 | 9.35 | 17.3 | 12.45 |
| Romania | 22.5 | Romania | 26.14 | 3.79 | 43.56 | 16.74 | 21.42 | 2.86 | 2.41 | 17.57 | 10.6 | 12.1 |
| Slovakia | 20.5 | Slovakia | 20.33 | 7.77 | 30.5 | 5.7 | 17.64 | 6.67 | 0 | 21.82 | 26.8 | 9.76 |
| Slovenia | 20.2 | Slovenia | 11.68 | 3 | 59.92 | 19.39 | 24.26 | 15.04 | 1.26 | 2.99 | 16.5 | 9.46 |
| Spain | 23.8 | Spain | 18.72 | 4.85 | 54.78 | 21.07 | 31.94 | 0.45 | 1.09 | 9.47 | 12.2 | 13.49 |
| Sweden | 20.6 | Sweden | 12.74 | 22.5 | 49.89 | 8.54 | 1.63 | 27.86 | 11.67 | 1.27 | 4.71 | 5.61 |
| UK | 27.8 | UK | 16.95 | 26.82 | 38.41 | 20.72 | 4.61 | 4.76 | 8.16 | 2.28 | 17 | 2.73 |
| Pearson’s, r | 0.122 | 0.056 | −0.96 | 0.011 | 0.004 | −0.291 | −0.132 | 0.268 | 0.183 | 0.246 | ||
| Pearson’s, p | 0.52 | 0.767 | 0.113 | 0.956 | 0.902 | 0.118 | 0.488 | 0.153 | 0.334 | 0.191 | ||
| ANOVA, p | 0.246 | 0.426 | 0.193 | 0.732 | 0.471 | 0.291 | 0.476 | 0.222 | 0.048 | 0.558 | ||
| OR | 1.005 | 1.002 | 0.962 | 1.023 | 0.992 | 0.957 | 0.913 | 1.030 | 1.090 | 1.027 | ||
| CI 95% | 0.875–1.155 | 0.902–1.114 | 0.885–1.045 | 0.921–1.138 | 0.920–1.070 | 0.863–1.061 | 0.709–1.174 | 0.931–1.140 | 0.948–1.254 | 0.883–1.193 | ||
| OR, p | 0.938 | 0.964 | 0.962 | 0.668 | 0.838 | 0.402 | 0.476 | 0.564 | 0.227 | 0.733 |
| Countries | Childhood Obesity 5–9 Years in % (2019) | Countries | J01CR % | J01D % | J01M % | J01A % | J01CA % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Italy | 17.7 | Spain | 31.94 | Greece | 24.53 | Malta | 23.14 | UK | 26.82 | Lithuania | 33.26 |
| Greece | 17.7 | Portugal | 34.06 | Malta | 23.48 | Cyprus | 16.77 | Finland | 24.72 | France | 29.3 |
| Malta | 16.95 | Italy | 31.85 | Slovakia | 21.82 | Hungary | 15.83 | Netherlands | 24.48 | Latvia | 26.26 |
| Cyprus | 15.8 | Malta | 30.15 | Cyprus | 21.11 | Italy | 14.67 | Sweden | 22.5 | Belgium | 21.28 |
| Croatia | 14.95 | Croatia | 26.46 | Germany | 20.56 | Bulgaria | 14.22 | Latvia | 21.22 | Spain | 21.07 |
| Spain | 14.8 | Austria | 25.52 | Bulgaria | 17.9 | Spain | 13.49 | Iceland | 20.3 | UK | 20.72 |
| Portugal | 14.7 | Hungary | 24.39 | Romania | 17.57 | Portugal | 12.45 | Norway | 19.33 | Denmark | 20.54 |
| Hungary | 14.25 | Cyprus | 24.35 | Croatia | 17.24 | Romania | 12.1 | Germany | 17.73 | Slovenia | 19.39 |
| Bulgaria | 13.65 | Slovenia | 24.26 | Luxembourg | 14.9 | Luxembourg | 11.41 | Estonia | 16.58 | Bulgaria | 19.28 |
| Iceland | 12.55 | Belgium | 23.27 | Hungary | 14.27 | Belgium | 10.69 | Ireland | 14.2 | Estonia | 17.36 |
| Ireland | 12.45 | Luxembourg | 22.53 | Poland | 13.85 | Germany | 10.03 | France | 13.69 | Poland | 17.11 |
| Slovenia | 12.45 | Romania | 21.42 | Finland | 13.54 | Slovakia | 9.76 | Czechia | 13.38 | Romania | 16.74 |
| Poland | 12.4 | Ireland | 20.87 | Austria | 12.95 | Slovenia | 9.46 | Cyprus | 12.12 | Germany | 16.72 |
| Czechia | 12.25 | France | 19.3 | Italy | 10.68 | Latvia | 9.27 | Poland | 11.52 | Iceland | 16.45 |
| Finland | 11.7 | Slovakia | 17.64 | Estonia | 10.26 | Netherlands | 8.34 | Denmark | 10.75 | Finland | 16.24 |
| UK | 11.45 | Czechia | 16.67 | Czechia | 10.17 | Estonia | 8.2 | Lithuania | 10.75 | Ireland | 14.22 |
| Germany | 11.35 | Poland | 14.76 | Lithuania | 9.67 | Croatia | 8.13 | Bulgaria | 9.98 | Norway | 13.73 |
| Luxembourg | 11.1 | Greece | 14.7 | Spain | 9.47 | Greece | 8.12 | Belgium | 9.17 | Greece | 13.66 |
| Norway | 11 | Iceland | 14.36 | Portugal | 9.35 | France | 7.32 | Hungary | 8.95 | Netherlands | 13.47 |
| Romania | 10.9 | Estonia | 12.38 | France | 9.27 | Lithuania | 7.05 | Austria | 8.19 | Luxembourg | 12.95 |
| Slovakia | 10.65 | Bulgaria | 11.57 | Belgium | 6.64 | Poland | 6.4 | Luxembourg | 7.89 | Italy | 12.9 |
| Austria | 10.4 | Latvia | 11.51 | Ireland | 6.28 | Czechia | 6.35 | Slovakia | 7.77 | Croatia | 11.74 |
| France | 10.3 | Netherlands | 11.38 | Latvia | 5.02 | Sweden | 5.61 | Greece | 7.42 | Cyprus | 10.39 |
| Denmark | 9.25 | Lithuania | 9.37 | Slovenia | 2.99 | Finland | 4.93 | Croatia | 6.96 | Portugal | 9.35 |
| Lithuania | 9.1 | Finland | 5.27 | Iceland | 2.88 | Ireland | 4.52 | Malta | 6.56 | Sweden | 8.54 |
| Netherlands | 9.1 | UK | 4.61 | UK | 2.28 | Iceland | 4.18 | Spain | 4.85 | Czechia | 7.16 |
| Latvia | 9 | Denmark | 4.21 | Sweden | 1.27 | Denmark | 3.26 | Portugal | 4.73 | Hungary | 7.03 |
| Belgium | 8.6 | Germany | 2.56 | Norway | 0.64 | Norway | 3.08 | Romania | 3.79 | Austria | 6.48 |
| Estonia | 8.4 | Sweden | 1.63 | Netherlands | 0.39 | UK | 2.73 | Slovenia | 3 | Slovakia | 5.7 |
| Sweden | 8.2 | Norway | 0.04 | Denmark | 0.21 | Austria | 0.52 | Italy | 2.41 | Malta | 2.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ternák, G.; Németh, M.; Rozanovic, M.; Márovics, G.; Bogár, L. “Growth-Promoting Effect” of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey. Antibiotics 2022, 11, 1321. https://doi.org/10.3390/antibiotics11101321
Ternák G, Németh M, Rozanovic M, Márovics G, Bogár L. “Growth-Promoting Effect” of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey. Antibiotics. 2022; 11(10):1321. https://doi.org/10.3390/antibiotics11101321
Chicago/Turabian StyleTernák, Gábor, Márton Németh, Martin Rozanovic, Gergely Márovics, and Lajos Bogár. 2022. "“Growth-Promoting Effect” of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey" Antibiotics 11, no. 10: 1321. https://doi.org/10.3390/antibiotics11101321
APA StyleTernák, G., Németh, M., Rozanovic, M., Márovics, G., & Bogár, L. (2022). “Growth-Promoting Effect” of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey. Antibiotics, 11(10), 1321. https://doi.org/10.3390/antibiotics11101321







